Diet Quality and Health in Older Americans
Abstract
:1. Introduction
2. Materials and Methods
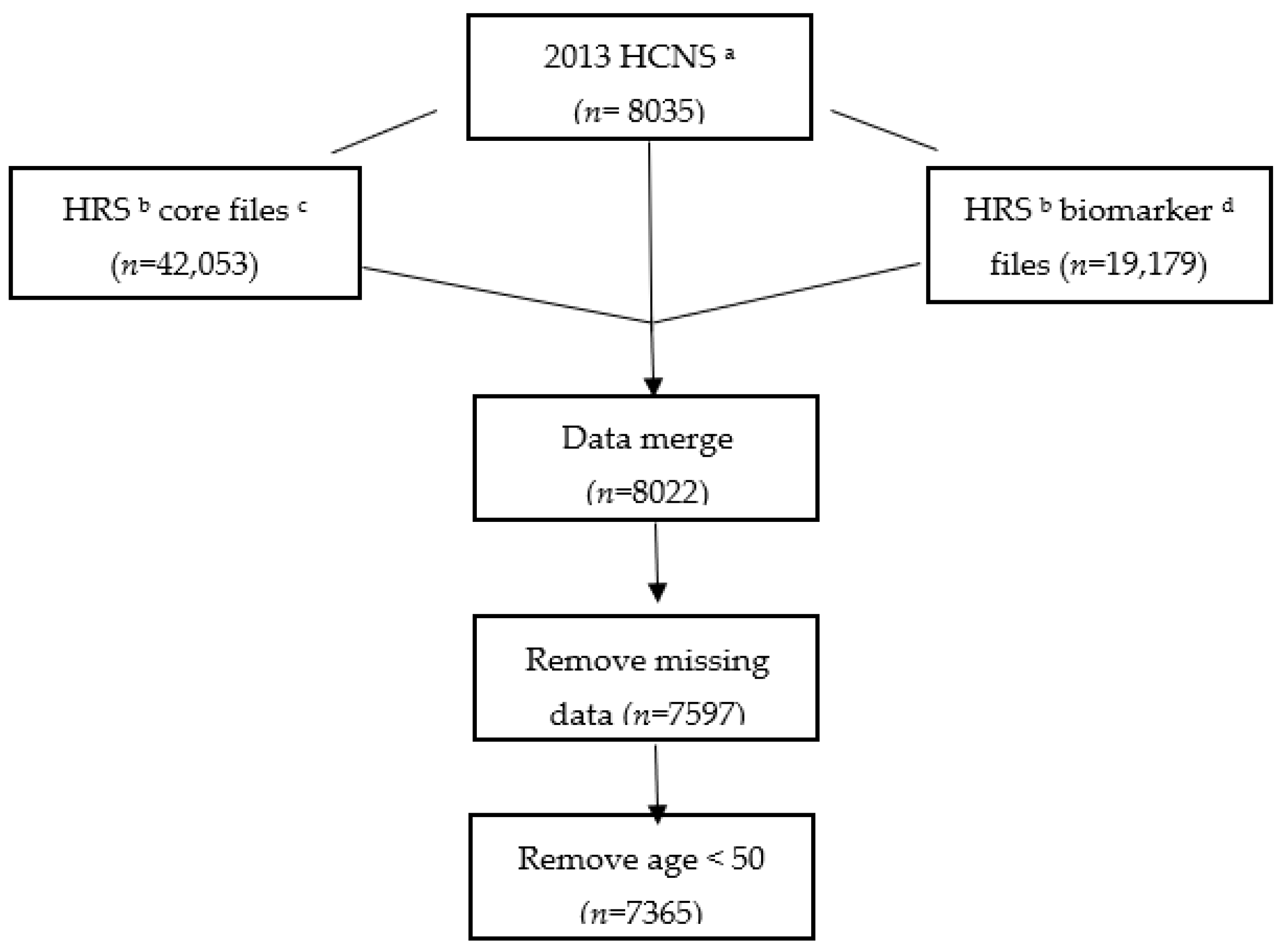
2.1. Data and Sample Selection
2.2. Measures
2.2.1. Diet Quality
2.2.2. Health Assessment
2.3. Analyses
3. Results
3.1. Demographic Characteristics
3.2. ADL Disability, Depression, and Biomarker Indicators across HEI/Diet Quality Groups: Unadjusted Results
3.3. Model Estimation: Predicted Probabilities of ADL Disability, Depression, and Biomarker Health Indicators
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Component (Range) | Female | Hispanic | ||
|---|---|---|---|---|
| Yes (n = 4322) | No (n = 3043) | Yes (n = 846) | No (n = 6519) | |
| Adequacy | ||||
| Total fruit (0–5) | 4.1 (4.0, 4.1) | 3.7 *** (3.6, 3.8) | 4.1 (4.0, 4.2) | 3.9 *** (3.8, 3.9) |
| Whole fruit (0–5) | 4.6 (4.6, 4.7) | 4.4 *** (4.4, 4.5) | 4.7 (4.6, 4.8) | 4.5 *** (4.5, 4.6) |
| Total vegetables (0–5) | 3.9 (3.8, 4.0) | 3.4 *** (3.4, 3.5) | 3.9 (3.8, 4.0) | 3.7 *** (3.6, 3.7) |
| Greens and beans (0–5) | 4.0 (3.9, 4.1) | 3.5 *** (3.5, 3.6) | 3.7 (3.5, 3.8) | 3.8 (3.7, 3.8) |
| Whole grains (0–10) | 4.4 (4.3, 4.5) | 4.4 (4.2, 4.6) | 5.7 (5.3, 6.1) | 4.3 *** (4.2, 4.4) |
| Dairy (0–10) | 5.7 (5.6, 5.8) | 5.2 *** (5.1, 5.3) | 5.3 (5.1, 5.5) | 5.5 (5.4, 5.6) |
| Total protein foods (0–5) | 3.3 (3.2, 3.3) | 3.5 *** (3.4, 3.5) | 3.2 (3.0, 3.3) | 3.4 *** (3.3, 3.4) |
| Seafood and plant proteins (0–5) | 2.4 (2.4, 2.5) | 2.4 (2.3, 2.4) | 2.1 (1.9, 2.3) | 2.4 *** (2.4, 2.5) |
| Fatty acids (0–10) | 3.7 (3.6, 3.8) | 3.8 (3.7, 3.9) | 4.1 (3.8, 4.3) | 3.7 ** (3.6, 3.8) |
| Moderation | ||||
| Refined grains (0–10) | 9.1 (9.0, 9.2) | 9.0 (8.8, 9.1) | 7.3 (6.8, 7.8) | 9.2 *** (9.1, 9.2) |
| Sodium (0–10) | 8.3 (8.2, 8.4) | 8.2 * (8.1, 8.3) | 8.2 (8.0, 8.5) | 8.3 (8.2, 8.3) |
| Added sugars (0–10) | 7.4 (7.3, 7.6) | 7.3 * (7.1, 7.4) | 7.9 (7.7, 8.1) | 7.3 *** (7.2, 7.4) |
| Saturated fats (0–10) | 5.8 (5.7, 5.9) | 5.9 (5.8, 6.0) | 6.7 (6.5, 6.9) | 5.8 *** (5.7, 5.8) |
| HEI–2015 score | 66.7 (66.3, 67.2) | 64.7 *** (64.1, 65.3) | 66.8 (65.9, 67.7) | 65.7 ** (65.3, 66.2) |
| Body Mass Index (BMI) | ||||
| Component (Range) | Normal Weight (n = 1920) | Obese (n = 2566) | Overweight (n = 2641) | Underweight (n = 76) |
| Adequacy | ||||
| Total fruit (0–5) | 3.9 (3.8, 4.0) | 3.9 (3.8, 3.9) | 3.9 (3.8, 4.0) | 3.8 (3.2, 4.3) |
| Whole fruit (0–5) | 4.5 (4.5, 4.6) | 4.6 (4.5, 4.6) | 4.6 (4.5, 4.6) | 4.3 (3.9, 4.7) |
| Total vegetables (0–5) | 3.7 (3.6, 3.8) | 3.7 (3.6, 3.8) | 3.7 (3.6, 3.7) | 3.2 ** (2.8, 3.6) |
| Greens and beans (0–5) | 3.8 (3.8, 3.9) | 3.8 (3.7, 3.9) | 3.8 * (3.7, 3.8) | 3.4 ** (3.0, 3.8) |
| Whole grains (0–10) | 4.4 (4.2, 4.6) | 4.4 (4.3, 4.6) | 4.4 (4.3, 4.6) | 3.9 (3.2, 4.6) |
| Dairy (0–10) | 5.5 (5.3, 5.7) | 5.4 (5.3, 5.6) | 5.5 (5.4, 5.6) | 4.9 * (4.2, 5.5) |
