1. Introduction
The number of people with dementia is now over 50–70 million worldwide, and it is presumed to increase by 10 million every year. As the quality of life in old age decreases through cognitive impairment, protection through nutrition and the avoidance of lifestyle-related diseases is essential to continue a healthy life and to save medical expenses. Reflecting these situations, studies concerning protection from the negative impacts of aging and cognitive impairment through the daily intake of certain foods and their components have been conducted by many researchers. As a result of these studies, protection from the negative impacts of aging and cognitive impairment by polyphenol and flavonoid intake [
1,
2]; improvements in cognitive functions using green tea and its components, such as tea catechins [
3,
4]; the prevention of learning and memory impairment in senescence-accelerated mice (SAMP8) using green tea catechin [
5] and the attenuation of age-associated muscle loss through the administration of epigallocatechin-3 gallate contained in green tea [
6] have been reported. However, further precise examination is needed to clarify the mechanisms of action of these compounds in the hippocampus [
7]. Matcha is a traditional green tea from Japan, obtained by the preparation of green tea leaves (
Camellia sinensis), which are cultivated under shade for approximately 3 weeks before harvest and processed into green tea fine powder and consumed in a thick suspension in hot water, delivering richer tea catechins and theanine, as well as fat-soluble compounds, such as lutein and vitamin K, which are not contained in green tea infusions. Recently, it was shown that matcha consumption improves cognition in human volunteers [
4,
8]. However, information on the underlying mechanism is still limited. Therefore, we tried to examine the improvement effect of matcha and decaffeinated matcha on cognitive impairment and their action mechanism mainly from the comprehensive analysis of hippocampus proteins by proteomics in SAMP8 mice.
To date, the influence of the consumption of green tea catechin on the expression of hippocampus proteins has mostly been examined by measuring its effects on the expression of a few brain (hippocampus) proteins, which were set as markers for aging and cognitive impairment in individual experiments [
5], suggesting that an extensive analysis of proteins using proteomics might be necessary to confirm the proteins influenced by ingested samples and to examine their action mechanism. However, the influence of matcha intake on protein expression in the mouse hippocampus, which may be involved in improving aging and cognitive impairment, has never been examined extensively.
Thus, the influence of long-term matcha intake on protein expression and its mechanism was investigated mainly by proteomics in the hippocampus of SAMP8 mice.
Senescence-accelerated prone-8 (SAMP8) mice have been known to show age-related changes in learning and memory abilities [
9,
10,
11], suggesting that SAMP8 is a valuable animal model for the investigation of the effect of food and its components on aging and cognitive impairment. Senescence-accelerated-resistant mice (SAMR1), which are reported to show a normal aging profile [
12], were set as the control. Thus, in this study, we investigated the functions of matcha and decaffeinated matcha in SAMP8 mice.
In this study, first, the influence of the intake of matcha and decaffeinated matcha on appearance, such as glossiness of the back coat, coarseness of hair on the head and memory ability, was examined; then, hippocampus proteins influenced by the administered samples were mainly examined using proteomics to confirm the proteins influenced by administering samples, to examine their action mechanism and finally to find the benefits of matcha and decaffeinated matcha. Additionally, proteins showing differential expression between SAMP8 and SAMR1 through differential expression analysis were also examined to determine the proteins that could vary through aging and cognitive impairment and could be improved by matcha intake and decaffeinated matcha.
2. Materials and Methods
2.1. Preparation of Matcha and Decaffeinated Matcha
Matcha and decaffeinated matcha (low in caffeine content) were obtained from Kyoeiseicha Co., Ltd. (Osaka, Japan). Matcha is a green tea powder that has a high content of whole leaves and is favored to drink as a thick suspension. The matcha was produced as follows: first, specific green tea leaves harvested after cultivation in an environment without sunlight for many weeks in May (the first tea in the year) were steamed and dried to obtain tencha, followed by removing the leaf vein and grinding it to produce matcha powder. The decaffeinated matcha was prepared by washing tencha green tea leaves with hot water to remove caffeine, then producing matcha green tea powder [
13].
