Abstract
Diet is a determinant for bodyweight and gut microbiota composition. Changes in dietary patterns are useful for the prevention and management of overweight and obesity. We aim to evaluate diet behavior and its potential association with selected gut bacteria and body weight among Mexican young adults. Mexican college students aged between 18 and 25 (normal-weight, overweight, and obese) were recruited. Anthropometric variables were recorded. A validated food frequency questionnaire was applied to all the participants. The percentages of macronutrients, fiber, and energy were calculated, and fecal samples were analyzed by real-time-qPCR to quantify selected gut bacteria. All the participants showed an unbalanced dietary pattern. However, the consumption of fruits, non-fat cereals, and oils and fats without protein were higher in the normal-weight individuals. In the overweight/obese participants, fiber intake did not correlate with the microbial variables, while Kcal from protein and Clostridium leptum correlated positively with Lactobacillus. Similarly, Clostridium coccoides-Eubacterium rectale correlated with Akkermansia muciniphila. In the normal-weight participants, Clostridium leptum and Lactobacillus correlated positively with Clostridium coccoides-Eubacterium rectale and Bifidobacterium, respectively, and Bacteroidetes negatively with Akkermansia muciniphila. In conclusion, a higher fiber intake had a positive impact on body weight and bacterial gut composition in this Mexican population of college students.
1. Introduction
Overweight and obesity are considered major public health concerns as they are key risk factors for the development of non-communicable diseases (which together cause over 60% of total mortality globally) [1].
The main cause of obesity and weight gain is a positive energy balance consequence of an increased energy intake and a decreased energy output associated with a loss of physical activity [2]. Thus, diet is one of the major determinants for body weight gain as well as a key tool in the prevention, management, and treatment of overweight and obesity [3]. In this context, the insufficient intake of whole grains, fruits, and vegetables, but abundant intake of discretionary foods, such as sugar-sweetened beverages, is ubiquitous, particularly in populations under 30 years old [4]. Accordingly, in recent decades, young adults aged 18–24 are gaining weight faster than their former generations. Indeed, weight gain is prominent among those aged 18–35 in most developed countries [4].
In the students’ population, this phenomenon might be related to the great change in all aspects that university life brings about.
Transition to university in young adults involves a greater autonomy concerning food choices, low food budget, and exposure to new social groups than usual [5,6]. Indeed, the transition from living at home with parents to autonomous university life is often associated with changes such as an increase in alcohol and sugar intake while at the same time a decrease in the consumption of fruits and vegetables [7,8,9].
On the other hand, in the last two decades the gastrointestinal (GI) microbiota (known as the community of microorganisms that subsists within the GI ecosystem) has emerged as a contributor to obesity and obesity-associated diseases [10].
A balanced bacterial composition is important for maintaining intestinal immunity and homeostasis. In healthy individuals, the role of the GI microbiota is to maintain a dynamic balance with the host, playing both local and remote functions in physiological processes such as inflammation and modulation of the immune response [11]. In contrast, an altered GI microbiota profile, referred to as dysbiosis, is found in obesity [12,13,14] and other metabolic diseases (e.g., type II diabetes mellitus and cardiovascular disease [15,16]).
Obese individuals showed different GI microbiota profiles than leans, Bacteroidetes, Firmicutes, and Actinobacteria being the most abundant phyla [16]. A greater Firmicutes/Bacteroidetes ratio, Firmicutes, Fusobacteria, Proteobacteria, Mollicutes, Lactobacillus (strains L. reuteri, L. plantarum, and L. paracasei, among others), and less Verrucomicrobia (Akkermansia muciniphila), Faecalibacterium prausnitzii, Bacteroidetes, and Methanobrevibacter smithii relative abundances have been found in obesity. Moreover, some bacteria present a positive correlation and others a negative correlation with obesity [17].
Having all this in mind, the increased interest in targeting the GI microbiome for the treatment and prevention of obesity is understandable. In this regard, one of the major influences on the microbial signatures of individuals is diet [18], and one way to alter the microbiome is through an increment in dietary fiber intake [19]. According to the United States Food and Drug Administration (FDA), dietary fiber is a carbohydrate component of an edible plant that is resistant to digestion and absorption. Thus, dietary fiber cannot be digested by the host but can be fermented by gut bacteria in the distal intestine, resulting in the production of short-chain fatty acids (SCFAs), which are known to benefit energy homeostasis and metabolism [19].
