Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunnumakkara, A.B.; Bordloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive Review on Physiological Effects of Curcumin. Drug Res. 2020, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Mutsuga, M.; Chambers, J.K.; Uchida, K.; Tei, M.; Makibuchi, T.; Mizorogi, T.; Takashima, A.; Nakayama, H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J. Vet. Med. Sci. 2012, 74, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithu, V.S.; Sarkar, B.; Bhowmik, D.; Das, A.K.; Chandrakesan, M.; Maiti, S.; Madhu, P.K. Curcumin alters the salt bridge-containing turn region in amyloid beta(1-42) aggregates. J. Biol. Chem. 2014, 289, 11122–11131. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Backskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Zha, W.L.; Ke, Z.Q.; Min, Q.; Li, C.R.; Sun, H.R.; Liu, C. Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. J. Diabetes Res. 2016, 2016, 4158591. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Hao, J.T.; Wang, J.; Zhang, W.F.; Yan, C.P.; Zhao, J.H. Curcumin inhibits cardiac hypertrophy and improves cardiovascular function via enhanced Na+/Ca2+ exchanger expression after transverse abdominal aortic constriction in rats. Pharmacol. Rep. 2018, 70, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Peng, X.F.; Du, W.M.; Wu, Y.; Huang, B.; Xue, L.; Wu, Q.; Qiu, H.M.; Jiang, Q.S. Curcumin attenuates cardiomyocyte hypertrophy induced by high glucose and insulin via the PPAR gamma/Akt/NO signaling pathway. Diabetes Res. Clin. Pract. 2015, 108, 235–242. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Sunagawa, Y.; Hasegawa, K.; Morimoto, T. Application of curcumin to heart failure therapy by targeting transcriptional pathway in cardiomyocytes. Biol. Pharm. Bull. 2013, 36, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Meng, B.; Li, J.; Cao, H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2113. [Google Scholar]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Hashemzaei, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef]
- Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhang, M.; Luo, J.; Zhang, L.; Shao, Y.; Li, G. Curcumin ameliorates memory deficits via neuronal nitric oxide synthase in aged mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Wong, R.H.X.; Howe, P.R.C. Can Curcumin Counteract Cognitive Decline? Clinical Trial Evidence and Rationale for Combining ω-3 Fatty Acids with Curcumin. Adv. Nutr. 2018, 9, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, doubleblind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Wahlqvist, M.L.; Chou, Y.C.; Fang, W.H.; Lee, J.T.; Kuan, J.C.; Liu, H.Y.; Lu, T.M.; Xiu, L.; Hsu, C.C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Niti, M.; Yap, K.B.; Kua, E.H.; Tan, C.H.; Ng, T.P. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int. Psychogeriatr. 2008, 20, 237–251. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Nyunt, M.S.; Feng, L.; Gao, Q.; Lim, M.L.; Collinson, S.L.; Chong, M.S.; Lim, W.S.; Lee, T.S.; et al. Metabolic Syndrome and the Risk of Mild Cognitive Impairment and Progression to Dementia: Follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol. 2016, 73, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.P.; Niti, M.; Chiam, P.C.; Kua, E.H. Ethnic differences in cognitive performance on Mini-Mental State Examination in Asians. Am. J. Geriatr. Psychiatry 2007, 15, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Collinson, S.L.; Feng, L.; Ng, T.P. Preliminary normative neuropsychological data for an elderly chinese population. Clin. Neuropsychol. 2012, 26, 321–334. [Google Scholar] [CrossRef] [PubMed]
- HPB-MOH Clinical Practice Guidelines on Obesity. Available online: http://www.moh.gov.sg/cpg (accessed on 3 January 2022).
