Abstract
A polysaccharide is a macromolecule composed of more than ten monosaccharides with a wide distribution and high structural diversity and complexity in nature. Certain polysaccharides are immunomodulators and play key roles in the regulation of immune responses during the progression of some diseases. In addition to stimulating the growth of certain intestinal bacteria, polysaccharides may also promote health benefits by modulating the gut microbiota. In the last years, studies about the triad gut microbiota–polysaccharides–health have increased exponentially. In consequence, in the present review, we aim to summarize recent knowledge about the function of dietary polysaccharides on gut microbiota composition and how these effects affect host health.
1. Introduction
1.1. Food Polysaccharides, an Overview
Carbohydrates are divided in several categories based on their number of sugar units: (a) monosaccharides have one sugar molecule; (b) disaccharides have two sugar molecules; (c) oligosaccharides have three to ten sugar units and may be produced by the breaking down polysaccharides; and (d) polysaccharides are macromolecules of monosaccharides consisting of more than ten units [1]. Polysaccharides are the major components of dietary fiber [2]. They bind to bile acids in the small intestine, thereby lowering serum cholesterol and normalizing blood lipid levels [3]. Most of the structures of polysaccharides are associated with numerous biological benefits for gut health and are frequently found in more complex structures that also contain digestible carbohydrates and proteins [4].
Food products contain polysaccharides derived from many sources, including farms, forests, oceans, fermentation vats, and chemical modification of natural polysaccharides, such as cellulose and starch [5]. Of the source and polysaccharide types, examples include algal (seaweed extracts) derived from agar, algins, carrageenans, and furcellaran, higher insoluble plants derived from cellulose, fruit extracts derived from pectin, corn starches, rice starches, wheat starches, beta-glucans, guar gum, locust bean gum, tara gum, psyllium seed gum, and tamarind seed polysaccharides [6].
For instance, hydrocolloids (plant-derived ingredients such as pectin, guar gum, locust bean gum, and konjac mannan) are a class of food ingredients mainly composed of polysaccharides and some proteins that are widely used in several food products [7]. Other polysaccharides are also commonly found in dietary products including starch, cellulose, chitosan, xyloglucan, glucan, xanthan, arabinoxylan, carrageenan, inulin, agar, and plant gums [8]. Indeed, starch is the second most abundant natural polysaccharide after cellulose and is the world’s primary source of food carbohydrates [9], while non-starch polysaccharides (NSPs) are non-glucan polysaccharides [10]. There are several hundred thousand monosaccharides units in NSPs that are linked through glycosidic bonds, making them more complex than starch [11]. The diverse categories of NSPs differ in terms of water solubility, size, and structure.
The ability of dissolved polysaccharides to thicken solutions and form gels is one of their most critical functional characteristics both in terms of formulation functionality and health-related functionality [12]. A large hydrodynamic volume of polysaccharides results in increased viscosity at low concentrations, while a small hydrodynamic volume results in decreased viscosity. As a consequence, high solubility (i.e., favorable interaction with the solvent which results in the polysaccharide expanding and a higher hydrodynamic volume) is beneficial for thickening [13]. Additionally, associative interactions may enhance the thickening properties of some modified starches and celluloses. A polysaccharide’s ability to form gels is dependent on its solubility. This is essential to the gel structure’s ability to hold water and the formation of a continuous network in the solution. It is therefore necessary for the polysaccharides to interact in some way in order to form associations [14]. In order for molecules to associate, they must exhibit either a hydrophobic effect, partial local crystallization, calcium bridges, or double or triple helices [2]. For instance, cellulose, galactomannans, xylans, xyloglucans, and lignin are water-insoluble fibers, while pectins, arabinogalactans, arabinoxylans, and -(1,3)(1,4)-D-glucans (-glucans) are water-soluble fibers [15].
As mentioned, polysaccharides are widely used in food technology and recognized for their bioactivity, which has been linked to a reduced risk of non-communicable chronic diseases [16]. NSPs and resistant starch (RS) are beneficial mediators of anti-inflammation, gut epithelial barrier protection, and immune modulation [17]. They possess antibacterial and anticancer properties. Polysaccharides also have antithrombotic, antioxidant, antiangiogenic, and antiviral properties [8]. The soluble part of dietary polysaccharides is related to an increase in transit time over the intestine. The insoluble fiber from dietary polysaccharides is linked to a decrease in transit time over the gastrointestinal tract. This is related to an augmentation in the excretion of bile acid and fecal bulk [18]. On the other hand, starch has a critical function in digestive processes because of the intermediation of ion exchange and holding water [19]. Additionally, bacterial polysaccharides, usually found in the cell wall, act as immune modulators. By interacting with gut microbes, host-derived polysaccharides protect host cells from pathogenic microbial neighbors, as well as affect overall intestinal health [17]. Indigestible but fermentable polysaccharides (termed prebiotics) can stimulate the growth and activity of beneficial bacteria in the colon. Accordingly, the inclusion of polysaccharides in the diet is therefore beneficial to the host metabolism, fat accumulation, and insulin resistance, among other benefits [8].
1.2. Food Polysaccharides and Gut Microbiota
The intestinal or gut microbiota is “the set of microbes that colonize our digestive tract and interact with each other and with the host” [20,21]. Indeed, the microbes that reside in our gut have a remarkable potential to influence physiology, both in disease and the health of the host. The gut microbiota modulates, directly or indirectly, most of our physiologic functions, including metabolic and pathogenic functions, as well as the immune system maturation [22]. The microbiome also encompasses all of the genetic information contained in the microbiota [23], creating a dynamic, interactive microecosystem capable of changing in time and scale, along with being integrated into macro-ecosystems including eukaryotic hosts, and being crucial to their health and functioning [24]. A gut ecosystem with a wide variety of species may be more resilient to environmental influences than one that lacks diversity, since functionally linked microbes within an intact ecosystem may be able to balance the function of other species that have become extinct. In consequence, a higher diversity is commonly regarded as an indicator of a healthy digestive system [25,26]. Thus, an equilibrated microbiota community frequently exhibits high taxonomic diversity, stable core microbiota, and high microbial gene richness [27,28]. In healthy conditions, the intestinal microbiota is stable, resilient, and interacts symbiotically with the host [27,28]. By contrast, an imbalance in gut microbiota composition and function (dysbiosis) has been linked to cardiovascular disease [29], cancer [30,31], respiratory diseases [32,33], diabetes [34], inflammatory bowel disease [35], brain disorders [36], chronic kidney disease [37], and liver disease [38], among others.