Nevertheless, to this date, to what extent an intervention with fiber may impact the human GI microbiota and therefore the metabolic regulation is not completely described [20], and it is mandatory, before developing an intervention, to explore as many contributory factors as possible for the entire population in general and for the university young adults in this particular subpopulation. Hence, it is important to analyze the behavior of food consumption together with the other social, biological, and psychological factors with a potential impact on the development of obesity. In consequence, the purpose of this study was to explore diet behavior among Mexican college students and their relationship with the proliferation of various bacterial rows based on body weight. We addressed this issue from nutritional and biological perspectives.
2. Materials and Methods
2.1. Study Design and Subjects
Subjects (females and males) aged between 18 and 25 years were selected in Ciudad Guzmán, a city located in Southern Mexico, from January to February 2014. Initially, 568 subjects were recruited in the first stage of the study, and then 50 subjects were divided according to anthropometric measures, such as body mass index (BMI) [21], into normal-weight and overweight/obese. The study was analytical, descriptive, and transversal. A schematic representation of this study is shown in Figure 1 This study was conducted in accordance with the Declaration of Helsinki and follows the rules of Law 14/2007 on Biomedical Research and the Organic Law 15/1999, RD 1720/2007 on the protection of personal data as well as international rules for research using samples from human beings. The participants’ accepted their inclusion in the study, signing the approved consent protocol. The confidentiality of the data obtained and any personal data used in this study has been kept and respected.
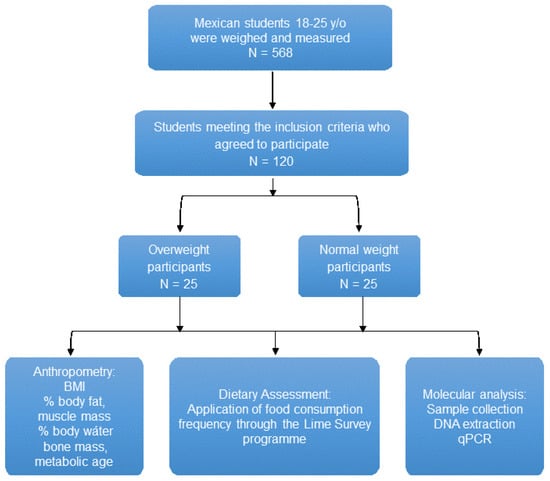
Figure 1.
Schematic diagram of the present study. Abbreviations: BMI, body mass index; DNA, deoxyribonucleic acid; qPCR, quantitative polymerase chain reaction; y/o, years old; %, percentage.
2.2. Variables and Data Collection
2.2.1. Dietary Variables
A food frequency questionnaire (FFQ) validated for the Mexican population was applied [22]. A previous validation was carried out on 40 students from the University of Guadalajara in order to identify local foods and brands to be included in the questionnaire. The percentages of macronutrients (carbohydrates, lipids, proteins), fiber, ethanol, and food groups consumed were obtained after the FFQ analysis. With the information about all the major brands of fermented dairy foods that the participants consume, the colony-forming units (CFUs) variable was determined. A previous validation in 40 students from the University of Guadalajara was made to know the food groups in our population with a 24 h recall questionnaire. The food groups were: dairy products (whole milk, skimmed milk, yogurt), fruits (orange, banana, apple), vegetables (chard, spinach, lettuce), cereals with and without fats (potatoes, corn tortilla, rice, cookies, box bread), animal protein foods (egg, chicken, beef and pork), vegetable protein foods (chia, almonds, nuts), oils and fats with protein (butter, mayonnaise, lard), oils and fats without protein (olive oil, sunflower oil, canola oil), sugars (honey, table sugar, soft drink), and alcoholic beverages (beer, red wine, tequila) [23,24]. The fiber was calculated according to the ingested foods [25].
The participant made a detailed description of the dietary intake (ingredients, method of preparation, and brands); this information allowed the correct coding and weight assignment for each food item. The information obtained was structured as mealtimes (breakfast, mid-morning, lunch, mid-afternoon, dinner, and other moments), which helped us to calculate the distribution of energy and nutrients in the different moments of the day.
To analyze the individual FFQ, foods were grouped according to the United States Department of Agriculture and Human Services (USDA, 2010 [26]), as well as the Mexican System of Equivalents [27], calculating each of the servings (grams) per day for each participant. Likewise, for the CFU of fermented dairy foods, the brands registered by the Procuraduria Federal del Consumidor (PROFECO) [28] were taken as a reference. Total energy was calculated by multiplying the total in grams of each food group by the nutritional contribution of each macronutrient. It is worth mentioning that for the final analysis, a grouping by categorical BMI was performed, dividing the study groups into normal-weight and overweight/obese, to express the results in a general way by study group, presenting medians and percentiles for each of them.