- Tay, J.C.; Sule, A.A.; Chew, E.K.; Tey, J.S.; Lau, T.; Lee, S.; Lee, S.H.; Leong, C.K.; Lim, S.T.; Low, L.P.; et al. Ministry of Health Clinical Practice Guidelines: Hypertension. Singap. Med. J. 2018, 59, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, S.Y.; Ang, S.B.; Bee, Y.M.; Chen, Y.T.; Gardner, D.S.; Ho, E.T.; Adaikan, K.; Lee, Y.C.; Lee, C.H.; Lim, F.S.; et al. Ministry of Health Clinical Practice Guidelines: Diabetes Mellitus. Singap. Med. J. 2014, 55, 334–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, E.S.; Chia, B.L.; Bastian, A.C.; Chua, T.; Ho, S.C.; Koh, T.S.; Low, L.P.; Tey, J.S.; Poh, K.K.; Tan, C.E.; et al. Ministry of Health Clinical Practice Guidelines: Lipids. Singap. Med. J. 2017, 58, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Nyunt, M.S.Z.; Jin, A.Z.; Fones, C.S.L.; Ng, T.P. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment. Health 2009, 13, 376–382. [Google Scholar] [CrossRef]
- Sudo, F.K.; Amado, P.; Alves, G.S.; Laks, J.; Engelhardt, E. A continuum of executive function deficits in early subcortical vascular cognitive impairment: A systematic review and meta-analysis. Dement. Neuropsychol. 2017, 11, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.Y.; Yang, F.Q.; Wang, Y.T.; Li, S.P. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 486–492. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef] [PubMed]
| Never/Rarely | <Once/Month | ≥Once/Month to <Once/Week | ≥Once/Week to Daily | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| N of participants | 519 | 1306 | 559 | 367 | |||||
| Sex: Male | 31.2 | (162) | 33.8 | (442) | 39.4 | (220) | 48.2 | (177) | <0.001 |
| Age, years | 67.9 | ±8.2 | 65.7 | ±7.1 | 65.2 | ±7.2 | 64.9 | ±7.3 | <0.001 |
| Ethnicity: Chinese | 98.3 | (510) | 97.6 | (1275) | 95.5 | (534) | 84.5 | (310) | <0.001 |
| Non-Chinese | 1.7 | (9) | 2.4 | (31) | 4.5 | (25) | 15.5 | (57) | |
| Education: None | 28.3 | (147) | 15.9 | (208) | 13.4 | (75) | 15.5 | (57) | <0.001 |
| 1–6 years | 42.4 | (220) | 39.4 | (514) | 38.3 | (214) | 33.2 | (122) | |
| >6 years | 29.3 | (152) | 44.7 | (584) | 48.3 | (270) | 51.2 | (188) | |
| Smoking: Never | 81.1 | (421) | 82.5 | (1077) | 81.9 | (458) | 77.4 | (284) | 0.216 |
| Past smoker | 11.8 | (61) | 9.7 | (127) | 10.7 | (60) | 11.2 | (41) | |
| Current smoker | 7.1 | (37) | 7.8 | (102) | 7.3 | (41) | 11.4 | (42) | |