Physiological properties of the gastrointestinal tract are revealed by the composition of the microbiota in a given region, which is stratiform both transversely and longitudinally. Microbiota density and composition are influenced by nutritional, chemical, and immunological gradients along the gut [39]. The large intestine has high levels of oxygen, acids, and antimicrobials, as well as a longer transit time than the small intestine [40]. However, facultative anaerobes with the ability to adhere to epithelial or mucus surfaces are thought to survive in the large intestine, as are rapidly growing bacteria [40]. Besides, according to animal studies, the microbial community of the small intestine is essentially dominated by Lactobacillaceae (traditionally classified as oxygen-tolerant anaerobes) [41]. A diverse and dense bacteria community occurs in the colon, primarily anaerobes with the ability to utilize complex carbohydrates, which are undigested in the small intestine. It has been reported that Prevotelaceae, Lachnospiraceae, and Rikenellaceae constitute the majority of species in the colon [39,42].
There is a spatial preservation of microbiota diversity and composition in the colorectal mucosa region [43,44]. On the contrary, the compositions of the mucosal and fecal/luminal regions are drastically different [45]. Bacteroidetes are more abundant in fecal/luminal samples than in the mucosa samples. Firmicutes, specifically Clostridium cluster XIVa, are enriched in the mucus compared with the luminal/fecal regions [46,47].
Gut microbiota is also involved in the metabolism of choline, phosphatidylcholine, and carnitine and can produce trimethylamine-N-oxide (TMAO). Smooth muscle cells and endothelial cells can respond to TMAO by triggering the nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways [48]. Several recent reviews have faced this topic in detail elsewhere [49,50,51,52,53,54].
Concerning the interaction of gut microbiota–food polysaccharides, several dietary polysaccharides are fermented by the gut microbiota [55]. In this regard, the results of recent interventional studies suggest that dietary fiber increments may reduce diversity. This is because the microbes that digest fiber become exclusively enriched, resulting in a change in intestinal composition and, through competitive interactions, decreased diversity [56]. Gut bacterial degradation by dietary polysaccharides happens in two phases: (1) internal anaerobic glycolysis and (2) polysaccharides are hydrolyzed extracellularly to produce mono- and disaccharides [57].
Bearing in mind the above mentioned, by increasing the growth of certain intestinal bacteria during intestinal fermentation (among others), polysaccharides can alter the microbiota profile of the intestinal microbiota and change the physiology of the host, both locally and remotely [58].
On the other hand, Bifidobacterium longum, an example of bacteria with the ability of microbial fermentation, has the advantage of using the fucosylated oligosaccharides present in human milk to inhibit the growth of specific bacteria such as Escherichia coli and Clostridium perfringens [59]. In addition, Bacteroides species may consume those fucosylated oligosaccharides as a carbon source [60]. Infants born to mothers with nonfunctional fucosyltransferase 2 (FUT2), which is required for the fucosylation of milk oligosaccharides, have low levels of Bacteroides and Bifidobacterium in their feces [61]. In humans, patients with insulin resistance show elevated levels of Dorea and Coprococcus. Polysaccharide-containing bacteria possess degradation properties, and their associations with fecal sugar derivatives were generally positive, while Alistipes showed a negative correlation [62]. Dorea strain administration on a high-fat diet mice intensified insulin resistance and obesity compared with Alistipes administration. The authors of this work reported that the gut microbes’ effects on metabolic diseases are mediated through polysaccharides’ microbial fermentation and their derivatives [62]. Several studies in mice involving species of Bacteroides have shown that controlling the intake of polysaccharides in the mouse diet allows species selection that are capable of metabolizing the complex glycans present, such as human milk oligosaccharides [60], fructans [63], fucosylated mucin glycans [64] and mannan [65], among others.
For a comprehensive understanding of the effects of polysaccharides on gut health and the host, more detailed information is required. Therefore, the present review aims to elucidate the knowledge of the function played by dietary polysaccharides on gut microbiota composition and how these effects affect host health. We addressed the impact of several polysaccharides in health-promoting effects through the modulation of gut microbiota. Finally, we summarize recently reported studies in the field conducted on humans.
2. Health-Promoting Effects of Polysaccharides through the Modulation of Gut Microbiota
2.1. Dietary Polysaccharides and Short-Chain Fatty Acids (SCFAs)
Short-chain fatty acids (SCFAs) are metabolites produced by bacteria that can pass through the intestinal barrier and interact with host cells, thereby affecting the immune response [66]. When fiber is anaerobically fermented by gut microbiota, polysaccharides and proteins are metabolized into SCFAs [1]. In Figure 1 we summarize the bacterial degradation of polysaccharides in the intestine by fermentation.
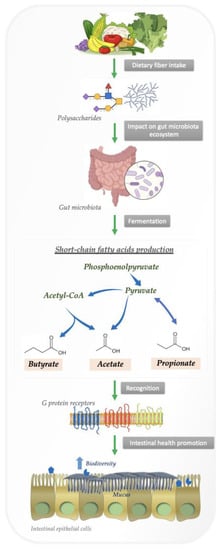
Figure 1.
Bacterial degradation of polysaccharides in the intestine by fermentation.
A growing body of evidence suggests that SCFAs are capable of modulating the inflammatory response of immune cells, including neutrophils, dendritic cells, macrophages, monocytes, and T cells [67,68,69].