| Total protein foods (0–5) | 3.1 (3.0, 3.2) | 3.6 *** (3.5, 3.7) | 3.3 *** (3.3, 3.4) | 2.9 (2.6, 3.3) |
| Seafood and plant proteins (0–5) | 2.4 (2.3, 2.5) | 2.4 (2.4, 2.5) | 2.4 (2.4, 2.5) | 2.2 (1.9, 2.6) |
| Fatty acids (0–10) | 4.0 (3.9, 4.2) | 3.6 *** (3.4, 3.7) | 3.8 ** (3.6, 3.9) | 3.6 (2.7, 4.5) |
| Moderation | ||||
| Refined grains (0–10) | 9.1 (9.0, 9.2) | 8.9 * (8.8, 9.1) | 9.0 (8.9, 9.1) | 8.9 (8.4, 9.5) |
| Sodium (0–10) | 8.5 (8.4, 8.7) | 7.9 *** (7.8, 8.1) | 8.3 *** (8.2, 8.4) | 8.9 ** (8.6, 9.3) |
| Added sugars (0–10) | 7.4 (7.2, 7.5) | 7.4 (7.3, 7.6) | 7.3 (7.2, 7.5) | 6.4 *** (5.6, 7.1) |
| Saturated fats (0–10) | 6.0 (5.9, 6.1) | 5.5 *** (5.4, 5.7) | 6.0 (5.8, 6.1) | 6.2 (5.2, 7.2) |
| HEI-2015 score | 66.5 (65.9, 67.1) | 65.1 *** (64.5, 65.7) | 66.0 (65.4, 66.6) | 62.7 ** (59.2, 66.1) |
| Age | ||||
| Component (range) | Age 50–60 (n = 2374) | Age 61–70 (n = 2094) | Age 71–80 (n = 2026) | Age above 81 (n = 871) |
| Adequacy | ||||
| Total fruit (0–5) | 3.7 (3.6, 3.8) | 3.8 (3.8, 3.9) | 4.2 (4.1, 4.3) | 4.3 (4.3, 4.4) |
| Whole fruit (0–5) | 4.4 (4.3, 4.5) | 4.5 *** (4.5, 4.6) | 4.7 *** (4.7, 4.8) | 4.8 *** (4.7, 4.9) |
| Total vegetables (0–5) | 3.6 (3.5, 3.7) | 3.7 * (3.7, 3.8) | 3.7 * (3.7, 3.8) | 3.7 (3.6, 3.8) |
| Greens and beans (0–5) | 3.8 (3.7, 3.9) | 3.8 (3.7, 3.9) | 3.8 (3.7, 3.8) | 3.7 (3.6, 3.8) |
| Whole grains (0–10) | 4.4 (4.2, 4.6) | 4.4 (4.2, 4.6) | 4.5 (4.3, 4.6) | 4.3 (4.1, 4.5) |
| Dairy (0–10) | 5.4 (5.3, 5.6) | 5.3 (5.1, 5.5) | 5.6 ** (5.5, 5.8) | 5.9 *** (5.7, 6.2) |
| Total protein foods (0–5) | 3.5 (3.4, 3.5) | 3.4 (3.4, 3.5) | 3.2 (3.1, 3.3) | 3.1 (3.0, 3.2) |
| Seafood and plant proteins (0–5) | 2.4 (2.3, 2.5) | 2.5 (2.4, 2.6) | 2.4 (2.3, 2.5) | 2.3 * (2.1, 2.4) |
| Fatty acids (0–10) | 3.7 (3.6, 3.9) | 3.9 (3.8, 4.1) | 3.8 (3.7, 4.0) | 3.3 (3.1, 3.5) |
| Moderation | ||||
| Refined grains (0–10) | 9.1 (8.9, 9.2) | 9.0 (8.8, 9.1) | 9.1 (8.9, 9.2) | 9.0 (8.8, 9.2) |
| Sodium (0–10) | 8.3 (8.1, 8.4) | 8.1 * (8.0, 8.2) | 8.3 (8.2, 8.4) | 8.5 ** (8.4, 8.6) |
| Added sugars (0–10) | 7.2 (7.0, 7.4) | 7.6 *** (7.4, 7.8) | 7.4 ** (7.3, 7.6) | 7.2 (7.0, 7.4) |
| Saturated fats (0–10) | 5.8 (5.6, 5.9) | 5.9 (5.7, 6.0) | 6.0 ** (5.8, 6.1) | 5.8 (5.6, 6.1) |
| HEI-2015 score | 65.2 (64.4, 65.9) | 66.1 ** (65.5, 66.6) | 66.7 *** (66.0, 67.4) | 65.9 (65.1, 66.6) |
| Component (range) | Ratio of Household Income to the Poverty | |||
| <1.30 (n = 1126) | 1.30–3.49 (n = 2692) | ≥3.50 (n = 3547) | ||
| Adequacy | ||||
| Total fruit (0–5) | 3.7 (3.6, 3.9) | 3.9 ** (3.8, 4.0) | 3.9 ** (3.9, 4.0) | |
| Whole fruit (0–5) | 4.4 (4.2, 4.5) | 4.6 ** (4.5, 4.6) | 4.6 *** (4.5, 4.6) | |
| Total vegetables (0–5) | 3.3 (3.2, 3.5) | 3.6 *** (3.5, 3.7) | 3.8 *** (3.8, 3.9) | |
| Greens and beans (0–5) | 3.4 (3.3, 3.6) | 3.6 ** (3.6, 3.7) | 4.0 *** (3.9, 4.0) | |
