Impact of Gut Microbiota on the Association between Diet and Depressive Symptoms in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Sample Size
2.3. Depressive Symptoms Assessment
2.4. Anxiety Symptoms Assessment
2.5. Dietary Intake Assessment and the Calculation of CHEI Component Scores
2.6. Plasma TRP/LNAA Ratio Determination
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Nutrient Intakes, CHEI Component Scores and Plasma Amino Acid Contents of the Patients
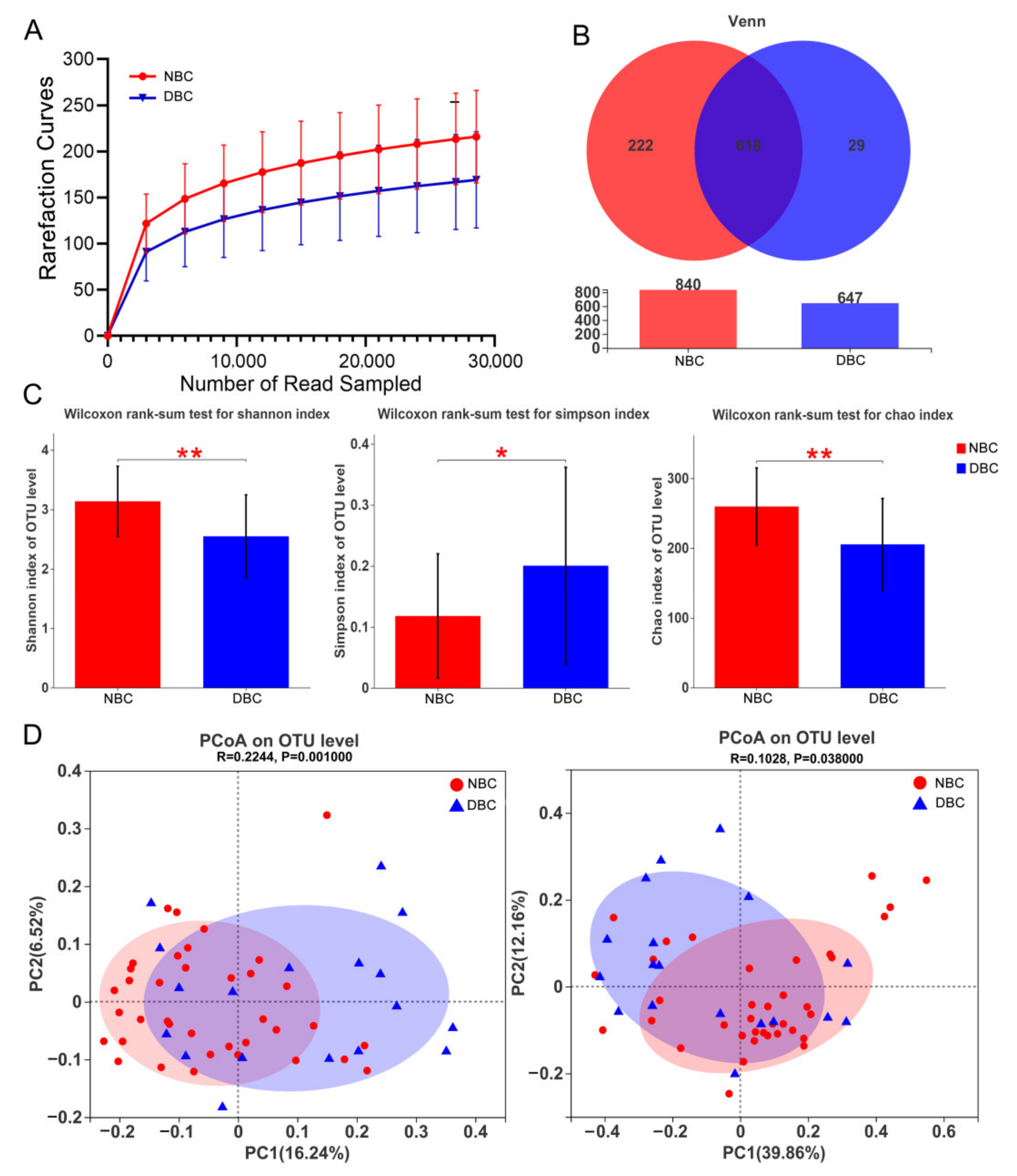
3.3. Gut Microbiota Diversity of the Patients
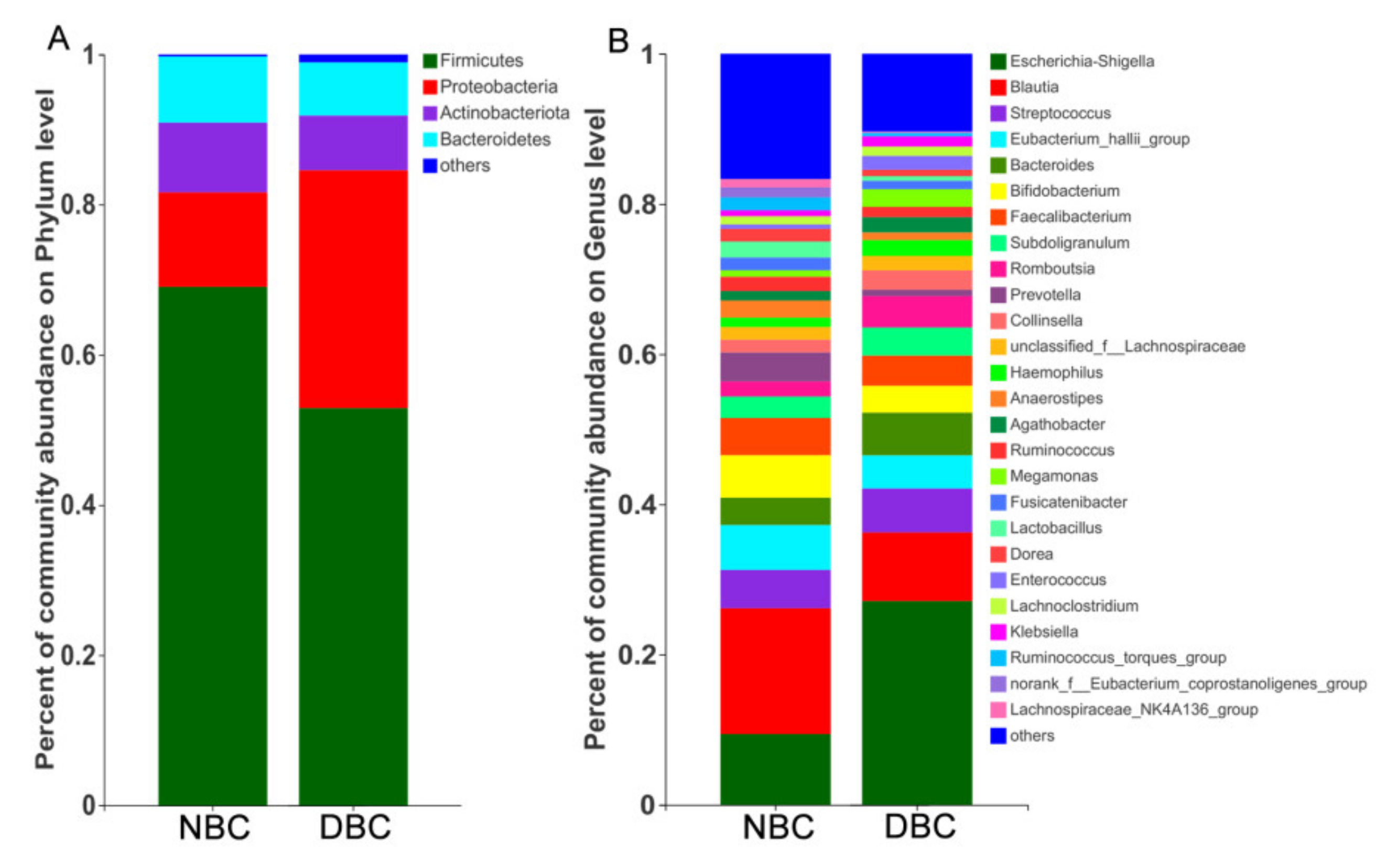
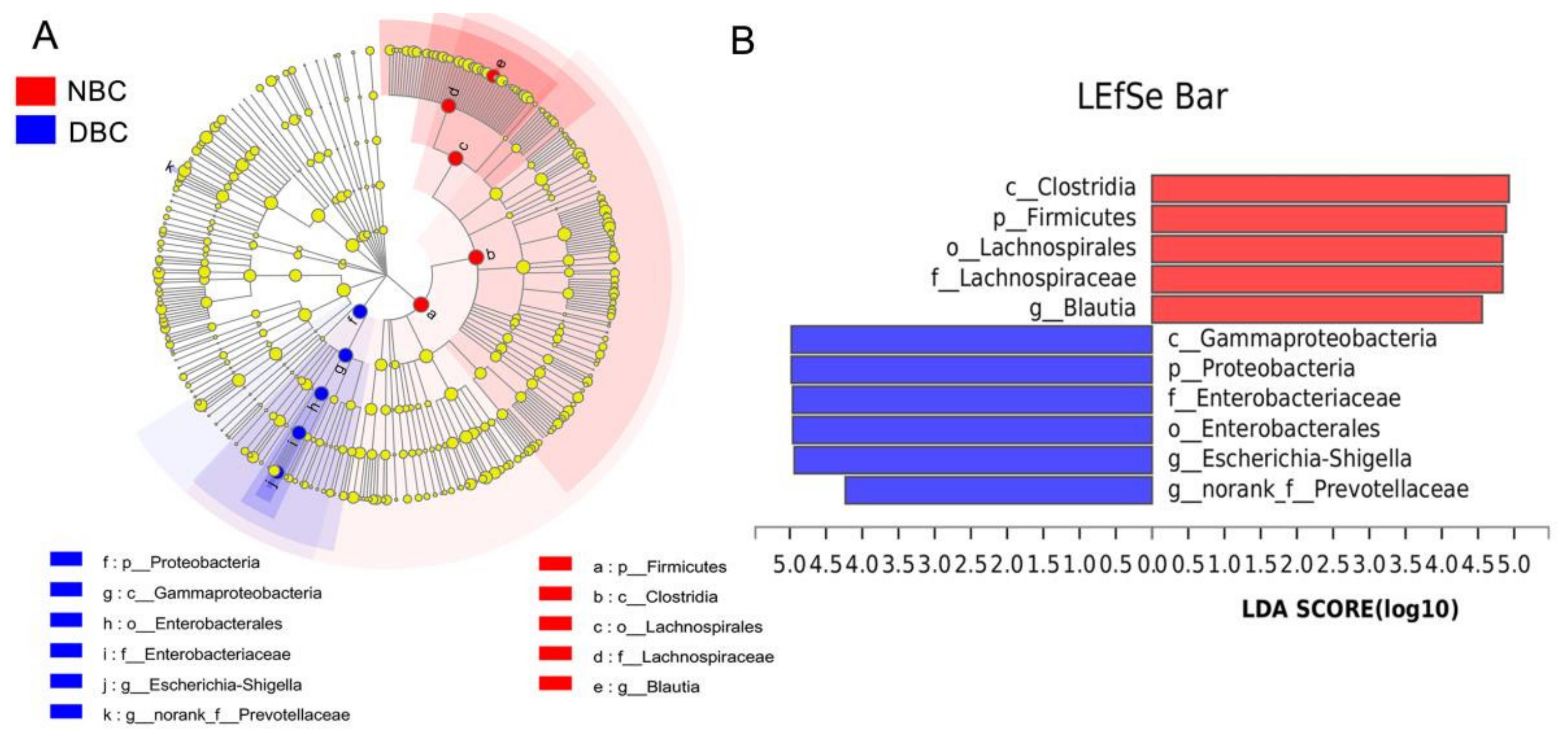
3.4. Gut Microbiota Composition of the Patients
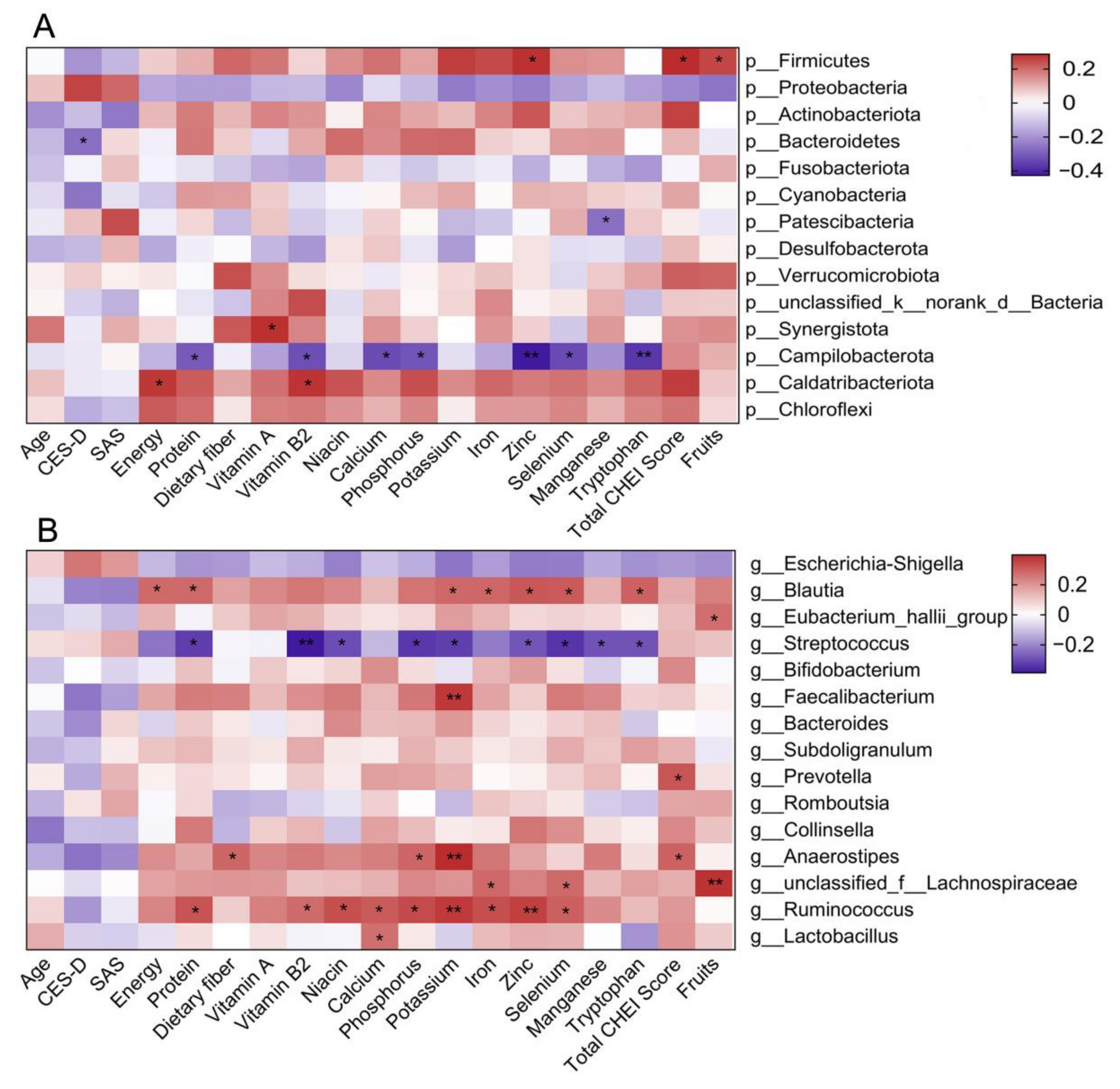
3.5. Associations of Gut Microbiota with CES-D Score and Dietary Intake
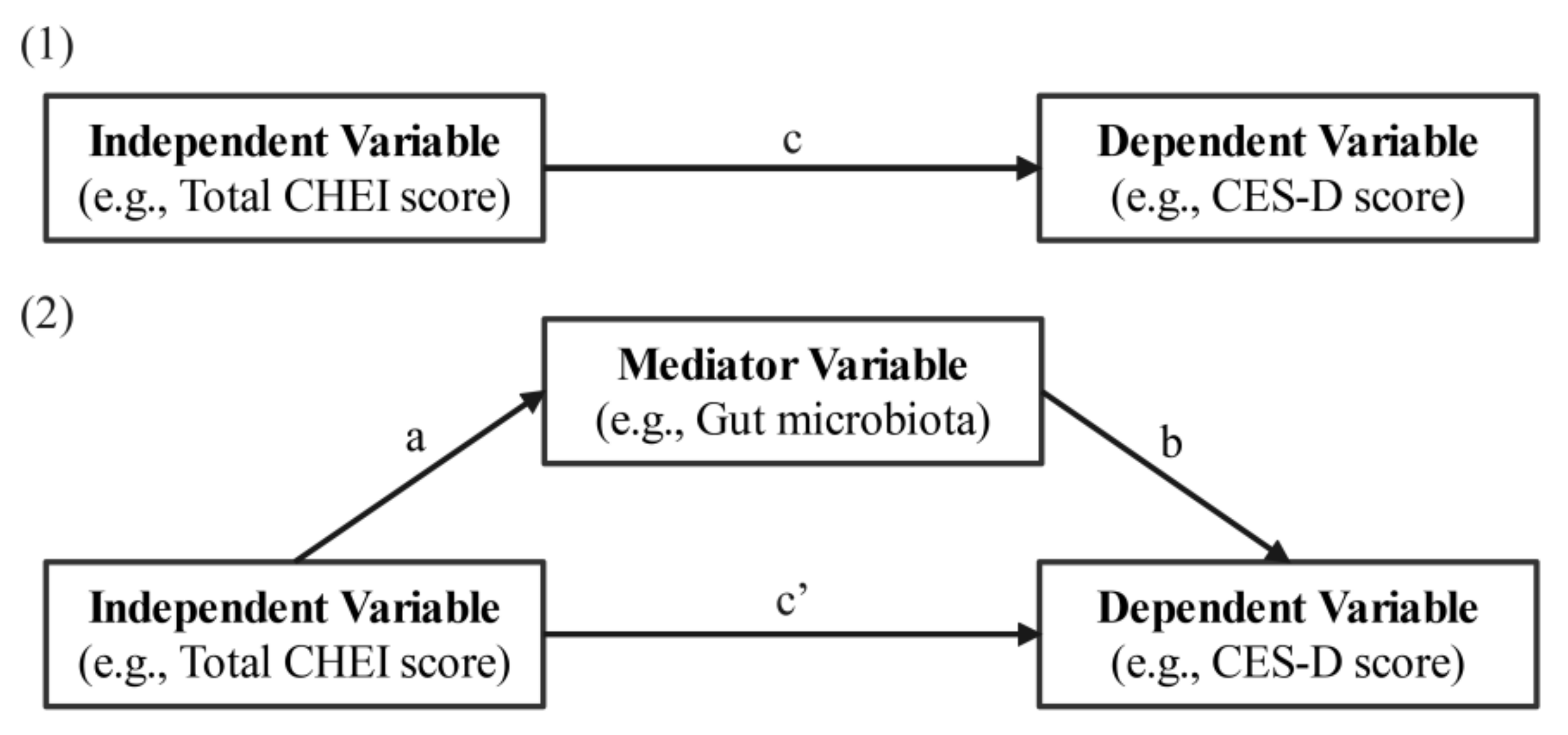
3.6. Associations between Diet and Depression with Gut Microbiota as a Mediator
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Behavioral symptoms in patients with breast cancer and survivors. J. Clin. Oncol. 2008, 26, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreira, H.; Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, 1311–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maass, S.W.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef]