Obligate anaerobes hydrolyze nondigestible carbohydrates into oligosaccharides, which are fermented in an anaerobic environment. Anaerobes convert hexoses to pyruvate by a process similar to glycolysis before oxidizing pyruvate to acetyl-CoA in conjunction with reduction of an electron carrier or, in many cases, hydrogen gas [70,71]. From there, acetyl CoA is converted into various SCFAs.
As soon as SCFAs are produced, they are absorbed by colonocytes, primarily through sodium-dependent monocarboxylate transporters or H+-dependent monocarboxylate transporters. SCFAs affect intestinal mucosal immunity and influence barrier integrity and function by binding to G protein-coupled receptors, including free fatty acid receptors 2 and 3, as well as GPR109a/HCAR2 and GPR164 [72,73]. In a mouse model of colitis induced by dextran sulfate sodium, SCFAs binding to GPR43 and GPR109A stimulated K+ efflux and hyperpolarization, resulting in NLRP3 inflammasome activation and increased levels of IL-18 in serum [74]. Hence, SCFAs and their receptors contribute to health benefits associated with dietary fiber, as well as the way in which metabolite signals feed through to a major path for gut homeostasis.
SCFAs have an important role in intestinal immune homeostasis maintenance [75,76,77]. By ratifying and purging antigens from neutrophils and monocytes, immunological responses could be triggered, and pathogens could be prevented from invading. [75,76,77].
The normal gut microbiome makes 50–100 mmol·L−1 SCFAs per day and works as a source of energy for the host’s gut epithelium [78]. These SCFAs can be rapidly absorbed in the colon and serve many diverse roles in regulating gut inflammation, motility, energy harvesting, and glucose homeostasis [79,80].
The most common SCFAs are acetates, butyrates, or propionates, and a large proportion of these acetates undergo lipogenesis in adipose tissue and undergo oxidization in muscle, while some are converted into butyrates by bacteria [28]. Both butyrate and propionate protect the host from hypertensive cardiovascular damage [81] and butyrates are also associated with intestinal barrier integrity and may have beneficial effects on the epithelium of the gut [82].
2.2. Dietary Polysaccharides Influence Immunity by Acting as Prebiotics by Changing Gut Microbiota Composition
Biologically, polysaccharides perform a wide variety of functions and are capable of producing prebiotics that stimulate the microbiota in the intestines. The intestinal microbiota also exerts beneficial effects by selectively degrading polysaccharides, which can be used by the intestinal microbiota as a source of energy to maintain the physiologic effects of the intestinal bacteria and regulate their composition [69]. Some polysaccharides, such as dietary fibers, resist hydrolysis in the stomach and the small intestine of humans. According to Dolan et al., prolonged deficiency of dietary fiber can permanently alter gut microbiota and result in gut dysbiosis [83].
Non-fermentable polysaccharides are excreted in the large intestine while fermentable polysaccharides are digested by the microbiota that inhabits in the large intestine and are fermented to produce diverse metabolites that provide the host with energy [84,85].
Certain polysaccharides act as immunomodulators and influence the regulation of immune responses during the progression of some diseases [86]. Moreover, natural polysaccharides are capable of enhancing immunity by promoting beneficial microorganisms and increasing immune cell function [87].
Sheng et al. have reported that Hericium erinaceus-derived polysaccharides can help to restore humoral and cellular immunity in a murine model by improving the phagocytic function of natural killer cells, phagocytes, secretory IgA, and increasing the activity of AKT and MAPK signaling pathways [88]. Several studies have demonstrated that polysaccharides from ginseng can enhance immunity in sows by increasing the levels of interleukin (IL)-2, IL-6, immunoglobulin (Ig)-G, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) in both milk and serum [89]. Some other polysaccharides isolated from Robinia pseudoacacia and young barley leaves have also been shown to enhance IgA-related cytokines, leukocytes, transforming growth factor-beta (TGF-β), and IL-10 levels [87,90,91].
Bacteroides possess the ability to degrade dietary polysaccharides, as well as the polysaccharides on the surface of other gut microbes, and this is the major factor that enables them to thrive within the gut environment [77]. These species could metabolize dietary polysaccharides to SCFAs [92].
Polysaccharides isolated from Artemisia sphaerocephala might prevent the diversity decrease associated with bacteria belonging to Proteobacteria and Helicobacter in an animal model of high-fat diet-induced obesity [93]. Also, Chlorella pyrenoidosa and Spirulina platensis can restructure the gut microbiota in an animal model of obesity using a high-fat diet, increasing beneficial bacteria from Bacteroidia, Clostridia, and Mollicutes, and decreasing some bacteria from Verrucomicrobia and Actinobacteria [94].
Some reports have shown that alginate in brown seaweed modulates the obesity-related with a high-fat diet by regulating SCFA production and changing the Bacteroidales and Clostridiales [95]. Laminaria japonica soluble polysaccharides diminish non-alcoholic fatty liver diseases in a high-fat diet animal model through decreasing the Firmicutes/Bacteroidetes ratio and stimulating Verrucomicrobia and propionate-producing bacteria Akkermansia (a bacterium of the phylum Verrucomicrobia) and Bacteroides [94].
Accordingly, Akkermansia muciniphila is involved in the metabolism of mucin and the maintenance of intestinal integrity [96]. The increment of Akkermansia muciniphila after polysaccharide interventions has been related to benefits to the host (e.g., [97,98,99]). By contrast, other studies define Verrucomicrobia phylum as “unfavorable” for the prevention of obesity, and higher levels of this bacteria have been associated with this disease [96]. These discrepancies may be due to the fact that not all subspecies of Verrucomicrobia (e.g., Akkermansia muciniphila) may display the same specific properties, the model used in the study (animal model, or humans), as well as the basal state of the microbiota (eubiosis or dysbiosis, healthy or not subject, etc.). Overall, the fact is that the bacteria belonging to the phylum Verrucomicrobia are widespread contributors to the cycling of carbon and have the capacity for starch degradation is a crucial component of plant biomass [100].