| Whole grains (0–10) | 4.4 (4.0, 4.8) | 4.3 (4.2, 4.5) | 4.5 (4.4, 4.6) | |
| Dairy (0–10) | 5.2 (5.0, 5.5) | 5.4 (5.3, 5.6) | 5.5 ** (5.4, 5.6) | |
| Total protein foods (0–5) | 3.3 (3.2, 3.4) | 3.3 (3.3, 3.4) | 3.4 * (3.4, 3.5) | |
| Seafood and plant proteins (0–5) | 2.2 (2.1, 2.3) | 2.2 (2.2, 2.3) | 2.6 *** (2.5, 2.6) | |
| Fatty acids (0–10) | 3.6 (3.4, 3.7) | 3.6 (3.5, 3.8) | 3.9 *** (3.7, 4.0) | |
| Moderation | ||||
| Refined grains (0–10) | 8.3 (7.9, 8.7) | 8.9 *** (8.8, 9.1) | 9.2 *** (9.2, 9.3) | |
| Sodium (0–10) | 8.4 (8.2, 8.6) | 8.2 * (8.1, 8.3) | 8.2 (8.1, 8.4) | |
| Added sugars (0–10) | 6.7 (6.4, 7.0) | 7.1 ** (6.9, 7.2) | 7.7 *** (7.6, 7.8) | |
| Saturated fats (0–10) | 5.9 (5.7, 6.2) | 5.8 (5.6, 5.9) | 5.9 (5.7, 6.0) | |
| HEI-2015 score | 62.9 (62.1, 63.8) | 64.7 *** (64.2, 65.2) | 67.2 *** (66.7, 67.8) | |
References
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Mortality and Burden of Disease Estimates for WHO Member States in 2004; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; December 2015. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 29 January 2022).
- Pilgrim, A.; Robinson, S.; Sayer, A.A.; Roberts, H. An overview of appetite decline in older people. Nurs. Older People 2015, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklett, E.J.; Kadell, A.R. Fruit and vegetable intake among older adults: A scoping review. Maturitas 2013, 75, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.E.; Kim, S.; Bishop, A.; Hermann, J. Poor nutritional status among low-income older adults: Examining the interconnection between self-care capacity, food insecurity, and depression. J. Acad. Nutr. Diet. 2019, 119, 1687–1694. [Google Scholar] [CrossRef]
- Schwartz, N.; Buliung, R.; Wilson, K. Disability and food access and insecurity: A scoping review of the literature. Health Place 2019, 57, 107–121. [Google Scholar] [CrossRef]
- Hiza, H.; Casavale, K.O.; Guenther, P.M.; Davis, C.A. Diet quality of Americans differs by age, sex, race/ethnicity, income and education level. J. Acad. Nutr. Diet. 2013, 113, 297–306. [Google Scholar] [CrossRef]
- Pooler, J.A.; Hartline-Grafton, H.; DeBor, M.; Sudore, R.L.; Seligman, H.K. Food insecurity: A key social determinant of health for older adults. J. Am. Geriatr. Soc. 2019, 67, 421. [Google Scholar] [CrossRef]
- Pieroth, R.; Radler, D.R.; Guenther, P.M.; Brewster, P.J.; Marcus, A. The relationship between social support and diet quality in middle-aged and older adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 1272–1278. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Social isolation and risk for malnutrition among older people. Geriatr. Gerontol. Int. 2017, 17, 286–294. [Google Scholar] [CrossRef]