- Scheier, M.F.; Helgeson, V.S.; Schulz, R.; Colvin, S.; Berga, S.; Bridges, M.W.; Knapp, J.; Gerszten, K.; Pappert, W.S. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J. Clin. Oncol. 2005, 23, 4298–4311. [Google Scholar] [CrossRef]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef] [Green Version]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional psychiatry: Towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Cebrino, J.; de la Cruz, S.P. Diet Quality and Sociodemographic, Lifestyle, and Health-Related Determinants among People with Depression in Spain: New Evidence from a Cross-Sectional Population-Based Study (2011–2017). Nutrients 2021, 13, 106. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Singh, G. Biological Mechanisms of Cancer-Induced Depression. Front. Psychiatry 2018, 9, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L. Tryptophan Intake in the US Adult Population Is Not Related to Liver or Kidney Function but Is Associated with Depression and Sleep Outcomes. J. Nutr. 2016, 146, 2609s–2615s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, E.L. Tryptophan supplementation and serotonin function: Genetic variations in behavioural effects. Proc. Nutr. Soc. 2018, 77, 174–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; He, J.; Yi, J.; Wang, Y.; Zhang, J.; Zhu, X. Emotional suppression and depressive symptoms in women newly diagnosed with early breast cancer. BMC Womens Health 2015, 15, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radloff, L.S. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Ying, Y.W. Depressive symptomatology among Chinese-Americans as measured by the CES-D. J. Clin. Psychol. 1988, 44, 739–746. [Google Scholar] [CrossRef]
- Rankin, S.H.; Galbraith, M.E.; Johnson, S. Reliability and validity data for a Chinese translation of the Center for Epidemiological Studies-Depression. Psychol. Rep. 1993, 73, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Wang, Z. Chinese version of Zung’s self-rating anxiety scale. J. Shanghai Psychiatry 1984, 2, 73–74. [Google Scholar]
- Yuan, Y.Q.; Li, F.; Wu, H.; Wang, Y.C.; Chen, J.S.; He, G.S.; Li, S.G.; Chen, B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients 2018, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Hutter, N.; Vogel, B.; Alexander, T.; Baumeister, H.; Helmes, A.; Bengel, J. Are depression and anxiety determinants or indicators of quality of life in breast cancer patients? Psychology Health Med. 2013, 18, 412–419. [Google Scholar] [CrossRef]
