Effect of Gut Microbial Enterotypes on the Association between Habitual Dietary Fiber Intake and Insulin Resistance Markers in Mexican Children and Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Sample and Data Collection
2.2. Clinical and Biochemical Measurements
2.3. Assessment of Habitual Dietary Intake
2.4. Gut Microbiota Characterization and Enterotype Stratification
2.5. Fecal Short-Chain Fatty Acids Analysis
2.6. Statistical Analysis
3. Results
3.1. Description of Study Sample
3.2. Enterotypes Stratification, Nutrient Intake and Phenotype Differences
3.3. Associations between Dietary Fiber Intake and Metabolic Traits in All Participants
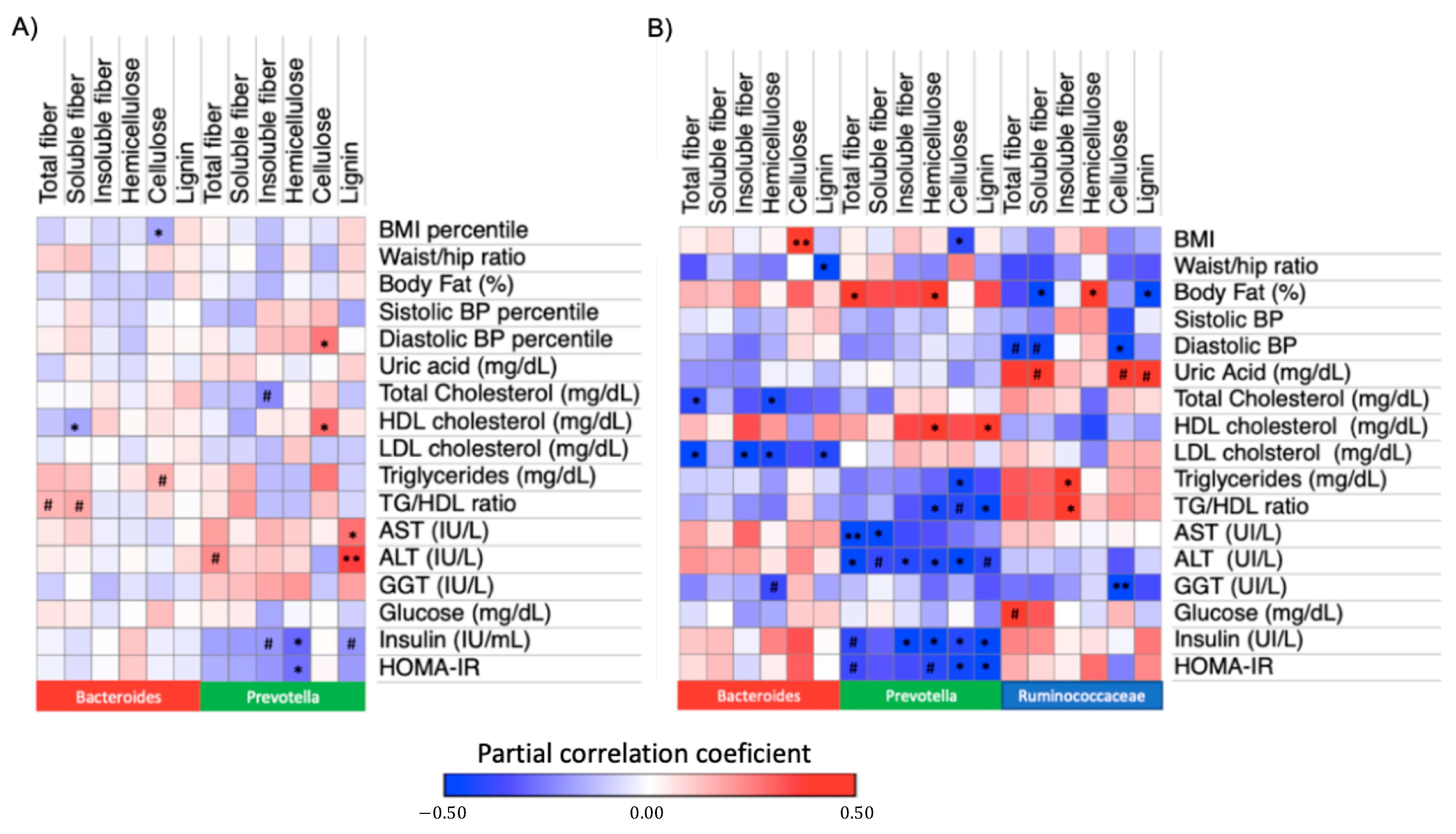
3.4. Associations between Dietary Fiber Intake and Metabolic Traits within Enterotypes
3.5. Association between Hemicellulose Intake and Insulin Resistance Markets among DMM Enterotypes
3.6. Association between Hemicellulose Intake and Insulin Resistance Markers Stratifying by Prevotella to Bacteroides Ratio
3.7. Association between Dietary Fiber Consumption and Fecal Short-Chain Fatty Acids (SCFA) among Enterotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [Green Version]
- Lahti-Koski, M.; Pietinen, P.; Heliövaara, M.; Vartiainen, E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK Studies. Am. J. Clin. Nutr. 2002, 75, 809–817. [Google Scholar] [CrossRef] [Green Version]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.; Ordovas, J.M.; Martínez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, L.; Gutin, B.; Zhu, H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur. J. Clin. Nutr. 2019, 73, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Fiber Intake and Insulin Resistance in 6374 Adults: The Role of Abdominal Obesity. Nutrients 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Tuncil, Y.E.; Thakkar, R.D.; Marcia, A.D.R.; Hamaker, B.R.; Lindemann, S.R. Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions. Sci. Rep. 2018, 8, 16655. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derrien, M.; Veiga, P. Rethinking Diet to Aid Human-Microbe Symbiosis. Trends Microbiol. 2017, 25, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Vuholm, S.; Roager, H.M.; Nielsen, D.S.; Krych, L.; Kristensen, M.; Astrup, A.; Hjorth, M.F. Prevotella Abundance Predicts Weight Loss Success in Healthy, Overweight Adults Consuming a Whole-Grain Diet Ad Libitum: A Post Hoc Analysis of a 6-Wk Randomized Controlled Trial. J. Nutr. 2019, 149, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2019, 43, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, M.F.; Christensen, L.; Kjølbæk, L.; Larsen, L.H.; Roager, H.M.; Kiilerich, P.; Kristiansen, K.; Astrup, A. Pretreatment Prevotella-to-Bacteroides ratio and markers of glucose metabolism as prognostic markers for dietary weight loss maintenance. Eur. J. Clin. Nutr. 2020, 74, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.E.; Ibarra-Gonzalez, I.; et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes 2020, 11, 900–917. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Ocampo-Medina, E.; Gutierrez-Aguilar, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; López-Contreras, B.E.; León-Mimila, P.; Vega-Badillo, J.; Gutierrez-Vidal, R.; Villarruel-Vazquez, R.; et al. An Amino Acid Signature Associated with Obesity Predicts 2-Year Risk of Hypertriglyceridemia in School-Age Children. Sci. Rep. 2017, 7, 5607. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Avila, M.; Resoles, M.; Parra, S.; Romieu, I. SNUT Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos; Instituto Nacional de Salud Públic: Cuernavaca, Mexico, 2003. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Lupton, J.R. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; Institute of Medicine: Washington, DC, USA, 2002. [Google Scholar]
- López-Contreras, B.E.; Morán-Ramos, S.; Villarruel-Vázquez, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; León-Mimila, P.; Vega-Badillo, J.; Sánchez-Muñoz, F.; Llanos-Moreno, L.E.; Canizalez-Román, A.; et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Holmes, I.; Harris, K.; Quince, C. Dirichlet multinomial mixtures: Generative models for microbial metagenomics. PLoS ONE 2012, 7, e30126. [Google Scholar] [CrossRef] [Green Version]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; van der Wal, P.; Jong, J.C.K.-D.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.P.; Jaddoe, V.W.V.; et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef] [Green Version]
- Koren, O.; Knights, D.; Gonzalez, A.; Waldron, L.; Segata, N.; Knight, R.; Huttenhower, C.; Ley, R.E. A guide to enterotypes across the human body: Meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput. Biol. 2013, 9, e1002863. [Google Scholar] [CrossRef] [Green Version]
- León-Mimila, P.; Villamil-Ramírez, H.; López-Contreras, B.E.; Morán-Ramos, S.; Macias-Kauffer, L.R.; Acuña-Alonzo, V.; Del Río-Navarro, B.E.; Salmerón, J.; Velazquez-Cruz, R.; Villarreal-Molina, T.; et al. Low Salivary Amylase Gene (AMY1) Copy Number Is Associated with Obesity and Gut Prevotella Abundance in Mexican Children and Adults. Nutrients 2018, 10, 1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syeda, T.; Sanchez-Tapia, M.; Pinedo-Vargas, L.; Granados, O.; Cuervo-Zanatta, D.; Rojas-Santiago, E.; Díaz-Cintra, S.A.; Torres, N.; Perez-Cruz, C. Bioactive Food Abates Metabolic and Synaptic Alterations by Modulation of Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 1657–1682. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Shen, W.; Heymsfield, S.B.; Heshka, S. Percentage body fat ranges associated with metabolic syndrome risk: Results based on the third National Health and Nutrition Examination Survey (1988–1994). Am. J. Clin. Nutr. 2003, 78, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Lairon, D.; Arnault, N.; Bertrais, S.; Planells, R.; Clero, E.; Hercberg, S.; Boutron-Ruault, M.C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am. J. Clin. Nutr. 2005, 82, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef]
- Martínez, I.; Stegen, J.C.; Maldonado-Gómez, M.X.; Eren, A.M.; Siba, P.M.; Greenhill, A.R.; Walter, J. The gut microbiota of rural papua new guineans: Composition, diversity patterns, and ecological processes. Cell Rep. 2015, 11, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Petrzelkova, K.J.; Burns, M.B.; Yeoman, C.J.; Amato, K.R.; Vlckova, K.; Modry, D.; Todd, A.; Robinson, C.A.J.; Remis, M.J.; et al. Gut Microbiome of Coexisting BaAka Pygmies and Bantu Reflects Gradients of Traditional Subsistence Patterns. Cell Rep. 2016, 14, 2142–2153. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.Y.; Rho, M.; Song, Y.M.; Lee, K.; Sung, J.; Ko, G. Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci. Rep. 2014, 4, 7348. [Google Scholar] [CrossRef] [Green Version]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Bel Lassen, P.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [Green Version]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total Sample (n = 204) | Bacteroides (n = 136) | Prevotella (n = 68) | p | |
|---|---|---|---|---|
| Sex; n (%) | ||||
| Boys | 109 (53.4) | 69.0 (50.7) | 40.0 (58.8) | 0.30 |
| Girls | 95.0 (46.6) | 67.0 (49.3) | 28.0 (41.2) | |
| Nutritional status; n (%) | ||||
| Underweight–normal weight | 130 (63.7) | 91.0 (66.9) | 39.0 (57.4) | 0.18 |
| Overweight–obesity | 74.0 (36.3) | 45.0 (33.1) | 29.0 (42.7) | |
| Age (years) | 9.40 (8.38–10.9) | 9.56 (8.40–10.9) | 9.26 (8.30–11.1) | 0.98 |
| Waist/hip ratio | 0.87 (0.83–0.92) | 0.87 (0.83–0.91) | 0.89 (0.83–0.93) | 0.14 |
| BMI (percentil) | 74.7 (31.7–92.4) | 68.8 (29.8–90.5) | 79.9 (37.0–93.9) | 0.19 |
| Body fat (%) | 31.5 (24.2–39.1) | 30.2 (23.5–38.4) | 33.8 (24.8–40.9) | 0.20 |
| Sistolic BP (percentil) | 45.9 (25.4–69.0) | 45.9 (25.0–69.0) | 45.9 (27.2–69.0) | 0.86 |