| Alcohol ≥ once/week | 4.6 | (24) | 1.8 | (24) | 3.2 | (18) | 4.1 | (15) | 0.006 |
| Physical activity score | 2.31 | 1.73 | 2.42 | 1.61 | 2.37 | 1.78 | 2.44 | 1.85 | 0.618 |
| Social activity score | 3.07 | 2.65 | 3.19 | 2.58 | 3.43 | 2.66 | 3.20 | 2.59 | 0.146 |
| Productive activity score | 3.83 | 1.85 | 3.96 | 1.84 | 4.21 | 1.83 | 4.03 | 1.88 | 0.005 |
| BMI, kg/m2 | 23.9 | 3.7 | 23.8 | 3.7 | 24.1 | 3.5 | 23.4 | 2.9 | 0.392 |
| Central obesity | 46.6 | (242) | 54.2 | (708) | 54.2 | (303) | 53.7 | (197) | 0.023 |
| Hypertension | 60.3 | (313) | 54.1 | (706) | 57.8 | (323) | 57.8 | (212) | 0.077 |
| Diabetes or FBG > 5.6 mmol/L | 25.0 | (130) | 26.3 | (343) | 28.1 | (157) | 26.2 | (96) | 0.723 |
| High triglyceride | 41.2 | (214) | 44.6 | (583) | 45.8 | (256) | 44.1 | (162) | 0.468 |
| Low HDL-Cholesterol | 44.9 | (233) | 49.4 | (645) | 49.7 | (278) | 47.1 | (173) | 0.297 |
| Cardiac diseases | 9.8 | (51) | 7.9 | (103) | 6.3 | (35) | 10.1 | (37) | 0.673 |
| GDS depression score | 1.45 | 2.25 | 0.88 | 1.64 | 1.03 | 2.08 | 1.29 | 2.40 | <0.001 |
| Neurocognitive Performance | Curry Consumption | Baseline | Between Group ANOVA, p | Follow-Up | Between-Group ANOVA, p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | ±SE | Linear | Vs Never/Rarely | n | Mean | ±SE | Linear | Vs Never/Rarely | ||
| MMSE | Never or rarely | 516 | 27.1 | ±3.27 | <0.001 | Reference | 272 | 27.7 | 3.18 | <0.001 | Reference |
| Occasionally (<once a month) | 1297 | 28.1 | ±2.40 | <0.001 | 862 | 28.8 | 2.41 | <0.001 | |||
| Often (>once a month, <once a week) | 555 | 28.4 | ±1.86 | <0.001 | 382 | 28.9 | 2.04 | <0.001 | |||
| Very often (>once a week to daily | 366 | 28.0 | ±2.70 | <0.001 | 231 | 28.9 | 2.26 | <0.001 | |||
| Digit span (forward) | Never or rarely | 478 | 0.07 | ±1.00 | 0.414 | Reference | 167 | −0.03 | 1.02 | 0.316 | Reference |
| Occasionally (<once a month) | 1108 | 0.05 | ±1.05 | 0.723 | 678 | −0.01 | 1.05 | 0.847 | |||
| Often (>once a month, <once a week) | 485 | 0.11 | ±1.07 | 0.551 | 293 | −0.05 | 1.07 | 0.847 | |||
| Very often (>once a week to daily | 324 | −0.03 | ±1.06 | 0.159 | 169 | −0.12 | 1.08 | 0.434 | |||
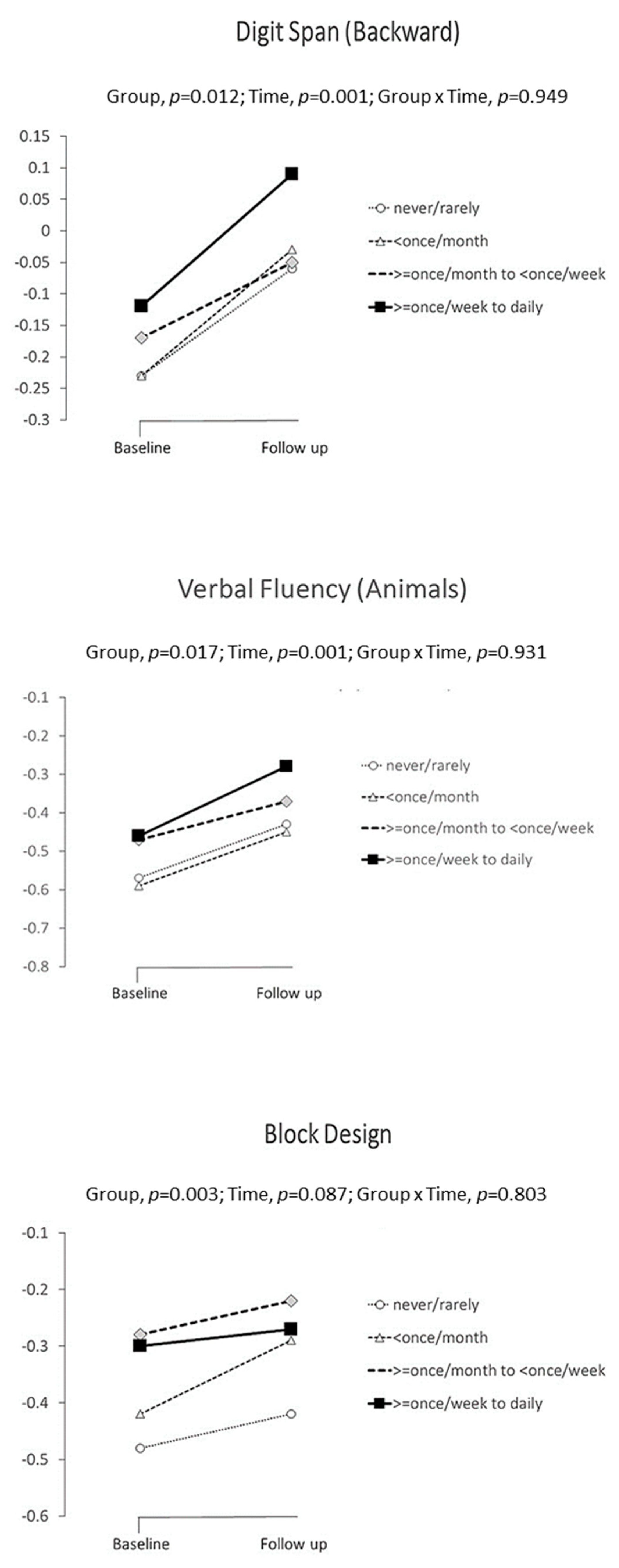
| Digit span (backward) | Never or rarely | 470 | −0.24 | ±1.02 | 0.004 | Reference | 166 | −0.11 | 0.93 | 0.007 | Reference |
| Occasionally (<once a month) | 1098 | −0.18 | ±1.00 | 0.289 | 674 | 0.05 | 0.98 | 0.062 | |||