- Milbury, K.; Kavanagh, A.; Meng, Z.; Chen, Z.; Chandwani, K.D.; Garcia, K.; Perkins, G.H.; McQuade, J.; Raghuram, N.V.; Nagarathna, R.; et al. Depressive symptoms and positive affect in Chinese and United States breast cancer survivors: A cross-cultural comparison. Support Care Cancer 2017, 25, 2103–2109. [Google Scholar] [CrossRef] [Green Version]
- Lan, B.; Jiang, S.; Li, T.; Sun, X.; Ma, F. Depression, anxiety, and their associated factors among Chinese early breast cancer in women under 35 years of age: A cross sectional study. Curr. Probl. Cancer 2020, 44, 100558. [Google Scholar] [CrossRef]
- Dumas, A.; Vaz Luis, I.; Bovagnet, T.; El Mouhebb, M.; Di Meglio, A.; Pinto, S.; Charles, C.; Dauchy, S.; Delaloge, S.; Arveux, P.; et al. Impact of Breast Cancer Treatment on Employment: Results of a Multicenter Prospective Cohort Study (CANTO). J. Clin. Oncol. 2020, 38, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.Y.; Skuli, S.J.; Thorner, E.D.; Zafman, N.; Riley, C.D.; Ruck, J.M.; Smith, K.C.; Snyder, C.; Smith, K.L.; Stearns, V.; et al. Late effects in a high-risk population of breast cancer