2.2.2. Anthropometric Variables
Body weight was measured in light clothes without shoes using Tanita BC-558 (Arlington Heights, IL, USA). Moreover, other variables were estimated with the aforementioned machine; they were Body fat percentage, Body water percentage, visceral fat percentage, muscle, basal metabolic rate, metabolic age, and bone mass. The participants were classified according to BMI, into overweight/obese (BMI > 25 kg/m2 and normal-weight (BMI = 18.5–24.9 kg/m2). In addition, a subgroup was created for the obese and overweight subjects.
2.3. Microbial Determination
2.3.1. Stool Collection
Fecal samples were collected from each volunteer at the end of the evaluation using a sterile kit (including a plastic bottle with a screw cap, a tongue depressor, a glove, and a plastic bag with sealing). The fecal samples were placed inside the plastic bag and the bag was immediately sealed. Samples were kept at −20 °C and then transferred to −80 °C until analysis.
2.3.2. DNA Extraction
The fecal samples were homogenized in a Stomacher-400 blender. DNA was extracted using a QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions, with the exception that the samples were mixed with the lysis buffer and incubated at a temperature of 95 °C instead of 70 °C to ensure lysis of both the Gram-positive and the Gram-negative bacteria. Quantification was conducted using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Kent, DE, USA) [29].
2.3.3. Identification of the Intestinal Microbiota
The identification of the intestinal microbiota was carried out using specific and detailed primers (Real time-qPCR) with the Applied BioSystems StepOne platform (Waltham, MA, USA). The aforementioned primers, detailed in Table 1, were: Firmicutes (Clostridium coccoides-Eubacterium rectale and Clostridium leptum), Bacteroidetes (Prevotella, Porphyromonas, and Bacteroides), Actinobacteria (Bifidobacterium spp.), Lactobacillus (Lactobacillus spp.), and Akkermansia muciniphila. The PCR reaction was carried out under the following conditions: forward (F) and reverse (R) primers (0.2 µL of each at a concentration of 20 µM), 2 µL of Master Mix (Roche-Applied, Pleasanton, CA, USA), containing SYBR Green, MgCl2, Taq polymerase, and dNTP’s, DNA, and H2O to a final volume of 10 µL were placed individually according to the initial concentration of each sample to obtain a final concentration of 50 ng of DNA in each qPCR reaction. All samples were run in duplicate, and in cases where the duplicate gave a deviation greater than 2 cycles, the reaction was repeated. All target species and PCR program cycles (temperatures/times) are included in Table 1. Primers were synthesized by Invitrogen Life Technologies (Waltham, MA, USA), and were selected according to previously published articles with the idea of obtaining a validated assay for the detection of different bacterial species.

Table 1.
Sequences of primers for real-time qPCR.
2.4. Statistical Analysis
Statistical tests were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA), and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were computed for each variable. All results are expressed as the mean ± standard deviation unless otherwise indicated. All P values were two-tailed, and statistical significance was considered at the 5% level (p < 0.05). To calculate the food consumption of the participants, a data dump was performed in the SPSS program, giving a coding for each response where: (never or almost never = 0, 1–3 times per month = 0.05, 1 time per week = 0.14, 2–4 times per week = 0.42, 5–6 times per week = 0. 78, 1 time per day = 1, 2–4 times per day = 3, 5–6 times per day = 5.5, and more than 6 times per day = 7, expressed in grams); this value was obtained by dividing the average number of times as appropriate (day, week, or month) by 1, 7, and 30 (e.g., 1–3 times per week = 2/30 = 0.06) to obtain the grams consumed per day.
Once each FFQ was coded, the grams consumed per day for each participant were calculated by multiplying the coding of each response by the amount in grams of each of the foods specified in the questionnaire. Subsequently, the foods were grouped according to the USDA to calculate the number of servings consumed per food group by each participant [26].
For the analysis of the fermented dairy products, each product was coded with the CFU reported by the brands asked; however, they were expressed in grams; so, the conversion of CFU contained in the grams of each product was performed and multiplied by the grams asked in the survey. The calculation of fiber was made taking into account the foods that contained more than 3 g of fiber of the FFQ applied, taking the Sistema Mexicano de Equivalentes [27] as a reference, and multiplied by the grams consumed per day by each participant.
Associations between dietary and microbial variables were tested using the Spearman correlation; these associations have shown significant variables, highlighted in red (negatively correlated) or blue (positively correlated), and the findings were corrected for multiple testing using the Benjamini–Hochberg procedure [34], using the corrplot function from the R studio [35]. Finally, differences between microbial variables were calculated using the U-Mann–Whitney test.
3. Results
Participants
In both groups, the sociodemographic and anthropometric data were analyzed (Table 2). No changes were observed in age and gender distribution. The anthropometric data showed differences between normal-weight and overweight/obese in all variables with the exception of height (Table 2).

Table 2.