| Often (>once a month, <once a week) | 480 | 0.02 | ±1.05 | <0.001 | 292 | 0.14 | 1.10 | 0.011 | |||
| Very often (>once a week to daily | 317 | −0.12 | ±1.06 | 0.117 | 169 | 0.17 | 1.05 | 0.011 | |||
| RAVLT, delayed recall | Never or rarely | 436 | −0.34 | ±1.13 | 0.084 | Reference | 162 | −0.14 | 1.09 | 0.985 | Reference |
| Occasionally (<once a month) | 1060 | −0.15 | ±1.02 | 0.002 | 676 | −0.02 | 1.07 | 0.221 | |||
| Often (>once a month, <once a week) | 465 | −0.15 | ±1.01 | 0.006 | 288 | −0.06 | 1.07 | 0.451 | |||
| Very often (>once a week to daily | 299 | −0.20 | ±1.10 | 0.084 | 169 | −0.09 | 1.02 | 0.703 | |||
| Visual Reproduction, delayed recall | Never or rarely | 421 | −0.25 | ±1.10 | 0.181 | Reference | 160 | 0.04 | 1.00 | 0.405 | Reference |
| Occasionally (<once a month) | 1043 | −0.15 | ±1.11 | 0.138 | 668 | 0.06 | 1.01 | 0.806 | |||
| Often (>once a month, <once a week) | 465 | −0.07 | ±1.05 | 0.019 | 289 | 0.13 | 1.09 | 0.367 | |||
| Very often (>once a week to daily | 291 | −0.18 | ±1.17 | 0.423 | 166 | 0.10 | 0.91 | 0.633 | |||
| Verbal Fluency-Animals | Never or rarely | 445 | −0.27 | ±1.07 | <0.001 | Reference | 167 | −0.06 | 1.07 | 0.005 | Reference |
| Occasionally (<once a month) | 1066 | −0.16 | ±0.99 | 0.044 | 675 | 0.00 | 1.00 | 0.498 | |||
| Often (>once a month, <once a week) | 458 | 0.00 | ±1.03 | <0.001 | 292 | 0.11 | 1.03 | 0.086 | |||
| Very often (>once a week to daily | 308 | −0.05 | ±1.01 | 0.003 | 167 | 0.19 | 0.86 | 0.021 | |||
| Trail-Making A | Never or rarely | 281 | −0.39 | ±1.44 | 0.005 | Reference | 159 | 0.10 | 1.38 | 0.013 | Reference |
| Occasionally (<once a month) | 899 | −0.09 | ±1.18 | <0.001 | 663 | 0.01 | 1.16 | 0.389 | |||
| Often (>once a month, <once a week) | 397 | −0.12 | ±1.02 | 0.005 | 291 | −0.06 | 1.32 | 0.180 | |||
| Very often (>once a week to daily | 238 | −0.17 | ±1.58 | 0.046 | 164 | −0.21 | 0.82 | 0.019 | |||
| Trail Making-B | Never or rarely | 239 | −0.32 | ±1.25 | 0.090 | Reference | 141 | 0.49 | 2.15 | 0.021 | Reference |
| Occasionally (<once a month) | 825 | −0.14 | ±1.20 | 0.056 | 630 | 0.15 | 1.46 | 0.014 | |||
| Often (>once a month, <once a week) | 362 | −0.11 | ±1.38 | 0.048 | 274 | 0.17 | 1.25 | 0.035 | |||
| Very often (>once a week to daily | 218 | −0.11 | ±1.24 | 0.077 | 157 | 0.00 | 1.13 | 0.004 | |||