survivors. Support Care Cancer 2022, 30, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2019, 24, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The Role of Nutrients in Protecting Mitochondrial Function and Neurotransmitter Signaling: Implications for the Treatment of Depression, PTSD, and Suicidal Behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [Green Version]
- Marozoff, S.; Veugelers, P.J.; Dabravolskaj, J.; Eurich, D.T.; Ye, M.; Maximova, K. Diet Quality and Health Service Utilization for Depression: A Prospective Investigation of Adults in Alberta’s Tomorrow Project. Nutrients 2020, 12, 2437. [Google Scholar] [CrossRef]
- Meller, F.d.O.; Manosso, L.M.; Schäfer, A.A. The influence of diet quality on depression among adults and elderly: A population-based study. J. Affect. Disord. 2021, 282, 1076–1081. [Google Scholar] [CrossRef]
- Taylor, A.; Thompson, S.; Edwards, C.; Musaad, S.; Khan, N.; Holscher, H. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2019, 23, 983–992. [Google Scholar] [CrossRef]
- Tangney, C.C.; Young, J.A.; Murtaugh, M.A.; Cobleigh, M.A.; Oleske, D.M. Self-reported dietary habits, overall dietary quality and symptomatology of breast cancer survivors: A cross-sectional examination. Breast Cancer Res. Treat. 