- Conklin, A.I.; Forouhi, N.G.; Surtees, P.; Khaw, K.; Wareham, N.J.; Monsivais, P. Social relationships and healthful dietary behaviour: Evidence from over-50s in the EPIC cohort, UK. Soc. Sci. Med. 2014, 100, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Crimmins, E.M.; Kim, J.K.; Ailshire, J.A. Food and nutrient intake and diet quality among older Americans. Public Health Nutr. 2021, 24, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.G.; Gomes, A.C.; Naves, M.M.; Mota, J.F. Nuts and legume seeds for cardiovascular risk reduction: Scientific evidence and mechanisms of action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, 2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.S.; Arroyave, W.D.; Lunn, R.M.; Park, Y.M.; Boyd, W.A.; Sandler, D.P. A prospective analysis of red and processed meat consumption and risk of colorectal cancer in women. Cancer Epidemiol. Prev. Biomark. 2020, 29, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Diet Collaborators GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Akbaraly, T.N.; Shipley, M.J.; Ferrie, J.E.; Virtanen, M.; Lowe, G.; Hamer, M.; Kivimaki, M. Long-term adherence to healthy dietary guidelines and chronic inflammation in the prospective Whitehall II study. Am. J. Med. 2015, 128, 152–160.e4. [Google Scholar] [CrossRef] [Green Version]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [Green Version]
- George, S.M.; Ballard-Barbash, R.; Manson, J.E.; Reedy, J.; Shikany, J.M.; Subar, A.F.; Tinker, L.F.; Vitolins, M.; Neuhouser, M.L. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: Evidence to inform national dietary guidance. Am. J. Epidemiol. 2014, 180, 616–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Féart, C.; Pérès, K.; Samieri, C.; Letenneur, L.; Dartigues, J.; Barberger-Gateau, P. Adherence to a Mediterranean diet and onset of disability in older persons. Eur. J. Epidemiol. 2011, 26, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, D.K.; Stevens, J.; Cai, J.; Haines, P.S. Dairy, fruit, and vegetable intakes and functional limitations and disability in a biracial cohort: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2005, 81, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.J.; Papachristou, E.; Atkins, J.L.; Papacosta, O.; Ash, S.; Lennon, L.T.; Whincup, P.H.; Ramsay, S.E.; Wannamethee, S.G. Healthier diet quality and dietary patterns are associated with lower risk of mobility limitation in older men. Eur. J. Nutr. 2019, 58, 2335–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomata, Y.; Watanabe, T.; Sugawara, Y.; Chou, W.; Kakizaki, M.; Tsuji, I. Dietary patterns and incident functional disability in elderly Japanese: The Ohsaki Cohort 2006 study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Grigorescu, E.; Sorodoc, V.; Floria, M.; Anisie, E.; Popa, A.D.; Onofriescu, A.; Ceasovschih, A.; Sorodoc, L. The inflammatory marker HSCRP as a predictor of increased insulin resistance in type 2 diabetics without atherosclerotic manifestations. Rev. Chim. 2019, 70, 1791–1794. [Google Scholar] [CrossRef]