| Block Design | Never or rarely | 324 | −0.38 | ±1.13 | 0.002 | Reference | 162 | −0.18 | 1.00 | 0.082 | Reference |
| Occasionally (<once a month) | 886 | −0.18 | ±1.03 | 0.004 | 671 | −0.05 | 0.99 | 0.120 | |||
| Often (>once a month, <once a week) | 343 | −0.06 | ±1.03 | <0.001 | 288 | 0.02 | 0.99 | 0.035 | |||
| Very often (>once a week to daily | 232 | −0.14 | ±1.11 | 0.010 | 166 | −0.01 | 0.92 | 0.113 | |||
| Neurocognitive Performance | Curry Consumption | Baseline Mean ± SE | Follow-Up Mean ± SE | Group | p | δ | 95% | CI | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | Never or rarely | A | 26.9 | ±0.198 | 25.6 | ±0.207 | Overall | 0.284 | ||||
| Occasionally (<once a month) | B | 27.1 | ±0.193 | 25.7 | ±0.200 | B vs. A | 0.141 | 0.124 | −0.041, | 0.289 | 0.048 | |
| Often (>once a month, <once a week) | C | 27.2 | ±0.204 | 25.7 | ±0.213 | C vs. A | 0.062 | 0.186 | −0.010, | 0.382 | 0.072 | |
| Very often (>once a week to daily | D | 27.0 | ±0.203 | 25.8 | ±0.217 | D vs. A | 0.314 | 0.115 | −0.109, | 0.339 | 0.045 | |
| Digit span (forward) | Never or rarely | A | −0.23 | ±0.108 | −0.38 | ±0.119 | Overall | 0.713 | ||||
| Occasionally (<once a month) | B | −0.30 | ±0.106 | −0.40 | ±0.111 | B vs. A | 0.299 | 0.048 | −0.139, | 0.043 | 0.046 | |
| Often (>once a month, <once a week) | C | −0.25 | ±0.111 | −0.37 | ±0.120 | C vs. A | 0.906 | 0.006 | −0.114, | 0.101 | 0.006 | |
| Very often (>once a week to daily) | D | −0.28 | ±0.110 | −0.38 | ±0.124 | D vs. A | 0.703 | 0.024 | −0.147, | 0.099 | 0.023 | |
| Digit span (backward) | Never or rarely | A | −0.23 | ±0.105 | −0.06 | ±0.114 | Overall | 0.012 | ||||
| Occasionally (<once a month) | B | −0.23 | ±0.103 | −0.03 | ±0.108 | B vs. A | 0.700 | 0.017 | −0.070, | 0.105 | 0.017 | |
| Often (>once a month, <once a week) | C | −0.17 | ±0.108 | −0.05 | ±0.115 | C vs. A | 0.010 | 0.135 | 0.032, | 0.239 | 0.132 | |
| Very often (>once a week to daily) | D | −0.12 | ±0.106 | 0.09 | ±0.119 | D vs. A | 0.028 | 0.133 | 0.014, | 0.252 | 0.130 | |
| RAVLT, delayed recall | Never or rarely | A | −0.44 | ±0.113 | −0.30 | ±0.123 | Overall | 0.156 | ||||
| Occasionally (<once a month) | B | −0.32 | ±0.111 | −0.23 | ±0.115 | B vs. A | 0.041 | 0.096 | 0.004, | 0.189 | 0.091 | |
| Often (>once a month, <once a week) | C | −0.34 | ±0.115 | −0.22 | ±0.124 | C vs. A | 0.118 | 0.087 | −0.022, | 0.196 | 0.083 | |