3. Human Studies Examining Polysaccharide Modulation of Gut Microbiota and Its Association with Improved Health
The intestinal microbiota plays a vital role in human physiology through the production of metabolites that regulate essential activities that facilitate a symbiotic relationship between the microbes and the host. Polysaccharides are key regulators of colon physiology and the changing intestinal environment [101], and they are selectively used by gut microbiota to enhance the selection, colonization, and survival of probiotic bacteria acting as prebiotics [102].
The consumption of prebiotics is currently increasing, as well as the interest in them as functional foods. Therefore, research aimed at deciphering the mechanisms involved and their precise health effects has augmented exponentially.
In this regard, numerous clinical trials have already been conducted addressing a wide range of diseases (from obesity to chronic kidney disease) through dietary intervention with different polysaccharides. These studies are mainly focused on evaluating the potential of these polysaccharides as modulators of the intestinal microbiota to counteract the detrimental effects of the pathology (Table 1).

Table 1.
Human studies addressing the modulation of gut microbiota by polysaccharides.
A clear example of the latter is inulin, a functional food found naturally in various plants and vegetables, which is a widely used ingredient in diverse efficacy studies thanks to its prebiotic properties [103]. Inulin is being investigated as a potential modulator of the gut microbiota with benefits for human health. The most notable recently reported changes induced by inulin are an increase in Bifidobacterium, an improvement in function, as well as benefits in host metabolism for a variety of metabolic diseases, including obesity, type 2 diabetes, kidney disease, intestinal disease, and non-alcoholic fatty liver disease [97,104,105,106,107,108,109,110,111,112,113,114].
In addition to being a dietary fiber beneficial to health, RS is also defined as the portion of starch that cannot be digested or absorbed by humans in their small intestine. By fermenting RS, the gut microbiota can produce SCFAs [115]. In recent years, clinical investigations addressing the use of RS as a microbiome-modifying strategy have proliferated. In this particular case, supplementation with RS in patients with renal disease has led to an elevation in Faecalibacterium and a decrease in systemic inflammation [116], as well as elevated SCFA producers’ microbes [107]. Additionally, a SCFA increment after RS intervention has been positively correlated with the relative abundance of Faecalibacterium, Ruminococcus, Roseburia, and Barnesiellaceae [117] and is effective in reducing body fat in healthy individuals [98].
The consumption of β-glucans has been shown to reduce calorie intake, lower cholesterol levels, and improve immunity [118]. Moreover, several clinical trials have also shown changes in gut microbiota composition and metabolic parameters. After dietary interventions with this prebiotic, changes in gut microbiota composition related to the increase of healthy bacteria (Bifidobacterium and Akkermansia) were observed in patients at high risk of developing metabolic syndrome [119,120]. Furthermore, in patients suffering from chronic kidney disease, β-glucan intake significantly altered the levels of the uremic toxin of intestinal origin and improved the state of the intestine [99].
On the other hand, non-invasive therapies such as prebiotic intake are becoming increasingly popular as a means to improve the quality of life of older adults [121]. In this regard, we found studies that examined the impact of polysaccharides on elderly people, but the results were conflicting. For instance, while Kiewiet et al. have reported changes in microbiota that were associated with improvements in health [111], Ganda et al. observed no significant effects following the intervention [122].
4. Future Perspectives in the Nutrition Field
Cell plant walls are composed of diverse types of polysaccharides and proteins which play vital roles in biology, including the regulation of cell expansion and tissue attachment, exchange of ions, as well as defense against pathogenic microorganisms. Further, there is evidence that fermentable dietary fiber from polysaccharides has biological activities that are low in toxicity, and have anti-oxidant, anti-inflammatory, anti-tumor, and antiviral effects. Moreover, evidence also suggests that polysaccharides play an active role in the symbiotic relationship between the gut microbiota and the host. Indeed, microbes convert complex polysaccharides into monosaccharides through a variety of biochemical pathways mediated by enzymatic activities. Together with polysaccharides, colonic bacteria also produce lactic acid, which reduces colonic pH and alters gut microbial composition. As immunomodulators, bacterial polysaccharides protect host cells from pathogenic microbial neighbors, and host-derived polysaccharides interact with gut microbes to influence gut health.
However, it is necessary to point out that fibers derived from polysaccharides obtained from different types of plants have different chemical compositions and physicochemical properties. Consequently, plant-based diets will provide a variety of dietary fibers as well as a variety of microbiota compositions. The genetics and the pre-diet microbiome of the host will also add variability to the effects of plant-based diets on microbiota composition.
On the other hand, although many of the physiological and nutritional effects of dietary polysaccharides are widely known, the different mechanisms of action have yet to be fully elucidated, as occurs, for example, with NSP. Along the same line, polysaccharides from marine algae are increasingly being used as prebiotics. These compounds are a rich source of dietary fiber, which are not decomposed by the enzymes of the upper gastrointestinal tract. Polysaccharides from marine seaweeds also have a detoxifying effect. Conversely, other factors, such as the complex chemical structure of some of these products must still be completely understood [138], as well as the high presence of sulfate residues in some of them, which may limit their fermentation by the gut microbiota and increase toxicity.
Several factors may affect the consistency of the results regarding polysaccharides’ effects on the gut microbiota, including methodological sampling and bioinformatic pipelines. To make conclusions regarding this issue, it is necessary to take these factors into account.
To conclude, despite the recent work in this field, we are only beginning to understand how dietary polysaccharides affect health by modulating gut microbiota. Progress is challenged by the wide variety of dietary polysaccharides, their interactions with other molecules such as proteins, and by the vast variations in gut microbiota profiles. In this sense, it is essential to emphasize the importance of carefully selecting a sampling method when analyzing the composition of the microbiota in order to avoid contradictory results and to be able to obtain a solid understanding of all the processes and the precise role of all the “players” involved.