- Ceasovschih, A.; Sorodoc, V.; Tesloianu, D.; Tuchilus, C.; Anisie, E.; Petris, A.; Statescu, C.; Jaba, E.; Stoica, A.; Grigorescu, E. Biomarker utility for peripheral artery disease diagnosis in real clinical practice: A prospective study. Diagnostics 2020, 10, 723. [Google Scholar] [CrossRef]
- Bugliari, D.; Campbell, N.; Chan, C.; Hayden, O.; Hayes, J.; Hurd, M.; Karabatakis, A.; Main, R.; Mallett, J.; McCullough, C. RAND HRS Longitudinal File 2016 (V1) Documentation; RAND Center for the Study of Aging: Santa Monica, CA, USA, 2019. [Google Scholar]
- Health and Retirement Study, 2013 Health Care and Nutrition Study V5.0: Nutrient Totals Data Description. Ann Arbor, MI 2018. Available online: https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/2013HCNS_data_description_nt.pdf (accessed on 29 January 2022).
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Harvard TH & Chan School of Public Health Nutrition. Nutrient Tables. 2015. Available online: https://regepi.bwh.harvard.edu/health/nutrition.html (accessed on 29 January 2022).
- Crimmins, E.; Faul, J.; Kim, J.K.; Guyer, H.; Langa, K.; Ofstedal, M.B.; Sonnega, A.; Wallace, R.; Weir, D. Documentation of biomarkers in the 2006 and 2008 Health and Retirement Study; Survey Research Center University of Michigan: Ann Arbor, MI, USA, 2013. [Google Scholar]
- US Department of Agriculture. Choose MyPlate Tips and Resources. Available online: https://www.myplate.gov (accessed on 29 January 2022).
- National Cancer Institute. Healthy Eating Index Scoring Algorithm. Available online: https://epi.grants.cancer.gov/hei/hei-scoring-method.html (accessed on 29 January 2022).
- Bishop, N.J.; Zuniga, K.E. Caloric Intake and Dietary Patterns Are Associated with Onset of Physical Disability and Mortality in a Representative Sample of Older U.S. Adults. In Proceedings of the Association of America 2019 Annual Meeting, Austin, TX, USA, 10–13 April 2019. [Google Scholar]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Gould, C.E.; Rideaux, T.; Spira, A.P.; Beaudreau, S.A. Depression and anxiety symptoms in male veterans and non-veterans: The Health and Retirement study. Int. J. Geriatr. Psychiatry 2015, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Bennett, C.M.; Guo, M.; Dharmage, S.C. HbA1c as a screening tool for detection of type 2 diabetes: A systematic review. Diabetic Med. 2007, 24, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Data—National Center for Health Statistics, What We Eat in America/National Health and Nutrition Examination Survey, 2013–2014. Healthy Eating Index-2015, Scores—U.S. Department of Agriculture, Center for Nutrition Policy and Promotion. Available online: https://www.fns.usda.gov/healthy-eating-index-hei (accessed on 29 January 2022).