| Very often (>once a week to daily) | D | −0.27 | ±0.114 | −0.23 | ±0.127 | D vs. A | 0.069 | 0.117 | −0.009, | 0.242 | 0.111 | |
| VR, delayed recall | Never or rarely | A | −0.42 | ±0.116 | −0.16 | ±0.123 | Overall | 0.303 | ||||
| Occasionally (<once a month) | B | −0.42 | ±0.113 | −0.25 | ±0.117 | B vs. A | 0.335 | −0.045 | −0.137, | 0.047 | 0.041 | |
| Often (>once a month, <once a week) | C | −0.35 | ±0.118 | −0.14 | ±0.124 | C vs. A | 0.422 | 0.044 | −0.064, | 0.153 | −0.040 | |
| Very often (>once a week to daily) | D | −0.37 | ±0.118 | −0.20 | ±0.126 | D vs. A | 0.859 | 0.011 | −0.114, | 0.137 | −0.010 | |
| Verbal Fluency-Animals | Never or rarely | A | −0.57 | ±0.108 | −0.43 | ±0.117 | Overall | 0.017 | ||||
| Occasionally (<once a month) | B | −0.59 | ±0.106 | −0.45 | ±0.110 | B vs. A | 0.705 | −0.017 | 0.103, | 0.070 | 0.016 | |
| Often (>once a month, <once a week) | C | −0.47 | ±0.111 | −0.37 | ±0.118 | C vs. A | 0.112 | −0.083 | 0.019, | 0.185 | 0.081 | |
| Very often (>once a week to daily) | D | −0.46 | ±0.109 | −0.28 | ±0.121 | D vs. A | 0.026 | 0.160 | 0.134, | 0.251 | 0.131 | |
| Trail-Making A | Never or rarely | A | −0.61 | ±0.178 | −0.53 | ±0.181 | Overall | 0.375 | ||||
| Occasionally (<once a month) | B | −0.41 | ±0.172 | −0.31 | ±0.174 | B vs. A | 0.315 | 0.060 | −0.057, | 0.176 | 0.047 | |
| Often (>once a month, <once a week) | C | −0.42 | ±0.176 | −0.43 | ±0.180 | C vs. A | 0914 | −0.007 | −0.143, | 0.128 | 0.006 | |
| Very often (>once a week to daily) | D | −0.46 | ±0.176 | −0.49 | ±0.182 | D vs. A | 0.496 | −0.055 | −0.214, | 0.104 | 0.044 | |
| Trail Making-B | Never or rarely | A | −0.64 | ±0.198 | −0.20 | ±0.209 | Overall | 0.975 | ||||
| Occasionally (<once a month) | B | −0.52 | ±0.191 | −0.19 | ±0.198 | B vs. A | 0.071 | 0.008 | −0.131, | 0.148 | 0.007 | |
| Often (>once a month, <once a week) | C | −0.49 | ±0.195 | −0.29 | ±0.208 | C vs. A | 0.083 | −0.023 | −0.186, | 0.139 | 0.019 | |
| Very often (>once a week to daily) | D | −0.45 | ±0.194 | −0.31 | ±0.212 | D vs. A | 0.097 | −0.013 | −0.203, | 0.176 | 0.011 | |
| Block Design | Never or rarely | A | −0.48 | ±0.142 | −0.42 | ±0.146 | Overall | 0.003 | ||||
| Occasionally (<once a month) | B | −0.42 | ±0.140 | −0.29 | ±0.141 | B vs. A | 0.039 | 0.096 | 0.005, | 0.188 | 0.090 | |
| Often (>once a month, <once a week) | C | −0.28 | ±0.146 | −0.22 | ±0.146 | C vs. A | <0.001 | 0.198 | 0.089, | 0.308 | 0.186 | |