Author Contributions
A.I.Á.-M. and J.P.-D. participated in the bibliographic search, discussion, and writing of the manuscript. A.I.Á.-M. and J.P.-D. designed the work and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Ana I. Álvarez-Mercado was awarded by the Regional Ministry of Health and Families (Andalucía, Spain). CSyF 2021—Postdoctoral (RPS 24665). Julio Plaza-Diaz is part of the “UGR Plan Propio de Investigación 2016” and the “Excellence actions: Unit of Excellence on Exercise and Health (UCEES), University of Granada”. Julio Plaza-Diaz is supported by a fellowship awarded to postdoctoral researchers at foreign universities and research centers from the “Fundación Ramón Areces”, Madrid, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aranceta, J.; Serra-Majem, L. Working Party for the Development of Food-Based Dietary Guidelines for the Spanish, P. Dietary Guidelines for the Spanish Population. Public Health Nutr. 2001, 4, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Edwards, C.; DE Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of Polysaccharides in Food, Digestion, and Health. Crit. Rev. Food Sci. Nutr. 2015, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tan, H.; Nie, S. Beneficial Effects of Seaweed-Derived Dietary Fiber: Highlights of the Sulfated Polysaccharides. Food Chem. 2021, 373, 131608. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Díaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Goff, H.D.; Guo, Q. Chapter 1. The Role of Hydrocolloids in the Development of Food Structure. In Handbook of Food Structure Development, 1st ed.; Royal Society of Chemistry: London, UK, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Mishra, S.K.; Yadav, H. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obes. Control Ther. Open Access 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Tiwari, U.; Brennan, C. Pulses nonstarch polysaccharides. In Pulse Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 177–192. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary Roles of Non-Starch Polysachharides in Human Nutrition: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Xu, Z. Solubility of Polysaccharides; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Capuano, E. The Behavior of Dietary Fiber in the Gastrointestinal Tract Determines Its Physiological Effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Do, M.H.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel Health and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 1212–1224. [Google Scholar] [CrossRef]
- Anderson, J.W. Physiological and Metabolic Effects of Dietary Fiber. Fed. Proc. 1985, 44, 2902–2906. [Google Scholar]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Sokol, H. Definition and Roles of the Gut Microbiota. La Rev. Du Prat. 2019, 69, 776–782. [Google Scholar]
- Aragón-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Álvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Solis-Urra, P.; Aragón-Vela, J.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Álvarez-Mercado, A. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines 2021, 9, 145. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Sommer, F.; Rühlemann, M.; Bang, C.; Höppner, M.; Rehman, A.; Kaleta, C.; Schmitt-Kopplin, P.; Dempfle, A.; Weidinger, S.; Ellinghaus, E.; et al. Microbiomarkers in Inflammatory Bowel Diseases: Caveats Come with Caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma Antibodies to Oral Bacteria and Risk of Pancreatic Cancer in a Large European Prospective Cohort Study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nelson, C.E.; Brodie, E.L.; DeSantis, T.Z.; Baek, M.S.; Liu, J.; Woyke, T.; Allgaier, M.; Bristow, J.; Wiener-Kronish, J.P.; et al. Airway Microbiota and Bronchial Hyperresponsiveness in Patients with Suboptimally Controlled Asthma. J. Allergy Clin. Immunol. 2011, 127, 372.e3–381.e3. [Google Scholar] [CrossRef]
- De Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; Van Raalte, D.H.; Scheithauer, T.P. Distinct Fecal and Oral Microbiota Composition in Human Type 1 Diabetes, an Observational Study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef]
- Kleessen, B.; Kroesen, A.J.; Buhr, H.J.; Blaut, M. Mucosal and Invading Bacteria in Patients with Inflammatory Bowel Disease Compared with Controls. Scand. J. Gastroenterol. 2002, 37, 1034–1041. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Cheng, Y.; Jiang, X.; Jiang, H.; Wang, Y.; Li, L. Decreased Diversity of the Oral Microbiota of Patients with Hepatitis B Virus-Induced Chronic Liver Disease: A Pilot Project. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef]
- Boscaini, S.; Leigh, S.-J.; Lavelle, A.; García-Cabrerizo, R.; Lipuma, T.; Clarke, G.; Schellekens, H.; Cryan, J.F. Microbiota and Body Weight Control: Weight Watchers Within? Mol. Metab. 2021, 57, 101427. [Google Scholar] [CrossRef]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial Variation of the Colonic Microbiota in Patients with Ulcerative Colitis and Control Volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef]
- Vuik, F.; Dicksved, J.; Lam, S.Y.; Fuhler, G.M.; Van Der Laan, L.; Van De Winkel, A.; Konstantinov, S.R.; Spaander, M.; Peppelenbosch, M.P.; Engstrand, L.; et al. Composition of the Mucosa-Associated Microbiota Along the Entire Gastrointestinal Tract of Human Individuals. United Eur. Gastroenterol. J. 2019, 7, 897–907. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Keku, T.O.; Chang, Y.-H.; Packey, C.D.; Sartor, R.B.; Ringel, Y. Molecular Analysis of the Luminal- and Mucosal-Associated Intestinal Microbiota in Diarrhea-Predominant Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G799–G807. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Burrough, E.R.; Arruda, B.L.; Plummer, P.J. Comparison of the Luminal and Mucosa-Associated Microbiota in the Colon of Pigs with and without Swine Dysentery. Front. Veter- Sci. 2017, 4, 139. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, The Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Nam, H.S. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J. 2021, 20, 301–319. [Google Scholar]