| Diastolic BP (percentil) | 81.1 (64.4–90.1) | 81.5 (66.2–90.3) | 80.1 (62.0–89.8) | 0.53 |
| Creatinin (mg/dL) | 0.46 (0.40–0.51) | 0.45 (0.40–0.51) | 0.47 (0.40–0.53) | 0.13 |
| Uric acid (mg/dL) | 4.5 (3.9–5.2) | 4.50 (3.90–5.20) | 4.50 (4.00–5.50) | 0.50 |
| Total cholesterol (mg/dL) | 164 (147–184) | 163 (144–185) | 166 (150–183) | 0.41 |
| HDL (mg/dL) | 48.0 (42.0–56.0) | 47.5 (41.0–56.0) | 48.0 (42.0–57.0) | 0.99 |
| LDL (mg/dL) | 97.0 (83.0–113) | 97.5 (84.0–114) | 97.5 (82.25–113) | 0.75 |
| Triglycerides (mg/dL) | 78.0 (54.3–107) | 70.5 (51.3–99.5) | 91.0 (67.3–134) | 1 × 10−3 |
| AST (IU/L) | 29.0 (26.0–34.0) | 29.0 (27.0–35.0) | 29.0 (26.0–34.0) | 0.39 |
| ALT (IU/L) | 20.0 (16.0–25.0) | 20.0 (16.0–26.0) | 19.0 (16.0–24.0) | 0.62 |
| GGT (IU/L) | 13.0 (11.0–16.0) | 13.0 (12.0–16.0) | 13.0 (11.0–16.0) | 0.45 |
| Glucose (mg/dL) | 88.0 (85.0–92.0) | 88.0 (85.0–92.0) | 89.0 (84.3–93.0) | 0.56 |
| Insulin (IU/mL) | 5.50 (3.60–7.88) | 5.15 (3.45–7.45) | 5.85 (3.83–9.03) | 0.21 |
| LPS (pg/mL) | 0.99 (0.48–1.36) | 0.70 (0.51–1.38) | 0.82 (0.40–1.23) | 0.79 |
| C-reactive protein (mg/dL) | 0.07 (0.02–0.17) | 0.07 (0.01–0.16) | 0.07 (0.02–0.18) | 0.61 |
| HOMA-IR | 1.17 (0.76–1.77) | 1.13 (0.76–1.69) | 1.24 (0.82–2.01) | 0.23 |
| Total energy intake (kcal/day) | 1824.14 (1431.29–2201.73) | 1846.04 (1440.87–2154.09) | 1809.95 (1406.36–2328.50) | 0.85 |
| Total DF (g/1000 kcal/day) | 10.4 (8.73–11.8) | 10.4 (8.81–11.6) | 10.2 (8.47–12.0) | 0.86 |
| Soluble fiber (g/1000 kcal/day) | 2.95 (2.44–3.53) | 3.01 (2.50–3.44) | 2.90 (2.37–3.59) | 0.55 |
| Insoluble fiber (g/1000 kcal/day) | 5.67 (4.94–6.59) | 5.69 (4.99–6.59) | 5.62 (4.81–6.72) | 0.60 |
| Hemicellulose (g/1000 kcal/day) | 1.82 (1.53–2.31) | 1.82 (1.52–2.29) | 1.82 (1.55–2.34) | 0.80 |
| Cellulose (g/1000 kcal/day) | 2.67 (1.96–4.24) | 2.66 (2.00–4.05) | 2.89 (1.85–4.45) | 0.87 |
| Lignin (g/1000 kcal/day) | 0.46 (0.37–0.57) | 0.46 (0.38–0.55) | 0.45 (0.35–0.60) | 0.91 |
| AI of dietary fiber; n (%) | 23.0 (11.2) | 13.0 (9.56) | 10.0 (14.7) | 0.27 |
| Total Sample (n = 75) | Bacteroides (n = 27) | Prevotella (n = 27) | Ruminococcaceae (n = 21) | p | |
|---|---|---|---|---|---|
| Sex; n (%) | |||||
| M | 13.0 (17.3) | 5.0 (18.52) | 6.00 (22.22) | 2.00 (9.52) | 0.50 |
| F | 62.0 (82.7) | 22.0 (81.48) | 21.0 (77.78) | 19.0 (90.48) | |
| Nutritional status; n (%) | |||||
| Underweight–normal weight | 20.0 (26.7) | 9.00 (33.3) | 5.00 (18.5) | 6.00 (28.6) | 0.46 |
| Overweight–obesity | 55.0 (73.3) | 18.0 (66.7) | 22.0 (81.5) | 15.0 (71.4) | |