- Hartmann, C.; Siegrist, M.; van der Horst, K. Snack frequency: Associations with healthy and unhealthy food choices. Public Health Nutr. 2013, 16, 1487–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr. J. 2019, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ziliak, J.P.; Gundersen, C. The state of senior hunger in America in 2015. National Foundation to End Senior Hunger. Available online: https://www.feedingamerica.org/sites/default/files/research/senior-hungerresearch/state-of-senior-hunger-2015.pdf (accessed on 1 March 2019).
- Xu, B.; Houston, D.; Locher, J.L.; Zizza, C. The association between Healthy Eating Index-2005 scores and disability among older Americans. Age Ageing 2012, 41, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Bennett, D.A.; Morris, M.C. Dietary patterns and self-reported incident disability in older adults. J. Gerontol. Ser. A 2019, 74, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, A.; Sun, Y.; Liang, H.; Wang, Q.; Yi, X.; Han, X. Magnesium status and dietary intake of mid-old people in a rural area of China. Magnes. Res. 2009, 22, 66–71. [Google Scholar] [CrossRef] [Green Version]
| Component (Score Range) | Standard: Maximum Score | Standard: Minimum Score of Zero | All Respondents Mean (95% CI) (n = 7365) |
|---|---|---|---|
| Adequacy | |||
| Total fruit (0–5) | ≥0.8 cup equiv. per 1000 kcal | No fruit | 3.9 (3.8, 4.0) |
| Whole fruit (0–5) | ≥0.4 cup equiv. per 1000 kcal | No whole fruit | 4.5 (4.5, 4.6) |
| Total vegetables (0–5) | ≥1.1 cup equiv. per 1000 kcal | No vegetables | 3.7 (3.6, 3.7) |
| Greens and beans (0–5) | ≥0.2 cup equiv. per 1000 kcal | No greens and beans | 3.8 (3.7, 3.8) |
| Whole grains (0–10) | ≥1.5 oz equiv. per 1000 kcal | No whole grains | 4.4 (4.3, 4.5) |
| Dairy (0–10) | ≥1.3 cup equiv. per 1000 kcal | No dairy | 5.5 (5.4, 5.6) |
| Total protein foods (0–5) | ≥2.5 oz equiv. per 1000 kcal | No protein foods | 3.4 (3.3, 3.4) |
| Seafood and plant proteins (0–5) | ≥0.8 oz equiv. per 1000 kcal | No seafood or plant proteins | 2.4 (2.4, 2.5) |
| Fatty acids (0–10) | (PUFAs + MUFAs)/SFAs ≥2.5 | (PUFAs + MUFAs)/SFAs ≤1.2 | 3.8 (3.7, 3.9) |
| Moderation | |||
| Refined grains (0–10) | ≤1.8 oz equiv. per 1000 kcal | ≥4.3 oz equiv. per 1000 kcal | 9.0 (8.9, 9.1) |
| Sodium (0–10) | ≤1.1 g per 1000 kcal | ≥2.0 g per 1000 kcal | 8.3 (8.2, 8.3) |
| Added sugars (0–10) | ≤6.5% of energy | ≥26% of energy | 7.4 (7.3, 7.5) |
| Saturated fats (0–10) | ≤8% of energy | ≥16% of energy | 5.8 (5.8, 5.9) |
| HEI-2015 score | - | - | 65.8 (65.4, 66.3) |
| Characteristics, Mean | Low HEI Group | Medium HEI Group | High HEI Group |
|---|---|---|---|
| Mean (95% CI) (n = 2173) | Mean (95% CI) (n = 2607) | Mean (95% CI) (n = 2585) | |
| Outcomes | |||
| Health, disability, and depression | |||
| Count of health conditions | 2.3 (2.2, 2.4) | 2.2 (2.2, 2.3) | 1.9 *** (1.9, 2.0) |
| Number of ADL functional limitations | 0.5 (0.4, 0.6) | 0.4 *** (0.3, 0.4) | 0.3 *** (0.2, 0.3) |
| ADL-based disability, % | 22.8 (20.1, 25.5) | 17.0 *** (15.5, 18.6) | 12.5 *** (11.0, 14.0) |
| Depression, % | 18.4 (16.3, 20.5) | 13.3 *** (11.7, 14.8) | 10.1 *** (8.5, 11.8) |