2.2. Animal Care
The research was performed with permission from the Institutional Animal Care and Use Committee of Yamagata University (Permit number: R2139) and carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and our guidelines at Yamagata University. Mice were kept individually in stainless steel cages with screen bottoms, with both their sides separated by a stainless-steel plate, under an environment with 12 h of lighting and off at 22 ± 2 °C and 40–60 humidity conditions. Abnormal behavior was not observed during feeding periods due to the use of these cages. All surgery was carried out under anesthesia using 2% isoflurane (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and all efforts were made to minimize the pain inflicted on mice.
2.3. Animals and Diet
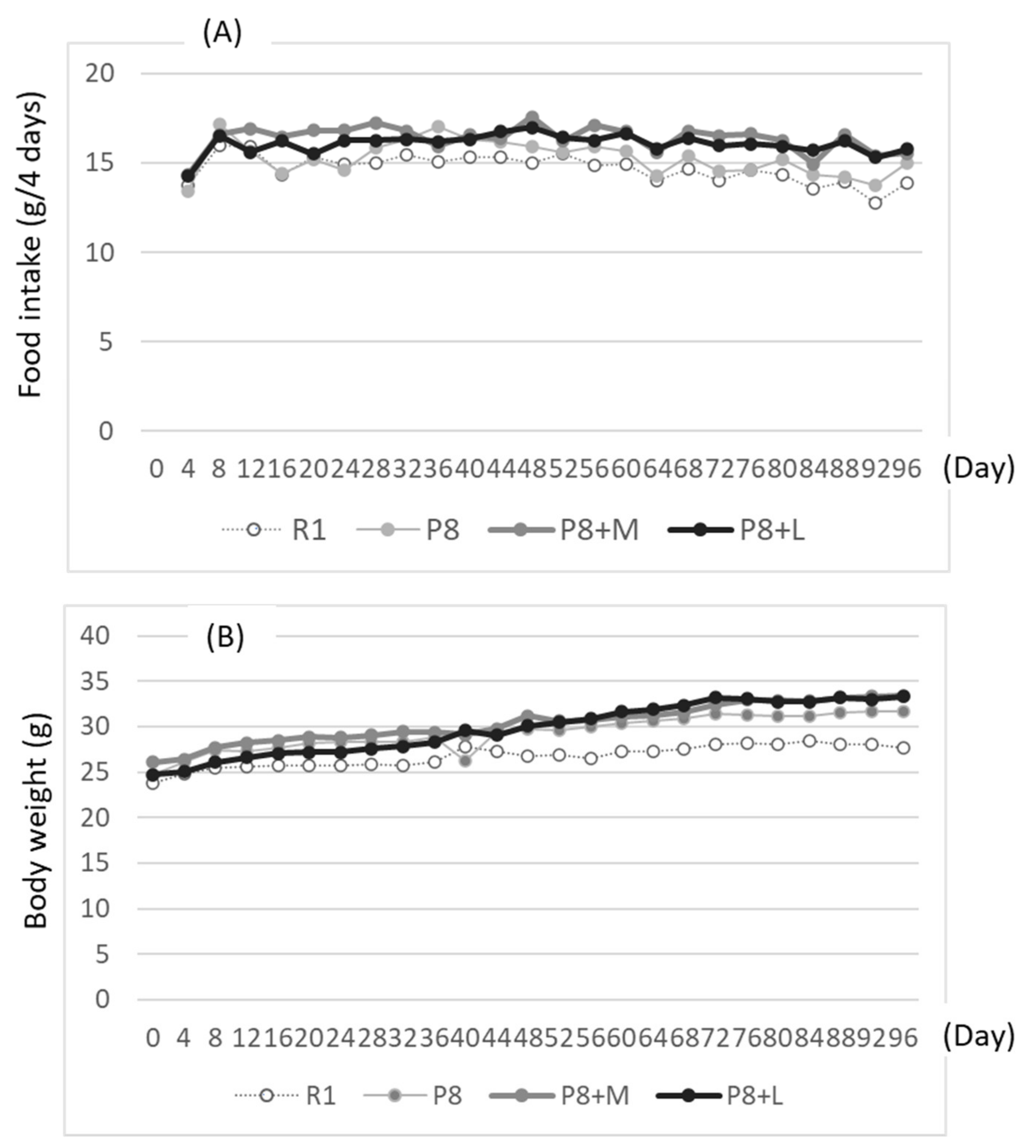
Eight-month-old senescence-accelerated mice (SAMP8, male) and senescence-accelerated-resistant mice (SAMR1, male) as the control (average weight, 25 g; SPF) were bought from the Shizuoka Laboratory Animal Center (SLC) (Hamamatsu, Shizuoka, Japan) and cared for according to the guidelines of Yamagata University for the care of experimental animals. After acclimating to the environment for three days, the mice were randomly divided into four groups of five or six mice and fed either a basal diet (SAMR1 = R1 and SAMP8 = P8 groups, n 6), a basal diet with matcha (SAMP8 + M = P8 + M group, n 6), or a basal diet with decaffeinated matcha (SAMP8 + L = P8 +L group, n 5). The diet composition of each experimental group is indicated in
Table 1. The amounts of matcha and decaffeinated matcha were set to avoid abnormality in the behavior of mice in the preliminary experiment.