2002, 71, 113–123. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Koochakpoor, G.; Salari-Moghaddam, A.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Dietary intake of branched-chain amino acids in relation to depression, anxiety and psychological distress. Nutr. J. 2021, 20, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020, 17, 288–297. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. Dysbiosis in the inflamed intestine: Chance favors the prepared microbe. Gut Microbes 2014, 5, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Teng, N.M.Y.; Price, C.A.; McKee, A.M.; Hall, L.J.; Robinson, S.D. Exploring the impact of gut microbiota and diet on breast cancer risk and progression. Int. J. Cancer 2021, 149, 494–504. [Google Scholar] [CrossRef] [PubMed]
| NBC (n = 145) | DBC (n = 60) | t/Z | p-Value | |
|---|---|---|---|---|
| Nutrients | ||||
| Energy (kcal/d) 1 | 1539.29 ± 428.12 | 1403.40 ± 273.64 | 2.569 | 0.011 |
| Protein (g/d) 1 | 75.24 ± 28.78 | 65.45 ± 16.64 | 2.891 | 0.004 |
| Fat (g/d) 1 | 57.78 ± 23.83 | 54.21 ± 17.43 | 0.982 | 0.327 |
| Carbohydrate (g/d) 1 | 174.75 ± 63.53 | 165.27 ± 59.86 | 0.929 | 0.354 |
| Dietary fiber (g/d) 1 | 12.52 ± 6.01 | 10.38 ± 6.26 | 2.162 | 0.032 |