Sociodemographic and anthropometric characteristics of the study subjects.
According to the food groups, changes were observed in fruits, cereals without fat, and oils and fats without protein. All these variables were higher in the normal-weight participants compared with overweight/obese (Table 3).

Table 3.
Consumption of food portions of the study groups according to the Food Guide recommended intake for Americans 2010 [26,36].
In the case of total energy, no differences were found between the groups, nor Kcal of different macromolecules and daily consumption calculated in grams. When the data were calculated according to recommendations of the USDA [21,29], the percentage of daily consumption was different for carbohydrates, lipids, and proteins (Table 4).

Table 4.
Total energy and macronutrient intake in the study groups.
Microbial changes were observed in Clostridium coccoides-Eubacterium rectale in the comparison between normal-weight and overweight/obese participants (Figure 2).
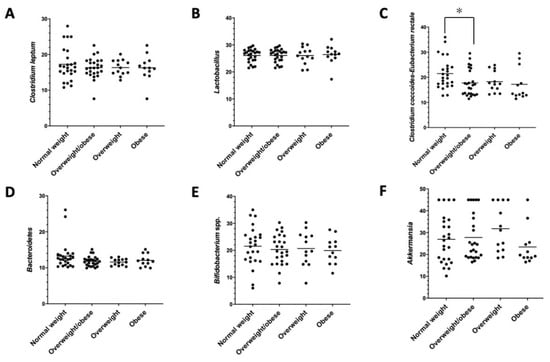
Figure 2.
Microbial differences by qPCR. (A) Clostridium leptum, (B) Lactobacillus spp., (C) Clostridium coccoides-Eubacterium rectale, (D) Bacteroidetes, (E) Bifidobacterium spp., and (F) Akkermansia muciniphila. * p < 0.05, normal-weight vs. overweight/obese subjects.
The correlations between the microbial and dietary variables have shown that fiber consumption, CFU, Kcal from protein, Kcal from carbohydrates, and total energy were correlated negatively with the Bacteroidetes level in the normal-weight group. In the same group, some microbial variables were associated; Clostridium leptum and Lactobacillus were correlated positively with Clostridium coccoides-Eubacterium rectale and Bifidobacterium, respectively. Finally, Bacteroidetes was correlated negatively with Akkermansia muciniphila.
In the case of overweight/obese participants, fiber and CFU do not correlate with the microbial variables; only Kcal from protein was correlated positively with Lactobacillus. Here, Bifidobacterium was positively correlated with Clostridium leptum and Lactobacillus and, in the same way, Clostridium coccoides-Eubacterium rectale and Akkermansia muciniphila (Figure 3).
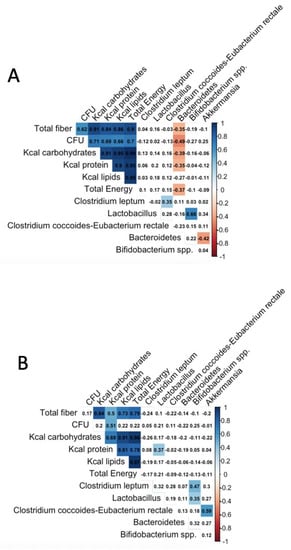
Figure 3.
Spearman correlations between nutritional and microbial variables. (A) Normal-weight subjects, (B) overweight/obese subjects. Associations between dietary and microbial variables were tested using Spearman correlation; these associations have shown significant variables highlighted in red (negatively correlated) or blue (positively correlated), and findings were corrected for multiple testing using the Benjamini–Hochberg procedure [34], using the corrplot function from the R studio [35]. Abbreviations: BMI, body mass index; qPCR, quantitative polymerase chain reaction.
4. Discussion
Diet is one of the major determinants for body weight gain as well as a key tool in the prevention, management, and treatment of overweight and obesity. Insufficient intake of whole grains, fruits, and vegetables, but abundant intake of discretionary foods, such as sugar-sweetened beverages, are globally extended, particularly in young adults and college students [37,38]. Furthermore, a balanced microbial composition is important for maintaining intestinal immunity and homeostasis [39], while an altered gut intestinal microbiota is found in obesity and other metabolic diseases [40]. In addition, it is well known that one of the major influences on the microbial signatures of individuals is diet [41], and one way to alter the microbiome is through an increment in dietary fiber intake [42].
Bearing in mind that which is mentioned above, in the present study we evaluated diet behavior among Mexican college students and their relationship with the proliferation of various bacterial rows based on body weight, addressing this issue from nutritional and biological perspectives.