| Very often (>once a week to daily) | D | −0.30 | ±0.145 | −0.27 | ±0.147 | D vs. A | 0.010 | 0.166 | 0.040, | 0.292 | 0.156 | |
| Neurocognitive Test | Curry > Once/Month to Daily | Baseline | Follow-Up | Group | p | δ | 95% | CI | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||||||||
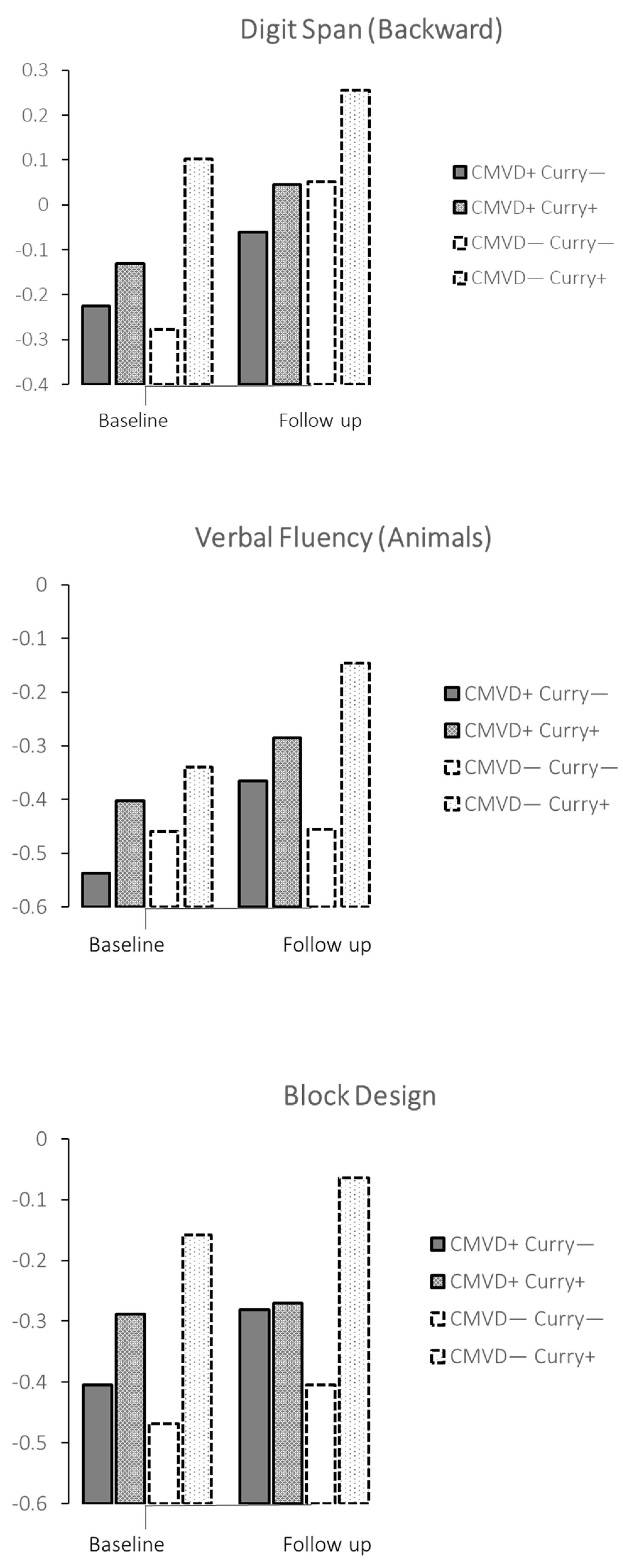
| Cardiometabolic or cardiac diseases | ||||||||||||
| MMSE | No | A | 26.9 | 26.6, 27.3 | 27.5 | 27.2, 27.9 | ||||||
| Yes | B | 27.1 | 27.0, 27.4 | 27.6 | 27.2, 28.0 | A vs. B | 0.213 | 0.095 | −0.054, | 0.244 | 0.037 | |
| Digit span (forward) | No | A | −0.24 | −0.44, −0.05 | −0.37 | −0.58, −0.17 | ||||||
| Yes | B | −0.25 | −0.45, −0.05 | −0.43 | −0.64, −0.21 | A vs. B | 0.450 | −0.031 | −0.110, | 0.049 | 0.029 | |
| Digit span (backward) | No | A | −0.23 | −0.41, −0.04 | −0.06 | −0.26, 0.14 | ||||||
| Yes | B | −0.13 | −0.32, 0.06 | −0.05 | −0.16, 0.25 | A vs. B | 0.010 | 0.101 | 0.024, | 0.178 | 0.098 | |
| RAVLT, delayed recall | No | A | −0.35 | −0.56, −0.14 | −0.22 | −0.44, −0.01 | ||||||
| Yes | B | −0.29 | −0.49, −0.98 | −0.22 | −0.44, 0.00 | A vs. B | 0.387 | 0.036 | −0.045, | 0.117 | 0.034 | |
| VR, delayed recall | No | A | −0.47 | −0.68, −0.26 | −0.23 | −0.45, −0.02 | ||||||
| Yes | B | −0.38 | −0.59, −0.17 | −0.21 | −0.43, 0.01 | A vs. B | 0.202 | 0.052 | −0.028, | 0.132 | 0.047 | |
| Verbal Fluency-Animals | No | A | −0.54 | 0.73, −0.34 | −0.37 | −0.57, −0.16 | ||||||
| Yes | B | −0.40 | −0.60, −0.20 | −0.28 | −0.49, −0.07 | A vs. B | 0.005 | 0.108 | 0.032, | 0.184 | 0.106 | |
| Trail-Making A | No | A | −0.54 | −0.87, −0.22 | −0.32 | −0.65, 0.00 | ||||||
| Yes | B | −0.49 | −0.81, −0.17 | −0.44 | −0.76, −0.11 | A vs. B | 0.558 | −0.030 | −0.129, | 0.070 | 0.024 | |
| Trail Making-B | No | A | −0.49 | −0.85, −0.12 | −0.05 | −0.42, 0.32 | ||||||
| Yes | B | −0.41 | −0.77, −0.05 | −0.07 | −0.45, 0.30 | A vs. B | 0.661 | 0.027 | −0.093, | 0.146 | 0.021 | |
| Block Design | No | A | −0.40 | −0.66, −0.14 | −0.28 | −0.54, −0.02 | ||||||
| Yes | B | −0.29 | −0.55, −0.02 | −0.27 | −0.53, −0.01 | A vs. B | 0.119 | 0.063 | −0.016, | 0.143 | 0.060 | |
| No cardiometabolic or cardiac diseases | ||||||||||||
| MMSE | No | C | 27.0 | 26.2, 27.1 | 27.3 | 26.8, 27.8 | ||||||
| Yes | D | 26.5 | 26.0, 27.0 | 27.2 | 26.6, 27.7 | C vs. D | 0.396 | −0.158 | −0.522, | 0.207 | 0.061 | |