- Zhu, Y.; Li, Q.; Jiang, H. Gut Microbiota in Atherosclerosis: Focus on Trimethylamine N-Oxide. APMIS 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2020, 228, 109–125. [Google Scholar] [CrossRef]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Funk, C.; Braune, A.; Grabber, J.H.; Steinhart, H.; Bunzel, M. Model Studies of Lignified Fiber Fermentation by Human Fecal Microbiota and Its Impact on Heterocyclic Aromatic Amine Adsorption. Mutat. Res. Mol. Mech. Mutagen. 2007, 624, 41–48. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Chen, C.; Kling, D.; Liu, B.; McCoy, J.M.; Merighi, M.; Heidtman, M.; Newburg, D.S. The Principal Fucosylated Oligosaccharides of Human Milk Exhibit Prebiotic Properties on Cultured Infant Microbiota. Glycobiology 2012, 23, 169–177. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Sonnenburg, E.D.; Pudlo, N.; Martens, E.C.; Desai, P.; Lebrilla, C.B.; Weimer, B.C.; Mills, D.A.; German, J.B.; et al. Bacteroides in the Infant Gut Consume Milk Oligosaccharides via Mucus-Utilization Pathways. Cell Host Microbe 2011, 10, 507–514. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.-S.; German, J.B.; et al. Maternal Fucosyltransferase 2 Status Affects the Gut Bifidobacterial Communities of Breastfed Infants. Microbiome 2015, 3, 1–21. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakanishi, Y.; Ikeda, K.; Tsugawa, H.; Suda, W.; Mizuno, Y.; Yamamichi, N.; Arita, M.; Hattori, M.; Kubota, N.; et al. 1876-P: Gut Microbial Fermentation of Polysaccharides Impacts Insulin Resistance in Humans. Diabetes 2020, 69, 1876. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of Polysaccharide Use in Intestinal Bacteroides Species Determines Diet-Induced Microbiota Alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Smits, S.A.; Sonnenburg, E.D.; Costello, E.K.; Higginbottom, S.K.; Domino, S.E.; Holmes, S.P.; Relman, D.A.; et al. Genetically Dictated Change in Host Mucus Carbohydrate Landscape Exerts a Diet-Dependent Effect on the Gut Microbiota. Proc. Natl. Acad. Sci. USA 2013, 110, 17059–17064. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human Gut Bacteroidetes Can Utilize Yeast Mannan Through a Selfish Mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B Cell-Intrinsic Epigenetic Modulation of Antibody Responses by Dietary Fiber-Derived Short-Chain Fatty Acids. Nat. Commun. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The Role of Short-Chain Fatty Acids in Immunity, Inflammation and Metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory Effects of Polysaccharides from Plants, Marine Algae and Edible Mushrooms on Gut Microbiota and Related Health Benefits: A Review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The Abundance and Variety of Carbohydrate-Active Enzymes in the Human Gut Microbiota. Nat. Rev. Genet. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay Between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.l.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis Through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Chen, F.; Yang, W.; Huang, X.; Cao, A.T.; Bilotta, A.J.; Xiao, Y.; Sun, M.; Chen, L.; Ma, C.; Liu, X.; et al. Neutrophils Promote Amphiregulin Production in Intestinal Epithelial Cells through TGF-β and Contribute to Intestinal Homeostasis. J. Immunol. 2018, 201, 2492–2501. [Google Scholar] [CrossRef]
- Desalegn, G.; Pabst, O. Inflammation Triggers Immediate Rather Than Progressive Changes in Monocyte Differentiation in the Small Intestine. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides Utilization for Dietary Polysaccharides and Their Beneficial Effects on Gut Health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Duncan, S.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of Ph in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, Enteroendocrine Functions and Metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.-R.; Sun, Y.; Rossi, C.; et al. Gut Microbiome–Derived Metabolites Modulate Intestinal Epithelial Cell Damage and Mitigate Graft-Versus-Host Disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, Gut Microbes, and the Pathogenesis of Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2016, 61, 1600129. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide Immunomodulators as Therapeutic Agents: Structural Aspects and Biologic Function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The Impacts of Natural Polysaccharides on Intestinal Microbiota and Immune Responses—A Review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, J.; Meng, Y.; Kang, Y.; Han, Z.; Tai, G.; Zhou, Y.; Cheng, H. Immunomodulatory Effects of Hericium Erinaceus Derived Polysaccharides Are Mediated by Intestinal Immunology. Food Funct. 2017, 8, 1020–1027. [Google Scholar] [CrossRef]
- Xi, Q.-Y.; Jiang, Y.; Zhao, S.; Zeng, B.; Wang, F.; Wang, L.-N.; Jiang, Q.-Y.; Zhang, Y.-L. Effect of Ginseng Polysaccharides on The Immunity and Growth of Piglets by Dietary Supplementation During Late Pregnancy and Lactating Sows. Anim. Sci. J. 2016, 88, 863–872. [Google Scholar] [CrossRef]