Our results show that both the normal-weight and overweight/obese groups are above the recommended dietary intakes (RDI) according to the percentage of adequacy in the consumption of alcoholic beverages, sugar, dairy products, grains and cereals, vegetables, and fruits. Besides, the consumption of fiber, oils, fats, and protein foods is below the RDI [43]. Neither of the total energy differences was found between the groups, which is in accordance with the results reported by Koo et al. (2019) [44]. These results indicate that both groups have an unhealthy diet and are at risk of presenting metabolic alterations, reflecting that adequate or healthy nutrition is not a priority at this stage of their lives. In accordance, college students tend to choose foods by cost and the ease of obtaining and consuming them; these are predominantly industrialized foods made by unhealthy preparations (e.g., fried), high in carbohydrates, fat, and energy and with low nutritional quality. Additionally, the influence of economics on food selection was evidenced by the fact that the consumption of proteins, which are generally more expensive, was markedly below the RDI. By contrast, more economical foods, such as vegetables, fruits, and sugars, were consumed to a greater extent. Moreover, and as reported by Sogari [45] et al., these strata of the population tend to migrate to have access to university, which is usually the first stage of life where they manage their food by themselves, which, together with the limited time available, can affect food selection and the establishment of adequate dietary patterns [45].
Nevertheless, significant differences were found between the groups (normal-weight and obese/overweight) in the consumption of fruits, non-fat cereals, and oils and fats without protein, reflecting the dietary contrasts in the sample studied. Consequently, this may influence to a greater or lesser extent the presence of obesity and metabolic disorders, which can also be reflected in the fact that the anthropometric data show differences between normal-weight and overweight/obese in all the variables with the exception of height.
On the other hand, the most common organisms in human gut microbiota are members of the Gram-positive Firmicutes and the Gram-negative Bacteroidetes phyla, with several other phyla, including the Actinobacteria, Fusobacteria, and Verrucomicrobia, that are present at subdominant levels [46]. Studies on the human intestinal microbiota have shown that obesity is associated with a reduction in Gram-negative bacteria, specifically members of the Bacteroidetes phyla [47]. Additionally, Lactobacillus can reduce body weight and alleviate fat accumulation in mice fed with a high-fat diet [48]. On the other hand, Firmicutes and Actinobacteria are the main responders to dietary fiber [49]. Body composition has been associated with higher levels of Akkermansia muciniphila, which may mediate the effects of dietary fiber [50].
Consequently, we selected the phyla Bacteroidetes (Prevotella, Porphyromonas, and Bacteroides), Firmicutes (Clostridium coccoides-Eubacterium rectale and Clostridium leptum), Actinobacteria (Bifidobacterium spp.), Lactobacillus spp., and Akkermansia muciniphila to compare the composition and expression of the intestinal microbiota of overweight/obese vs. normal-weight students, as well as the changes potentially associated with fiber intake, using bacterial probes that could identify several species (Table 1).
Interestingly, the phyla belonging to Firmicutes were found to be mostly expressed in overweight and obese individuals, with mainly Clostridium coccoides-Eubacterium rectale revealing a higher level in the composition of the intestinal microbiota of the overweight and obese individuals compared to the normal-weight subjects. Similar results have been found in a randomized clinical trial where Blautia, Romboutsia, Ruminococcus2, Clostridium sensu stricto, and Dorea were positively correlated with indicators of bodyweight [51].
In addition, the correlations between the microbial and dietary variables have shown that fiber consumption primarily in the form of non-fat cereal, fermented dairy foods, Kcal from protein, Kcal from carbohydrates, and total energy was correlated negatively with the Bacteroidetes level in the normal-weight group. In this respect, Wu and colleagues reported that high fecal Bacteroides abundance was positively associated with a protein- and animal-fat-rich diet and negatively with fiber [52], while other studies indicate that a fiber diet increases Bacteroides [53]. Moreover, in a large study conducted by Menni et al. (2017) fiber intake was positively correlated with measures of microbiome diversity; the conclusion was that gut microbiome diversity and high-fiber intake are related to lower long-term weight gain [54]. Other authors reported that although they found differences in the gut microbiome in obese individuals, fiber and fat/saturated fat diets were not key for central obesity [44].
Discrepancies could be attributed to the extent of the diet (short-term versus long-term). Given these controversial results, we agree with the reflection of Johnson and colleagues that reconciling the long-term and population-level patterns with the short-term observations requires a better understanding of every diet influence on digestive features such as changes in bile, pH, or substrate availability [47]. Moreover, the inconsistencies and contradictions between findings might also be explained because the influence of confounding factors, such as the composition of the diet, the energy content of the diet, the use of antibiotics, food availability, geographical areas, or age, are all factors that affect the gut microbial composition.