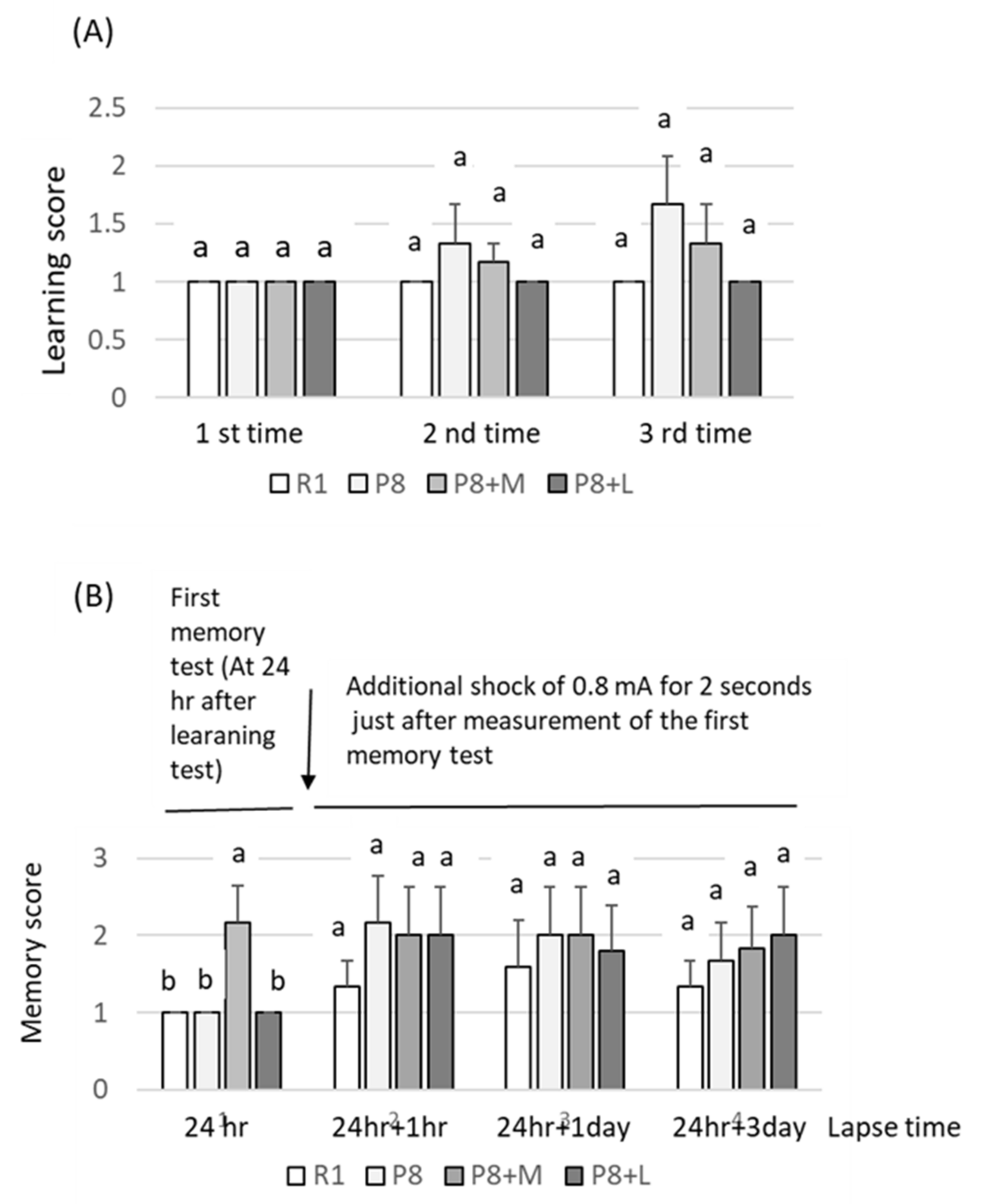
Food and water were taken freely for 98 days. Body weight and food intake were measured every four days. Learning and memory ability tests using the step-through test system (shock generator: MK-SG05, Muromachi Kikai Co., Ltd., Tokyo, Japan) were carried out from days 91 to 94 of the feeding. First, mice were placed in a light box, which was partitioned by an opener from a black box; then, the time needed to move from the lightbox to the black box was measured (first measurement). Shortly after, the mice moved to the dark box were exposed to electric stimulation at 0.08 mA for 0.3 s per mouse. Then, the mice were taken out of the black box and held for 30 s. The mice were placed again into the same light box as in the first measurement. This procedure was repeated thrice. The delays in the movement time to the black box from the light box were used to evaluate learning ability. After 3 measurements, mice were returned to the cage in which the mice were kept, then after one day were placed into the light box. The time required to move to the black box was used to measure memory ability (first measurement for memory ability test), followed by electrical stimulation at 0.8 mA for two seconds per mouse. Then, after three hours, memory ability was measured again (as the first measurement of the second memory ability test). After one and three days of the second memory test, the memory ability test was repeated without electric stimulation.
The method used to evaluate the degree of senescence in mice was in accordance with Hosokawa et al. [
14]. Although Hosokawa et al. used 11 categories of age-associated changes in behavior and appearance and evaluated their degree of severity in grades, in this experiment, only the difference in the appearance, such as glossiness of the back coat, coarseness of hair on the head and loss of hair on the head (ranked by five grades, from 1 to 5, depending on the severity of symptom in this order) was scored, and the tentative mean of the grade per mouse was shown as an aging score.