| Age (years) | 39.0 (34.0–42.0) | 40.0 (35.0–43.0) | 38.0 (32.0–41.0) | 39.0 (35.0–41.5) | 0.30 |
| Waist/hip radio | 0.85 (0.81–0.90) | 0.85 (0.82–0.90) | 0.85 (0.80–0.92) | 0.82 (0.79–0.89) | 0.54 |
| BMI (kg/m²) | 27.8 (24.8–30.4) | 26.6 (24.0–29.4) | 29.0 (26.3–32.5) | 27.1 (24.8–29.5) | 0.16 |
| Body fat (%) | 35.3 (30.5–41.2) | 35.0 (28.6–37.0) | 38.5 (33.0–42.5) | 35.3 (29.0–43.5) | 0.13 |
| Sistolic BP (mmHg) | 113 (101–122) | 113 (93.5–118) | 114 (101–126) | 116 (103–122) | 0.57 |
| Diastolic BP (mmHg) | 73.0 (67.0–79.3) | 76.0 (61.0–81.0) | 71.0 (67.8–78.0) | 73.0 (70.0 -80.0) | 0.64 |
| Creatinin (mg/dL) | 0.66 (0.58–0.74) | 0.69 (0.60–0.79) | 0.64 (0.57–0.81) | 0.66 (0.58–0.71) | 0.55 |
| Uric acid (mg/dL) | 4.80 (4.30–5.70) | 4.90 (4.40–5.50) a | 5.30 (4.40–6.20) a | 4.40 (3.35–4.85) b | 4 × 10−3 |
| Total cholesterol (mg/dL) | 192 (164–213) | 196 (170–228) | 183 (163–198) | 192 (163–216) | 0.19 |
| HDL (mg/dL) | 48.0 (39.0–56.0) | 50.0 (42.0–58.0) | 43.0 (35.0–54.0) | 49.0 (45.0–55.0) | 0.12 |
| LDL (mg/dL) | 117 (91.8–141) | 124 (100–151) | 106 (85.6–127) | 111 (96.1–139) | 0.13 |
| Triglycerides (mg/dL) | 117 (95.0–177) | 115 (83.0–177) | 120 (103–205) | 103 (85.0–162) | 0.33 |
| AST (IU/L) | 22.0 (19.0–27.8) | 23.5 (20.0–33.8) | 20.0 (19.0–24.0) | 22.0 (17.5–27.0) | 0.25 |
| ALT (IU/L) | 21.0 (15.0–28.0) | 25.0 (18.0–28.0) | 19.0 (15.0–27.0) | 18.0 (13.0–31.5) | 0.23 |
| GGT (IU/L) | 17.5 (12.0–27.0) | 21.0 (14.5–37.5) | 16.0 (12.0–31.3) | 15.0 (10.0–19.5) | 0.06 |
| Glucose (mg/dL) | 92.0 (88.0–99.0) | 93.0 (87.0–100) | 92.0 (88.0–101) | 91.0 (88.0–96.0) | 0.48 |
| Insulin (IU/mL) | 67.0 (46.0–96.0) | 57.0 (44.0–96.0) | 69.0 (44.0–106) | 69.0 (48.5–97.0) | 0.60 |
| HOMA-IR | 1.66 (1.09–2.34) | 1.55 (1.00–2.70) | 1.72 (1.19–2.71) | 1.58 (1.16–2.12) | 0.75 |
| Total energy intake (kcal/day) | 2085.78 (1583.04–2427.43) | 2085.78 (1581.05–2404.53) | 2223.51 (1602.68–2579.91) | 1772.75 (1575.70–2212.96) | 0.39 |
| Total DF (g/1000 kcal/day) | 11.5 (9.25–13.9) | 11.7 (9.44–13.5) | 11.4 (8.40–14.2) | 11.4 (9.52–14.5) | 0.86 |
| Soluble fiber (g/1000 kcal/day) | 3.68 (2.63–4.44) | 3.75 (2.66–4.42) | 3.66 (2.78–4.83) | 3.46 (2.57–4.69) | 0.89 |
| Insoluble fiber (g/1000 kcal/day) | 6.07 (4.88–7.25) | 6.46 (5.31–7.25) | 5.81 (4.29–7.90) | 6.29 (5.64–7.24) | 0.58 |
| Hemicellulose (g/1000 kcal/day) | 1.91 (1.61–2.50) | 1.92 (1.63–2.34) | 2.01 (1.31–2.76) | 1.79 (1.79–2.14) | 0.48 |
| Cellulose (g/1000 kcal/day) | 3.62 (2.24–5.12) | 2.94 (2.02–4.69) | 3.82 (2.40–6.59) | 2.79 (2.21–4.53) | 0.22 |
| Lignin (g/1000 kcal/day | 0.47 (0.34–0.65) | 0.47 (0.34–0.79) | 0.40 (0.28–0.63) | 0.51 (0.38–0.71) | 0.48 |