In the overweight/obese participants, fiber intake, and CFU do not correlate with the microbial variables, while Kcal from protein and Clostridium leptum was correlated positively with Lactobacillus. In the same way, Clostridium coccoides-Eubacterium rectale correlated with Akkermansia muciniphila. In the normal-weight participants, Clostridium leptum and Lactobacillus were correlated positively with Clostridium coccoides-Eubacterium rectale and Bifidobacterium, respectively, and Bacteroidetes was correlated negatively with Akkermansia muciniphila. In patients with type 2 diabetes, A. muciniphila was negatively associated with hemoglobin A1c [55]; its relative abundance is negatively associated with body mass [32,56,57,58].
Altogether, these results show a relationship between food consumption and the composition of the intestinal microbiota. This supports the fact that the gut microbiota composition responds to dietary patterns determined, among others, by the competition of the substrates that the bacterial species obtained from the diet.
In sum, the analysis of dietary intake from a biological and nutritional perspective, including a detailed study of energy, macronutrients, fiber, and CFU intake, and their relationship with gut microbiota and body weight, is the main contribution of this work. However, one of the main limitations of this study is the cross-sectional design which limited us to establishing an association between the variables at a specific time, but we were not able to establish cause–effect relationships between diet and the composition of the intestinal microbiota. It is also worthy to note that this study did not consider factors such as physical activity, social status, and the extent of obesity in time. Finally, the bacterial probes that were used in the study were selected in the literature, and they could be named as the Lactobacillus spp. or the Clostridium leptum group; however, these groups could include different species with several identification ratios.
We are aware that the bacterial population of the intestine has been performed by qPCR and not by 16S rRNA gene sequencing, the most-used sequence-based bacterial analysis for decades, which would give a broader view to this study. However, we believe that the selection of the analyzed bacteria has been sufficiently justified and our results sufficiently conclusive.
Another very interesting aspect that remains to be evaluated is the difference between the subgroups of obese and overweight. However, the number of subjects available was very limited, which did not allow us to carry out these analyses.
Nevertheless, although other studies have already reported alterations in the intestinal microbiota related to body weight, this study not only corroborates those results but extends them to a well-established population of young university students. Our study was conducted in a very homogeneous population in a well-limited geographical area, which limited the bias due to geographical origin–location.
Importantly, the participants are also in an important lifetime period, the transition from youth to maturity when newly acquired habits concerning diet and their effects may be crucial for the rest of their lives.
Consequently, the more knowledge we have the better to design appropriate dietary interventions in a specific context. Such strategies should not be limited only to variables related to energy and macronutrient intake but should be redirected to the integral analysis of nutrition in a multidisciplinary manner, considering aspects such as genetics, environment, and lifestyles, among others.
To conclude our study, we revealed an unbalanced dietary pattern in the normal-weight and overweight/obese subjects in a subpopulation of Mexican young adults. The phyla belonging to Firmicutes were found to be mostly expressed in overweight and obese individuals, indicating an alteration in the composition of the intestinal microbiota of these individuals compared to the normal-weight subjects. Increased fiber intake in the participants influences to a lesser extent the presence of obesity and overweight and a bacterial gut composition more associated with health.
Author Contributions
Conceptualization, A.R.-L. and Z.R.-C.; methodology, P.L.-U., A.V.-A. and Z.R.-C.; software, A.R.-L.; validation, A.R.-L., P.L.-U., A.V.-A. and Z.R.-C.; formal analysis, A.R.-L., J.P.-D., P.L.-U., A.V.-A., Z.R.-C. and A.I.Á.-M.; investigation, A.R.-L., P.L.-U., A.V.-A. and Z.R.-C.; data curation, J.P.-D. and A.I.Á.-M.; writing—original draft preparation, A.R.-L., J.P.-D. and A.I.Á.-M.; writing—review and editing, A.R.-L., J.P.-D., P.L.-U., A.V.-A., Z.R.-C. and A.I.Á.-M. All authors have read and agreed to the published version of the manuscript.
Funding
A.R.-L. was granted a fellowship from the National Council for Science and Technology (CONACYT). Grant No. 365902 and Registration No. 297325.
Institutional Review Board Statement
The present study was considered non-therapeutic research and adhered to the bases established for research on human beings indicated in article 100, numbers I to VII of the Fifth Title of the General Law of Health on Research for Health Matters [59,60], which supports the part related to the use of the methodology established in NOM-008-SSA2-1993. The integrity and well-being of each participant who took part in this study were respected by providing them with an informed consent form, which indicated the commitment to protect the participant’s privacy, notifying them that they could leave the study if they so wished. Likewise, there were no conflicts of interest involved; therefore, neither the participants nor the research team obtained any economic benefit. Therefore, the present study was approved by the ethics committee of the Centro de Investigación en Comportamiento Alimentario y Nutrición (CICAN). Finally, the procedures involved minimal risks, considering that they do not violate the ethical principles for medical research on human subjects established in the rules of Law 14/2007 on Biomedical Research and the Organic Law 15/1999, RD 1720/2007 on the protection of personal data as well as the international rules for research using samples from human beings.