On day 97 or 98 of the feeding period, half of the mice in each group were anesthetized using isoflurane 3–5 h after starving. After the collection of blood by heart puncture with a syringe, the brain was detached. The brain hippocampus was removed from the detached brain as soon as possible. The hippocampus was kept under –80 °C until analysis.
2.4. Measurements of Amino Acid and TBARS, and Proteomics
The frozen hippocampus was manually crushed and homogenized with cold 80% methanol (MeOH) using a plastic pestle, then ultra-sonicated for 15 min in an ice bath, followed by centrifugation at 11,000 rpm for 15 min to obtain the protein pellet and supernatant according to the method of Ivanisevic et al. [
15]. The protein pellet was re-homogenized with cold 80% MeOH and then re-centrifuged. The same procedure was repeated thrice. The obtained protein pellet and supernatant were used for proteomics and analyses of amino acid and TBARS, respectively. TBARS was measured by the method of Uchiyama and Mihara [
16], in which TBARS values were measured using the fluorometric method at Ex 532 and Em 560 nm. Then, the formed malondialdehyde (MDA)–TBARS complex was purified by transferal to an n-butyl alcohol layer. Amino acids were measured by the ninhydrin method using an amino acid analyzer (Hitachi L8900, Tokyo, Japan).
The protein pellet for proteomics was suspended in the RapiGest SF Surfactant (Waters, Milford, MA, USA) solution, then cysteine residue in protein was reduced with dithiothreitol and alkylated with iodoacetamide (Zhao et al., 2010) [
17]. Then, it was digested with sequencing-grade modified trypsin (Promega, Madison, WI, USA) for 16 h at 37 °C. The digested sample solution, which was added with 10% TFA (0.5% TFA at the final concentration), was incubated at 37 °C for 30 min and centrifuged at 10,000×
g for 20 min at 5 °C. The obtained supernatant was freeze-dried and dissolved with a small amount of Milli Q water and then centrifuged at 10,000×
g for 20 min at 5 °C. Finally, the obtained supernatant was applied to a UPLC ESI-Q-TOF MS/MS system (Xevo Q-Tof MS, Waters, Manchester, UK).