- Liang, M.-f.; Liu, G.-h.; Zhao, Q.-y.; Yang, S.-f.; Zhong, S.-x.; Cui, G.-l.; He, X.-h.; Zhao, X.; Guo, F.-x.; Wu, C. Effects of Taishan Robinia Pseudoacacia Polysaccharides on Immune Function in Chickens. Int. Immunopharmacol. 2013, 15, 661–665. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.-W.; Hong, H.-D.; Shin, K.-S. Effect of Arabinoxylan-and Rhamnogalacturonan I-Rich Polysaccharides Iso-Lated from Young Barley Leaf on Intestinal Immunostimulatory Activity. J. Funct. Foods 2017, 35, 384–390. [Google Scholar] [CrossRef]
- Zafar, H.; Jr, M.H.S. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Li, J.; Pang, B.; Shao, D.; Jiang, C.; Hu, X.; Shi, J. Artemisia Sphaerocephala Krasch Polysaccharide Mediates Lipid Metabolism and Metabolic Endotoxaemia in Associated with the Modulation of Gut Microbiota in Diet-Induced Obese Mice. Int. J. Biol. Macromol. 2020, 147, 1008–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhao, N.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J. Soluble Polysaccharide Derived from Laminaria japonica Attenuates Obesity-Related Nonalcoholic Fatty Liver Disease Associated with Gut Microbiota Regulation. Mar. Drugs 2021, 19, 699. [Google Scholar] [CrossRef]
- Zheng, W.; Duan, M.; Jia, J.; Song, S.; Ai, C. Low-Molecular Alginate Improved Diet-Induced Obesity and Metabolic Syndrome Through Modulating the Gut Microbiota In BALB/C Mice. Int. J. Biol. Macromol. 2021, 187, 811–820. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; Cauduro, C.G.D.S.; Mulders, M.D.; Zamariola, G.; Azzi, A.-S.; et al. Link Between Gut Microbiota and Health Outcomes in Inulin-Treated Obese Patients: Lessons from the Food4Gut Multicenter Randomized Placebo-Controlled Trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic Phenotypes and The Gut Microbiota in Response to Dietary Resistant Starch Type 2 In Normal-Weight Subjects: A Randomized Crossover Trial. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Nixon, S.L.; Daly, R.A.; Borton, M.A.; Solden, L.M.; Welch, S.A.; Cole, D.R.; Mouser, P.J.; Wilkins, M.J.; Wrighton, K.C. Genome-Resolved Metagenomics Extends the Environmental Distribution of the Verrucomicrobia Phylum to the Deep Terrestrial Subsurface. mSphere 2019, 4, e00613-19. [Google Scholar] [CrossRef]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef]
- Kumar, D.; Lal, M.K.; Dutt, S.; Raigond, P.; Changan, S.S.; Tiwari, R.K.; Chourasia, K.N.; Mangal, V.; Singh, B. Functional Fermented Probiotics, Prebiotics, and Synbiotics from Non-Dairy Products: A Perspective from Nutraceutical. Mol. Nutr. Food Res. 2022, 66, 2101059. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin Fructans in Diet: Role in Gut Homeostasis, Immunity, Health Outcomes and Potential Therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Rodriguez, J.; Neyrinck, A.M.; Van Kerckhoven, M.; Gianfrancesco, M.A.; Renguet, E.; Bertrand, L.; Cani, P.D.; Lanthier, N.; Cnop, M.; Paquot, N.; et al. Physical Activity Enhances the Improvement of Body Mass Index and Metabolism by Inulin: A Multicenter Randomized Placebo-Controlled Trial Performed in Obese Individuals. BMC Med. 2022, 20, 1–20. [Google Scholar] [CrossRef]
- Williams, C.J.; Torquati, L.; Li, Z.; Lea, R.A.; Croci, I.; Keating, E.; Little, J.P.; Eynon, N.; Coombes, J.S. Oligofructose-Enriched Inulin Intake, Gut Microbiome Characteristics, and the VO2 Peak Response to High-Intensity Interval Training in Healthy Inactive Adults. J. Nutr. 2021, 152, 680–689. [Google Scholar] [CrossRef]
- He, S.; Xiong, Q.; Tian, C.; Li, L.; Zhao, J.; Lin, X.; Guo, X.; He, Y.; Liang, W.; Zuo, X.; et al. Inulin-Type Prebiotics Reduce Serum Uric Acid Levels Via Gut Microbiota Modulation: A Randomized, Controlled Crossover Trial in Peritoneal Dialysis Patients. Eur. J. Nutr. 2021, 61, 665–677. [Google Scholar] [CrossRef]
- Kemp, J.A.; de Paiva, B.R.; dos Santos, H.F.; de Jesus, H.E.; Craven, H.; Ijaz, U.Z.; Borges, N.A.; Shiels, P.G.; Mafra, D. The Impact of Enriched Resistant Starch Type-2 Cookies on the Gut Microbiome in Hemodialysis Patients: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2021, 65, e2100374. [Google Scholar] [CrossRef]
- Hedin, C.R.; McCarthy, N.E.; Louis, P.; Farquharson, F.M.; McCartney, S.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Prebiotic Fructans Have Greater Impact on Luminal Microbiology and CD3+ T Cells in Healthy Siblings Than Patients with Crohn’s Disease: A Pilot Study Investigating the Potential for Primary Prevention of Inflammatory Bowel Disease. Clin. Nutr. 2021, 40, 5009–5019. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic Dietary Fibre Intervention Improves Fecal Markers Related to Inflammation in Obese Patients: Results from The Food4Gut Randomized Placebo-Controlled Trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.D.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Portheault, D.; Amadieu, C.; Bindels, L.B.; Leclercq, S.; et al. Prebiotic Effect on Mood in Obese Patients Is Determined by the Initial Gut Microbiota Composition: A Randomized, Controlled Trial. Brain Behav. Immun. 2021, 94, 289–298. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Elderman, M.E.; El Aidy, S.; Burgerhof, J.G.M.; Visser, H.; Vaughan, E.E.; Faas, M.M.; de Vos, P. Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long-Chain Inulin Intake. Mol. Nutr. Food Res. 2020, 65, e2000390. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Orr, D.; Plank, L.D.; Vatanen, T.; O’Sullivan, J.M.; Murphy, R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 937. [Google Scholar] [CrossRef]