Informed Consent Statement
The participants’ accepted their inclusion in the study, signing the approved protocol. The confidentiality of the data obtained and any personal data used in this study has been kept and respected.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
Julio Plaza-Diaz is part of the “UGR Plan Propio de Investigación 2016” and the “Excellence actions: Unit of Excellence on Exercise and Health (UCEES), University of Granada”. Julio Plaza-Diaz is supported by a fellowship awarded to postdoctoral researchers at foreign universities and research centers from the “Fundación Ramón Areces”, Madrid, Spain. Claudia Patricia Beltrán Miranda for her support and dedication to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iversen, K.N.; Carlsson, F.; Andersson, A.; Michaelsson, K.; Langton, M.; Riserus, U.; Hellstrom, P.M.; Landberg, R. A hypocaloric diet rich in high fiber rye foods causes greater reduction in body weight and body fat than a diet rich in refined wheat: A parallel randomized controlled trial in adults with overweight and obesity (the RyeWeight study). Clin. Nutr. ESPEN 2021, 45, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varkevisser, R.D.M.; van Stralen, M.M.; Kroeze, W.; Ket, J.C.F.; Steenhuis, I.H.M. Determinants of weight loss maintenance: A systematic review. Obes. Rev. 2019, 20, 171–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, L.M.Y.; Fong, S.S.M.; Law, Q.P.S. Younger Adults Are More Likely to Increase Fruit and Vegetable Consumption and Decrease Sugar Intake with the Application of Dietary Monitoring. Nutrients 2021, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Sprake, E.; Russell, J.; Cecil, J.; Cooper, R.; Grabowski, P.; Pourshahidi, L.K.; Barker, M. Dietary patterns of university students in the UK: A cross-sectional study. Nutr. J. 2018, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Arbués, E.; Granada-López, J.-M.; Martínez-Abadía, B.; Echániz-Serrano, E.; Antón-Solanas, I.; Jerue, B.A. Factors Related to Diet Quality: A Cross-Sectional Study of 1055 University Students. Nutrients 2021, 13, 3512. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Ali, B.; Cameron, A.; Armstrong, M.E.; Isaacs, P.; Thomas, K.S.; Gadbois, E.A.; Willis, P. ‘It’s not just about the dinner; it’s about everything else that we do’: A qualitative study exploring how Meals on Wheels meet the needs of self-isolating adults during COVID-19. Health Soc. Care Community 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Mitri, R.; Boulos, C.; Ziade, F. Mediterranean diet adherence among adolescents in North Lebanon: The role of skipping meals, meals with the family, physical activity, and physical wellbeing. Br. J. Nutr. 2021, 1–8, in press. [Google Scholar] [CrossRef] [PubMed]
- Nuss, T.; Morley, B.; Scully, M.; Wakefield, M. Energy drink consumption among Australian adolescents associated with a cluster of unhealthy dietary behaviours and short sleep duration. Nutr. J. 2021, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Granata, I.; Nardelli, C.; D’Argenio, V.; Tramontano, S.; Compare, D.; Guarracino, M.R.; Nardone, G.; Pilone, V.; Sacchetti, L. Duodenal Metatranscriptomics to Define Human and Microbial Functional Alterations Associated with Severe Obesity: A Pilot Study. Microorganisms 2020, 8, 1811. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornejo-Pareja, I.; Munoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Martinez, T.M.; Meyer, R.K.; Duca, F.A. Therapeutic Potential of Various Plant-Based Fibers to Improve Energy Homeostasis via the Gut Microbiota. Nutrients 2021, 13, 3470. [Google Scholar] [CrossRef] [PubMed]
- Poobalan, A.S.; Aucott, L.S.; Clarke, A.; Smith, W.C. Diet behaviour among young people in transition to adulthood (18–25 year olds): A mixed method study. Health Psychol. Behav. Med. 2014, 2, 909–928. [Google Scholar] [CrossRef]
- Hypponen, E.; Carslake, D.; Berry, D.J.; Power, C.; Davey Smith, G. Estimating the influence of body mass index (BMI) on mortality using offspring BMI as an instrumental variable. Int. J. Obes. 2022, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Macedo-Ojeda, G.; Vizmanos-Lamotte, B.; Marquez-Sandoval, Y.F.; Rodriguez-Rocha, N.P.; Lopez-Uriarte, P.J.; Fernandez-Ballart, J.D. Validation of a semi-quantitative food frequency questionnaire to assess food groups and nutrient intake. Nutr. Hosp. 2013, 28, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Molina-Montes, E.; Soto-Méndez, M.J.; Madrigal, C.; Hernández-Ruiz, Á.; Valero, T.; Lara Villoslada, F.; Leis, R.; Martínez de Victoria, E.; Moreno, J.M. Clustering of dietary patterns and lifestyles among spanish children in the EsNuPi study. Nutrients 2020, 12, 2536. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, C.; Soto-Méndez, M.J.; Hernández-Ruiz, Á.; Valero, T.; Ávila, J.M.; Ruiz, E.; Lara Villoslada, F.; Leis, R.; Martínez de Victoria, E.; Moreno, J.M. Energy intake, macronutrient profile and food sources of spanish children aged one to <10 years—Results from the EsNuPI Study. Nutrients 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tester, J.M.; Leak, T.M. Fiber-rich foods delivered to Low-Income Households: A feasibility study of children with prediabetes and spillover effect on their caregivers. Prev. Med. Rep. 2021, 24, 101511. [Google Scholar] [CrossRef] [PubMed]
- United States. Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2010; US Department of Health and Human Services, US Department of Agriculture: Washington, DC, USA, 2010.