| Biomarker indicators | |||
| Glycosylated hemoglobin (HbA1c), % | 5.9 (5.9, 6.0) | 6.0 * (5.9, 6.1) | 5.8 * (5.8, 5.9) |
| C-reactive protein (CRP), mg/L | 4.2 (3.8, 4.7) | 3.6 ** (3.3, 4.0) | 2.9 *** (2.6, 3.2) |
| High-density lipoprotein (HDL), mg/dL | 53.5 (52.1, 54.8) | 54.9 (53.6, 56.3) | 57.7 *** (56.4, 59.0) |
| Total cholesterol, mg/dL | 195.0 (191.1, 199.0) | 192.8 (189.8, 195.8) | 194.2 (191.3, 197.2) |
| Cystatin C, mg/L | 1.20 (1.17, 1.24) | 1.17 (1.13, 1.20) | 1.07 *** (1.04, 1.11) |
| Demographic and other covariates | |||
| Daily energy (kcal/100) | 20.2 (19.5, 20.9) | 18.2 *** (17.8, 18.7) | 17.8 *** (17.3, 18.3) |
| Age, years | 65.0 (64.3, 65.8) | 66.0 ** (65.3, 66.8) | 65.8 * (65.1, 66.5) |
| Socio-demographic covariates, % | |||
| Gender | |||
| Female | 48.6 (46.5, 50.7) | 54.5 *** (52.5, 56.6) | 59.7 *** (57.5, 61.9) |
| Male | 51.4 (49.3, 53.5) | 45.5 *** (43.4, 47.5) | 40.3 *** (38.1, 42.5) |
| Race/ethnicity | |||
| Non-Hispanic white | 78.4 (75.0, 81.9) | 77.4 (73.8, 80.9) | 77.0 (73.5, 80.4) |
| Non-Hispanic black | 10.4 (8.2, 12.6) | 10.6 (9.0, 12.2) | 10.4 (8.7, 12.0) |
| Hispanic of any race | 7.5 (5.2, 9.8) | 8.7 (6.0, 11.5) | 9.2 (6.4, 11.9) |
| Other race/ethnicity | 3.7 (2.4, 4.9) | 3.3 (2.2, 4.5) | 3.5 (2.5, 4.5) |
| Education | |||
| Less than high school education | 17.5 (15.6, 19.4) | 13.3 *** (11.3, 15.2) | 10.0 *** (8.2, 11.7) |
| High school or GED | 59.8 (56.9, 62.7) | 52.8 *** (50.1, 55.4) | 46.8 *** (44.2, 49.4) |
| College | 17.2 (14.4, 19.9) | 24.2 *** (21.5, 26.9) | 28.4 *** (25.9, 30.9) |
| Graduate degree | 5.5 (4.2, 6.8) | 9.7 *** (8.2, 11.2) | 14.8 *** (13.1, 16.6) |
| Household’s receipt of benefits from Supplemental Nutrition Assistance Program (SNAP) | 13.3 (11.2, 15.3) | 8.1 *** (6.3, 9.9) | 5.7 *** (4.3, 7.1) |
| Ratio of family income to poverty threshold | |||
| <1.3 | 19.5 (16.9, 22.2) | 12.8 *** (10.2, 15.4) | 10.0 *** (8.1, 11.9) |
| 1.3–3.49 | 35.8 (33.2, 38.5) | 33.3 (31.2, 35.5) | 27.6 *** (25.3, 29.8) |
| ≥3.5 | 44.6 (41.6, 47.7) | 53.9 *** (50.6, 57.1) | 62.5 *** (59.3, 65.6) |
| Body weight status | |||
| Underweight | 1.9 (1.2, 2.6) | 0.5 *** (0.2, 0.8) | 1.0 * (0.6, 1.5) |
| Normal weight | 27.1 (24.6, 29.6) | 25.1 (22.9, 27.3) | 30.4 ** (28.6, 32.2) |
| Overweight | 33.7 (31.4, 35.9) | 37.9 ** (35.1, 40.7) | 36.8 * (34.5, 39.2) |
| Obese | 37.4 (34.9, 39.8) | 36.5 (33.8, 39.3) | 31.7 *** (29.0, 34.5) |
| Marital status | |||
| Married/partnered | 61.9 (59.2, 64.5) | 67.8 *** (65.7, 70.0) | 67.8 *** (65.2, 70.3) |
| Divorced/separated | 16.5 (14.7, 18.3) | 13.2 *** (11.7, 14.7) | 12.0 *** (10.3, 13.6) |
| Widowed | 14.6 (12.7, 16.4) | 14.0 (12.2, 15.8) | 14.4 (12.6, 16.2) |
| Never married | 7.0 (5.6, 8.4) | 5.0 ** (3.8, 6.2) | 5.9 (4.7, 7.1) |
| Variables | Low HEI Group Estimated Marginal Means (95% CI) | Medium HEI Group Estimated Marginal Means (95% CI) | High HEI Group Estimated Marginal Means (95% CI) |
|---|---|---|---|
| Health, Disability and Depression Outcomes | |||
| Count of health conditions | |||
| Overall (n = 7140) | 2.18 (2.14, 2.22) | 2.19 (2.15, 2.22) | 2.13 (2.09, 2.18) |