- Hess, A.L.; Benítez-Páez, A.; Blædel, T.; Larsen, L.H.; Iglesias, J.R.; Madera, C.; Sanz, Y.; Larsen, T.M.; the MyNewGut Consortium. The Effect of Inulin and Resistant Maltodextrin on Weight Loss During Energy Restriction: A Randomised, Placebo-Controlled, Double-Blinded Intervention. Eur. J. Nutr. 2019, 59, 2507–2524. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a Diet Based on Inulin-Rich Vegetables on Gut Health and Nutritional Behavior in Healthy Humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Li, Y. Resistant Starch Formation in Rice: Genetic Regulation and Beyond. Plant Commun. 2022, 3, 100329. [Google Scholar] [CrossRef]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose Resistant Starch (HAM-RS2) Supplementation Increases the Proportion of Faecalibacterium Bacteria in End-Stage Renal Disease Patients: Microbial Analysis From a Randomized Placebo-Controlled Trial. Hemodial. Int. 2019, 23, 343–347. [Google Scholar] [CrossRef]
- Hughes, R.; Horn, W.; Finnegan, P.; Newman, J.; Marco, M.; Keim, N.; Kable, M. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients 2021, 13, 645. [Google Scholar] [CrossRef]
- Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A Comprehensive Review on the Impact of Β-Glucan Metabolism by Bacteroides and Bifidobacterium Species as Members of The Gut Microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gómez-Candela, C.; Smidt, H.; Marín, F.R.; Soler-Rivas, C. Modulation of Human Intestinal Microbiota in a Clinical Trial by Consumption of A Β-D-Glucan-Enriched Extract Obtained from Lentinula Edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in Gut Microbiota Composition and Metabolic Parameters After Dietary Intervention with Barley Beta Glucans in Patients with High Risk for Metabolic Syndrome Development. Anaerobe 2018, 55, 67–77. [Google Scholar] [CrossRef]
- Ale, E.C.; Binetti, A.G. Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights into Their Applications. Front. Microbiol. 2021, 12, 631254. [Google Scholar] [CrossRef]
- Mall, J.-P.G.; Fart, F.; Sabet, J.A.; Lindqvist, C.M.; Nestestog, R.; Hegge, F.T.; Keita, A.V.; Brummer, R.J.; Schoultz, I. Effects of Dietary Fibres on Acute Indomethacin-Induced Intestinal Hyperpermeability in the Elderly: A Randomised Placebo Controlled Parallel Clinical Trial. Nutrients 2020, 12, 1954. [Google Scholar] [CrossRef]
- Nilholm, C.; Manoharan, L.; Roth, B.; D’Amato, M.; Ohlsson, B. A Starch- And Sucrose-Reduced Dietary Intervention in Irritable Bowel Syndrome Patients Produced a Shift in Gut Microbiota Composition Along with Changes in Phylum, Genus, and Amplicon Sequence Variant Abundances, Without Affecting the Micro-RNA Levels. United Eur. Gastroenterol. J. 2022, 10, 363–375. [Google Scholar] [CrossRef]
- DeMartino, P.; Johnston, E.A.; Petersen, K.S.; Kris-Etherton, P.M.; Cockburn, D.W. Additional Resistant Starch from One Potato Side Dish per Day Alters the Gut Microbiota but Not Fecal Short-Chain Fatty Acid Concentrations. Nutrients 2022, 14, 721. [Google Scholar] [CrossRef]
- Fong, J.V.N.; Miketinas, D.; Moore, L.W.; Nguyen, D.T.; Graviss, E.A.; Ajami, N.; Patterson, M.A. Precision Nutrition Model Predicts Glucose Control of Overweight Females Following the Consumption of Potatoes High in Resistant Starch. Nutrients 2022, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Ponder, M.A.; McMillan, R.P.; Hughes, M.D.; Hulver, M.W.; Neilson, A.P.; Davy, K.P. Prebiotic Inulin Supplementation and Peripheral Insulin Sensitivity in adults at Elevated Risk for Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients 2021, 13, 3235. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Terasawa, M.; Okazaki, F.; Nakayama, H.; Zang, L.; Nishiura, K.; Matsuda, K.; Nishimura, N. Rhamnan Sulphate from Green Algae Monostroma Nitidum Improves Constipation with Gut Microbiome Alteration in Double-Blind Placebo-Controlled Trial. Sci. Rep. 2021, 11, 13384. [Google Scholar] [CrossRef] [PubMed]
- Yoon, L.; Michels, K. Characterizing the Effects of Calcium and Prebiotic Fiber on Human Gut Microbiota Composition and Function Using a Randomized Crossover Design—A Feasibility Study. Nutrients 2021, 13, 1937. [Google Scholar] [CrossRef]
- Biruete, A.; Cross, T.-W.L.; Allen, J.M.; Kistler, B.M.; de Loor, H.; Evenepoel, P.; Fahey, G.C.; Bauer, L.; Swanson, K.S.; Wilund, K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522. [Google Scholar] [CrossRef]
- Berding, K.; Long-Smith, C.M.; Carbia, C.; Bastiaanssen, T.F.S.; van de Wouw, M.; Wiley, N.; Strain, C.R.; Fouhy, F.; Stanton, C.; Cryan, J.F.; et al. A Specific Dietary Fibre Supplementation Improves Cognitive Performance—An Exploratory Randomised, Placebo-Controlled, Crossover Study. Psychopharmacology 2020, 238, 149–163. [Google Scholar] [CrossRef]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota—Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef]
- Reimer, R.A.; Soto-Vaca, A.; Nicolucci, A.C.; Mayengbam, S.; Park, H.; Madsen, K.L.; Menon, R.; Vaughan, E.E. Effect of Chicory Inulin-Type Fructan–Containing Snack Bars on the Human Gut Microbiota in Low Dietary Fiber Consumers in a Randomized Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 1286–1296. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Sasidharan, B.K.; Ramadass, B.; Viswanathan, P.; Samuel, P.; Gowri, M.; Pugazhendhi, S.; Ramakrishna, B.S. A Phase 2 Randomized Controlled Trial of Oral Resistant Starch Supplements in the Prevention of Acute Radiation Proctitis in Patients Treated for Cervical Cancer. J. Cancer Res. Ther. 2019, 15, 1383–1391. [Google Scholar] [CrossRef]
- Yasukawa, Z.; Inoue, R.; Ozeki, M.; Okubo, T.; Takagi, T.; Honda, A.; Naito, Y. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients 2019, 11, 2170. [Google Scholar] [CrossRef]
- Sandberg, J.; Kovatcheva-Datchary, P.; Björck, I.; Bäckhed, F.; Nilsson, A. Abundance of Gut Prevotella at Baseline and Metabolic Response to Barley Prebiotics. Eur. J. Nutr. 2018, 58, 2365–2376. [Google Scholar] [CrossRef]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The Pros and Cons of Using Algal Polysaccharides as Prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).