| Digit span (forward) | No | C | −0.30 | −0.53, −0.06 | −0.19 | −0.46, 0.07 | ||||||
| Yes | D | −0.20 | −0.46, 0.07 | −0.28 | −0.59, 0.04 | C vs. D | 0.919 | 0.010 | −0.185, | 0.205 | 0.010 | |
| Digit span (backward) | No | C | −0.28 | −0.50, −0.05 | 0.05 | −0.20, 0.30 | ||||||
| Yes | D | −0.10 | −0.16, 0.36 | 0.26 | −0.05, 0.56 | C vs. D | 0.003 | 0.291 | 0.101, | 0.480 | 0.284 | |
| RAVLT, delayed recall | No | C | −0.22 | −0.47, 0.03 | −0.11 | −0.38, 0.16 | ||||||
| Yes | D | −0.33 | −0.461, 0.05 | −0.14 | −0.47, 0.18 | C vs. D | 0.508 | −0.068 | −0.269, | 0.133 | 0.064 | |
| VR, delayed recall | No | C | −0.39 | −0.64, −0.13 | −0.34 | −0.61, −0.07 | ||||||
| Yes | D | −0.48 | −0.77, −0.19 | −0.06 | −0.38, 0.25 | C vs. D | 0.346 | 0.095 | −0.103, | 0.294 | 0.086 | |
| Verbal Fluency-Animals | No | C | −0.46 | −0.69, −0.22 | −0.46 | −0.71, −0.20 | ||||||
| Yes | D | −0.34 | −0.61, −0.07 | −0.15 | −0.45, −0.16 | C vs. D | 0.024 | 0.215 | 0.028, | 0.402 | 0.211 | |
| Trail-Making A | No | C | −0.49 | −0.87, −0.11 | −0.35 | −0.73, 0.02 | ||||||
| Yes | D | −0.41 | −0.84, 0.02 | −0.67 | −1.10, 0.24 | C vs. D | 0.372 | −0.118 | −0.376, | 0.141 | 0.093 | |
| Trail Making-B | No | C | −0.33 | −0.74, 0.08 | −0.14 | −0.58, 0.31 | ||||||
| Yes | D | −0.30 | −0.76, 0.16 | −0.52 | −1.03, 0.00 | C vs. D | 0.255 | −0.176 | −0.478, | 0.127 | 0.139 | |
| Block Design | No | C | −0.47 | −0.78, −0.16 | −0.40 | −0.70, −0.10 | ||||||
| Yes | D | −0.16 | −0.53, 0.22 | −0.06 | −0.40, 0.27 | C vs. D | 0.003 | 0.325 | 0.110, | 0.541 | 0.305 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, T.P.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.L.; Yap, K.B. Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study). Nutrients 2022, 14, 1189. https://doi.org/10.3390/nu14061189
Ng TP, Nyunt MSZ, Gao Q, Gwee X, Chua DQL, Yap KB. Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study). Nutrients. 2022; 14(6):1189. https://doi.org/10.3390/nu14061189
Chicago/Turabian StyleNg, Tze Pin, Ma Shwe Zin Nyunt, Qi Gao, Xinyi Gwee, Denise Qian Ling Chua, and Keng Bee Yap. 2022. "Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study)" Nutrients 14, no. 6: 1189. https://doi.org/10.3390/nu14061189
APA StyleNg, T. P., Nyunt, M. S. Z., Gao, Q., Gwee, X., Chua, D. Q. L., & Yap, K. B. (2022). Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study). Nutrients, 14(6), 1189. https://doi.org/10.3390/nu14061189