| Cholesterol (g/d) 2 | 617.89 ± 348.54 | 541.54 ± 282.61 | −0.912 | 0.362 |
| Vitamin A (μgRAE/d) 2 | 530.78 ± 290.36 | 378.49 ± 209.22 | −3.340 | 0.001 |
| Vitamin D (μg/d) 2 | 11.92 ± 25.71 | 9.38 ± 19.92 | −0.935 | 0.350 |
| Vitamin E (mg/d) 1 | 19.73 ± 11.53 | 17.06 ± 8.00 | 1.792 | 0.075 |
| Vitamin B1 (mg/d) 1 | 0.88 ± 0.39 | 0.77 ± 0.34 | 1.855 | 0.065 |
| Vitamin B2 (mg/d) 2 | 1.26 ± 0.66 | 0.99 ± 0.46 | −3.060 | 0.002 |
| Vitamin B6 (mg/d) 2 | 0.24 ± 0.20 | 0.20 ± 0.13 | −0.408 | 0.683 |
| Vitamin C (mg/d) 2 | 132.56 ± 85.26 | 119.36 ± 86.31 | −1.221 | 0.222 |
| Folate (μg/d) 1 | 154.05 ± 82.75 | 133.80 ± 78.43 | 1.479 | 0.141 |
| Niacin (mg/d) 2 | 15.00 ± 6.22 | 12.31 ± 4.20 | −2.756 | 0.006 |
| Calcium (mg/d) 1 | 621.73 ± 283.03 | 531.73 ± 267.13 | 1.978 | 0.049 |
| Phosphorus (mg/d) 1 | 1023.22 ± 321.55 | 877.03 ± 249.03 | 3.299 | 0.001 |
| Potassium (mg/d) 1 | 2164.18 ± 763.69 | 1842.64 ± 760.09 | 2.583 | 0.011 |
| Sodium (mg/d) 1 | 2692.12 ± 463.39 | 2699.23 ± 517.62 | −0.090 | 0.928 |
| Magnesium (mg/d) 2 | 301.73 ± 121.62 | 260.12 ± 104.34 | −1.850 | 0.064 |
| Iron (mg/d) 2 | 19.38 ± 8.23 | 15.91 ± 5.31 | −2.598 | 0.009 |
| Iodine (μg/d) 2 | 23.65 ± 15.83 | 19.71 ± 14.46 | −1.724 | 0.085 |