| Female (n = 4165) | 2.21 (2.16, 2.26) | 2.22 (2.17, 2.28) | 2.16 * (2.12, 2.21) |
| Male (n = 2975) | 2.15 (2.10, 2.21) | 2.14 (2.09, 2.19) | 2.10 (2.02, 2.17) |
| Number of functional limitations | |||
| Overall (n = 7140) | 0.41 (0.37, 0.45) | 0.38 (0.34, 0.42) | 0.33 *** (0.28, 0.37) |
| Female (n = 4165) | 0.47 (0.41, 0.53) | 0.45 (0.39, 0.52) | 0.35 *** (0.30, 0.41) |
| Male (n = 2975) | 0.33 (0.28, 0.39) | 0.29 (0.24, 0.35) | 0.29 (0.21, 0.38) |
| ADL-based disability, % | |||
| Overall (n = 7140) | 19.6 (17.9, 21.3) | 16.4 *** (15.1, 17.7) | 15.2 *** (14.0, 16.5) |
| Female (n = 4165) | 22.5 (19.9, 25.1) | 18.2 *** (16.3, 20.1) | 16.5 *** (14.7, 18.2) |
| Male (n = 2975) | 16.5 (14.2, 18.8) | 14.2 (12.2, 16.1) | 13.8 * (11.7, 15.9) |
| Depression, % | |||
| Overall (n = 6867) | 14.9 (13.4, 16.4) | 13.6 (12.0, 15.3) | 11.8 *** (10.0, 13.6) |
| Female (n = 4068) | 16.9 (14.5, 19.3) | 16.2 (13.9, 18.6) | 13.1 *** (11.2, 14.9) |
| Male (n = 2799) | 12.7 (10.1, 15.2) | 10.5 (8.5, 12.6) | 10.2 (7.5, 12.9) |
| Biomarker Indicators | |||
| Glycosylated hemoglobin (HbA1c), % | |||
| Overall (n = 4405) | 5.90 (5.85, 5.94) | 5.95 * (5.91, 5.99) | 5.89 (5.86, 5.93) |
| Female (n = 2626) | 5.88 (5.81, 5.96) | 5.93 (5.88, 5.97) | 5.87 (5.81, 5.92) |
| Male (n = 1779) | 5.91 (5.85, 5.97) | 5.99 * (5.91, 6.07) | 5.92 (5.86, 5.99) |
| C-reactive protein, mg/L | |||
| Overall (n = 4591) | 3.8 (3.4, 4.3) | 3.6 (3.2, 4.0) | 3.3 ** (2.9, 3.6) |
| Female (n = 2741) | 4.2 (3.5, 4.9) | 3.9 (3.3, 4.5) | 3.6 (3.3, 3.9) |
| Male (n = 1850) | 3.4 (2.9, 3.9) | 3.2 (2.7, 3.7) | 2.8 * (2.4, 3.3) |
| High-density lipoprotein, mg/dL | |||
| Overall (n = 4369) | 53.8 (52.9, 54.8) | 55.5 ** (54.3, 56.8) | 57.2 *** (56.0, 58.3) |
| Female (n = 2633) | 57.4 (56.1, 58.7) | 58.2 (56.9, 59.6) | 59.8 ** (58.3, 61.2) |
| Male (n = 1736) | 49.7 (48.1, 51.4) | 52.1 ** (50.4, 53.9) | 54.0 *** (52.3, 55.7) |
| Total Cholesterol, mg/dL | |||
| Overall (n = 4586) | 196.4 (192.6, 200.2) | 193.4 (190.8, 196.0) | 192.1 * (189.2, 194.9) |
| Female (n = 2742) | 201.4 (197.3, 205.4) | 195.7 ** (192.0, 199.5) | 195.8 ** (192.8, 198.7) |
| Male (n = 1844) | 190.6 (185.7, 195.5) | 190.5 (186.5, 194.5) | 187.7 (183.1, 192.3) |
| Cystatin C, mg/L | |||
| Overall (n = 4531) | 1.17 (1.14, 1.20) | 1.13 ** (1.11, 1.15) | 1.13 * (1.09, 1.16) |
| Female (n = 2715) | 1.16 (1.12, 1.20) | 1.14 (1.12, 1.17) | 1.13 (1.09, 1.17) |
| Male (n = 1816) | 1.18 (1.13, 1.22) | 1.12 ** (1.08, 1.15) | 1.12 (1.07, 1.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Andreyeva, T. Diet Quality and Health in Older Americans. Nutrients 2022, 14, 1198. https://doi.org/10.3390/nu14061198
Zhao H, Andreyeva T. Diet Quality and Health in Older Americans. Nutrients. 2022; 14(6):1198. https://doi.org/10.3390/nu14061198
Chicago/Turabian StyleZhao, Hang, and Tatiana Andreyeva. 2022. "Diet Quality and Health in Older Americans" Nutrients 14, no. 6: 1198. https://doi.org/10.3390/nu14061198
APA StyleZhao, H., & Andreyeva, T. (2022). Diet Quality and Health in Older Americans. Nutrients, 14(6), 1198. https://doi.org/10.3390/nu14061198