The peptides in the supernatant were separated on the UPLC (nano AQUITY, Waters, Manchester, UK). The UPLC conditions were as follows: column BEH C-18 (i.d. 2.1 × 100 mm, 1.7 µm, Waters, Ireland, UK); eluent, 0.1% HCOOH (eluent A), 0.1% HCOOH in acetonitrile (eluent B); flow rate, 0.3 mL/min; column temperature, 30 °C; 0–100% B for 45 min with linear gradient. The spectra were obtained in the positive ion mode in micro Q-TOF with a mass range of 50–2000. The source temperature was 120 °C. Protein identification was carried out using Protein Lynx (Waters, Milford, MA, USA) and a database on the mice (taxonomy ID: 10088), which was provided by the National Center for Biotechnology Information (NCBI), Bethesda, MD, USA. The carbamidomethylating of cysteine and methionine oxidation was considered to be caused by variable modifications of tryptic peptides in MS/MS analysis. Differential protein expression analyses were performed after data normalization using the Protein Lynx TM Global Server (ver. 2.3) (Waters, Manchester, UK).
2.5. Statics
Data are expressed as means ± SEM. Six animals in each group, except for the SAMP8+L group (five animals), were used. Bartlett’s test verified the homogeneity of variance between treatments. Data were statistically analyzed using one-way analysis of variance for the measurements of the aging score, hippocampus (GABA/Glu) ratio, hippocampus TBARS and learning and memory scores. Tukey’s multiple range test was used for a post hoc analysis of significance. All comparisons were considered significant at p < 0.05. A significant difference in differential protein expression analysis, which was performed after data normalization using the Protein Lynx TM Global Server (ver. 2.3), was considered significant at p < 0.05 or p > 0.95.
4. Discussion
As no difference in the aging score due to sample feeding was observed, breeding for a longer period or a higher dosage of the sample might have been necessary to clearly observe the difference in appearance, such as the aging score. The effects of matcha and decaffeinated matcha on learning and memory abilities, determined using the step-through test, loading 0.08 mA for 0.3 s per mouse (repeated thrice), was unclear in the first loading test; the memory ability tended to be improved in the second step-through test, which was performed through additional electric shocks of 0.8 mA for two seconds (repeated thrice) just after the first step-through test, suggesting that matcha and decaffeinated matcha may have the ability to improve memory ability, but this was not statistically significant. Stronger electric stimulation from the first electric shock without alternation in the latter stage might be necessary to obtain clearer results in the step-through test.
Although many reports have dealt with the physiological functions of green tea and its components, especially catechins on the protection of cognitive impairment [
3,
5], almost all of them were obtained by evaluating the effects of ingested samples on the expression of some proteins in the brain and hippocampus with learning and memory abilities in mouse models with cognitive impairments, such as SAMP8. To discover the protective effects of matcha and decaffeinated matcha against cognitive impairment, including their action mechanism, it is considered to be necessary to examine their effects comprehensively using methods such as proteomics, which makes it possible to examine the effects on the expression of many proteins holistically.
An outline of the results of the differential expression analyses between two groups are shown in
Table 7 as increase/decrease arrows. Although a significant improvement in aging and memory abilities due to the ingestion of matcha and decaffeinated matcha was not observed, differential analyses and fold changes between the control and ingested groups in the expression of hippocampus proteins indicated that proteins mainly associated with neuron degeneration, Parkinson’s disease and Alzheimer’s (brain acid-soluble protein 1), glial fibrillary acidic protein, Parkinson’s disease (autosomal recessive and early onset) 7 and microtubule-associated protein tau are downregulated by matcha and decaffeinated matcha, and that proteins mainly associated with antioxidation (peroxiredoxin 5 and glutathione S-transferase Mu 1) are upregulated by matcha and decaffeinated matcha.