| AI of dietary fiber; n (%) | 25.0 (33.3) | 9.0 (33.3) | 10.0 (37.0) | 6.00 (28.6) | 0.83 |
| Total Sample (n = 47) | Bacteroides (n = 12) | Prevotella (n = 26) | Ruminococcaceae (n = 9) | p | |
|---|---|---|---|---|---|
| Acetate (mmol/g wet stool) | 168 (138–210) | 140 (101–186) | 181 (153–231) | 192 (133–249) | 0.10 |
| Propionate (mmol/g wet stool) | 133 (107–160) | 114 (76–139) b | 143 (114–190) a | 117 (84.0–144) ab | 0.04 |
| Butyrate (mmol/g wet stool) | 86 (62–119) | 67 (39–115) | 92.1 (62.8–126) | 84.6 (68.3–111) | 0.42 |
| Total SCFA (mmol/g wet stool) | 389 (310–499) | 319 (236–418) | 425 (317–509) | 350 (307–515) | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Medina, J.N.; Flores-Lopez, R.; López-Contreras, B.E.; Villamil-Ramirez, H.; Guzman-Muñoz, D.; Macias-Kauffer, L.R.; León-Mimila, P.; Granados-Portillo, O.; Del-Rio-Navarro, B.E.; Gómez-Perez, F.J.; et al. Effect of Gut Microbial Enterotypes on the Association between Habitual Dietary Fiber Intake and Insulin Resistance Markers in Mexican Children and Adults. Nutrients 2021, 13, 3892. https://doi.org/10.3390/nu13113892
Martinez-Medina JN, Flores-Lopez R, López-Contreras BE, Villamil-Ramirez H, Guzman-Muñoz D, Macias-Kauffer LR, León-Mimila P, Granados-Portillo O, Del-Rio-Navarro BE, Gómez-Perez FJ, et al. Effect of Gut Microbial Enterotypes on the Association between Habitual Dietary Fiber Intake and Insulin Resistance Markers in Mexican Children and Adults. Nutrients. 2021; 13(11):3892. https://doi.org/10.3390/nu13113892
Chicago/Turabian StyleMartinez-Medina, Jennifer N., Regina Flores-Lopez, Blanca E. López-Contreras, Hugo Villamil-Ramirez, Daniela Guzman-Muñoz, Luis R. Macias-Kauffer, Paola León-Mimila, Omar Granados-Portillo, Blanca E. Del-Rio-Navarro, Francisco J. Gómez-Perez, and et al. 2021. "Effect of Gut Microbial Enterotypes on the Association between Habitual Dietary Fiber Intake and Insulin Resistance Markers in Mexican Children and Adults" Nutrients 13, no. 11: 3892. https://doi.org/10.3390/nu13113892
APA StyleMartinez-Medina, J. N., Flores-Lopez, R., López-Contreras, B. E., Villamil-Ramirez, H., Guzman-Muñoz, D., Macias-Kauffer, L. R., León-Mimila, P., Granados-Portillo, O., Del-Rio-Navarro, B. E., Gómez-Perez, F. J., Aguilar-Salinas, C. A., Torres, N., Tovar, A. R., Canizales-Quinteros, S., & Moran-Ramos, S. (2021). Effect of Gut Microbial Enterotypes on the Association between Habitual Dietary Fiber Intake and Insulin Resistance Markers in Mexican Children and Adults. Nutrients, 13(11), 3892. https://doi.org/10.3390/nu13113892