- Pérez-Lizaur, A.; Palacios-González, B.; Castro-Becerra, A.; Flores-Galicia, I. Sistema Mexicano de Alimentos Equivalentes (Mexican Equivalent Food System); Fomento de Nutrición y Salud, AC: Mexico City, Mexico, 2014; pp. 31–36. [Google Scholar]
- Procuraduría Federal del Consumidor. Gobierno de Mexico, PROFECO. Available online: https://www.gob.mx/profeco (accessed on 12 February 2022).
- Plaza-Diaz, J.; Fernandez-Caballero, J.A.; Chueca, N.; Garcia, F.; Gomez-Llorente, C.; Saez-Lara, M.J.; Fontana, L.; Gil, A. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.; Tannock, G.W.; Tilsala-Timisjarvi, A.; Rodtong, S.; Loach, D.M.; Munro, K.; Alatossava, T. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 2000, 66, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- McGuire, S. US department of agriculture and US department of health and human services, dietary guidelines for Americans, 2010. Washington, DC: US government printing office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A. Healthy lifestyles in Europe: Prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001, 4, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallus, S.J.; Brandt, L.J. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.; Blottiere, H. The influence of diet on the gut microbiota and its consequences for health. Curr. Opin. Biotechnol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yokomichi, H.; Matsubara, H.; Ishikuro, M.; Kikuya, M.; Isojima, T.; Yokoya, S.; Tanaka, T.; Kato, N.; Chida, S.; et al. Longitudinal changes in body mass index of children affected by the Great East Japan Earthquake. Int. J. Obes. 2017, 41, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar] [PubMed]
- Koo, S.H.; Chu, C.W.; Khoo, J.J.C.; Cheong, M.; Soon, G.H.; Ho, E.X.P.; Law, N.M.; De Sessions, P.F.; Fock, K.M.; Ang, T.L.; et al. A pilot study to examine the association between human gut microbiota and the host’s central obesity. JGH Open 2019, 3, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Sogari, G.; Velez-Argumedo, C.; Gomez, M.I.; Mora, C. College Students and Eating Habits: A Study Using An Ecological Model for Healthy Behavior. Nutrients 2018, 10, 1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frugé, A.D.; Van der Pol, W.; Rogers, L.Q.; Morrow, C.D.; Tsuruta, Y.; Demark-Wahnefried, W. Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J. Acad. Nutr. Diet. 2020, 120, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Sasaki, T.; Itoh, K.; Kitahara, T.; Takema, Y.; Hiramatsu, K.; Ishikawa, D.; Shibuya, T.; Kobayashi, O.; Osada, T.; et al. A Soluble Fiber Diet Increases Bacteroides fragilis Group Abundance and Immunoglobulin A Production in the Gut. Appl. Environ. Microbiol. 2020, 86, e00405-20. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, C.T.; Yeh, Y.T.; Lin, C.C.; Yang, L.Y.; Chiang, C.P. Akkermansia muciniphila is Negatively Correlated with Hemoglobin A1c in Refractory Diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, S.L.; Hervey, T.K.; Mackenbach, J.P.; McKee, M. Health law and policy in the European Union. Lancet 2013, 381, 1135–1144. [Google Scholar] [CrossRef]
- Burris, S.; Anderson, E. Legal regulation of health-related behavior: A half century of public health law research. Annu. Rev. Law Soc. Sci. 2013, 9, 95–117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).