Proteins associated with synapse transmission and nerve cell plasticity, except for proteins of synapsin-2 and synapsin-1 (tubulin alpha-1A chain and dynamin-2), were upregulated. In contrast, synapsin-2 and synapsin-1 were downregulated. As it is reported that the amounts of synapsin-2 are higher in the hippocampus of older rats than in younger ones, that the amounts of tubulin alpha are lower in the hippocampus of old rats than young ones [
22] and that dynamin is needed for memory formation [
23], the opposite amounts of these proteins in the hippocampus in mice administered matcha and decaffeinated matcha indicated that the administered samples have the ability to ameliorate aging and cognitive impairment, presumably by increasing nerve cell plasticity. Proteins associated with glutamate transport and metabolism, and with GABA formation and transport, were upregulated, except for sodium- and chloride-dependent GABA transporter-3, suggesting that matcha and decaffeinated matcha may contribute to the prevention of aging and cognitive impairment by upregulating the expression of these proteins (solute carrier family 1 (glial high-affinity glutamate transporter) and calcium/calmodulin-dependent protein kinase type II subunit gamma) (
Table 7). However, detailed mechanisms need to be examined in the future. In addition, it is necessary to examine the identification of effective components and their action mechanisms in the future.
Although solute carrier family-1 (glial high-affinity glutamate transporter) was upregulated by feeding with matcha and decaffeinated matcha, the expression of excitatory amino acid transporters was mostly not influenced by the ingestion of these samples, suggesting that glial glutamate transporters other than excitatory amino acid transporters may play an essential role in the clearance of glutamate in the synaptic cleft when mice were fed these samples. Although the administration of matcha and decaffeinated matcha influenced the expression of proteins involved in neuron degeneration, Parkinson’s disease and Alzheimer’s, antioxidation, synapse transmission and nerve cell plasticity and glutamate transport and metabolism, it is interesting to consider whether these influences were caused by the same compounds contained in the samples or not. These points remain to be solved in the future.
Some reports show that the long-term ingestion of caffeine partially improves hippocampus-dependent learning and memory abilities [
27], indicating the possibility that one reason for the difference in protein expression between matcha- and decaffeinated matcha-fed mice might be caused by the difference in caffeine content. Since matcha and decaffeinated matcha contain large amounts of catechins, such as epigallocatechin (EGC), epigallocatechin gallate (EGCG), epicatechin (EC) and epicatechin gallate (ECG), which can ameliorate aging and cognitive impairment [
28], these compounds may also be strongly related to the efficiency of matcha and decaffeinated matcha. However, as matcha and decaffeinated matcha also contain other compounds, such as theanine [
29,
30] and lutein [
31], it may also be necessary to consider the effects of these compounds. It is known that theanine, which is abundant in matcha, exhibits preventive effects on stress-induced brain atrophy [
32]; vitamin K and lutein, which are abundant in matcha, enhance angiogenic potential [
33] and cortical capillary aging was prevented in matcha-fed mice [
33], suggesting that studies administering individual compounds may be necessary to clarify active compounds.
As a conclusion of this experiment, it was found, mainly using proteomics of the hippocampus of SAMP8, that the ingestion of matcha and decaffeinated matcha could influence the expression of many proteins associated with memory, aging and cognitive impairment and could improve their expression, confirming the effect of matcha and decaffeinated matcha. Proteins showed differential expression upon the administration of matcha and decaffeinated matcha, which are expected to be used as markers for the evaluation of food and its components that show protective effects against aging and cognitive impairments.
5. Conclusions
The effects of the ingestion of matcha and decaffeinated matcha on aging, memory ability and the expression of hippocampus proteins that may be related cognitive impairment were investigated in a senescent-accelerated mouse model (SAMP8).
Although the improvement of memory ability by the ingestion of these samples was minor, the ingestion of these samples downregulated the expression of the hippocampus brain acid-soluble protein 1, microtubule-associated protein tau, syanpsin-2 and sodium- and chloride-dependent GABA transporter, and tended to downregulate the expression of Parkinson’s disease 7 and synapsin-1. Furthermore, it was shown that these samples upregulated the expression of hippocampus glutathione S-transferase Mu 1, tubulin alpha-1A chain, dynamin-2, calcium/calmodulin-dependent protein kinase type II subunit gamma and tyrosine 3-monooxygenase/tyrosine 5-monooxygenase activation protein epsilon polypeptide. These results indicate the possibility that matcha and decaffeinated matcha could reduce aging and cognitive impairment by regulating the expression of these proteins. Furthermore, these results suggest that these proteins could be used as markers for food evaluation and for the investigation of food components that are capable of reducing aging and cognitive impairment. In particular, we are the first to propose that the downregulation of synapsin-2 by matcha may be involved in the prevention of cognitive impairment.