| Zinc (mg/d) 1 | 10.39 ± 4.17 | 8.83 ± 3.05 | 2.810 | 0.006 |
| Selenium (μg/d) 1 | 55.43 ± 23.85 | 45.77 ± 20.41 | 2.557 | 0.011 |
| Copper (mg/d) 2 | 2.08 ± 1.67 | 1.85 ± 1.59 | −0.986 | 0.324 |
| Manganese (mg/d) 2 | 3.98 ± 1.98 | 3.23 ± 1.30 | 3.007 | 0.003 |
| Choline (mg/d) 2 | 30.31 ± 22.62 | 35.57 ± 29.12 | −0.730 | 0.465 |
| Biotin (μg/d) 2 | 5.11 ± 5.02 | 5.22 ± 4.77 | −0.062 | 0.951 |
| Tryptophan (mg/d) 1 | 659.99 ± 201.87 | 579.41 ± 156.87 | 2.501 | 0.013 |
| SFA (g/d) 1 | 13.28 ± 6.47 | 11.89 ± 5.22 | 1.345 | 0.180 |
| MUFA (g/d) 1 | 16.53 ± 7.50 | 15.15 ± 5.71 | 1.310 | 0.193 |
| PUFA (g/d) 1 | 10.96 ± 4.88 | 10.07 ± 4.17 | 1.109 | 0.269 |
| CHEI component scores | ||||
| Total CHEI score 1 | 67.40 ± 6.98 | 64.80 ± 7.26 | 2.195 | 0.029 |
| Total grains 2 | 3.69 ± 1.23 | 3.58 ± 1.25 | −0.583 | 0.560 |
| Whole grains and mixed beans 2 | 1.49 ± 1.76 | 1.38 ± 1.45 | −0.797 | 0.425 |
| Tubers 2 | 1.74 ± 2.18 | 1.57 ± 1.87 | −0.126 | 0.900 |
| Total vegetables 2 | 3.60 ± 1.49 | 3.58 ± 1.24 | −0.420 | 0.675 |
| Dark vegetables 2 | 3.93 ± 1.46 | 3.97 ± 1.41 | −0.186 | 0.853 |
| Fruits 2 | 9.43 ± 1.82 | 8.46 ± 2.80 | −2.275 | 0.023 |
| Eggs 2 | 3.92 ± 1.66 | 3.63 ± 1.99 | −0.601 | 0.548 |
| Soybeans 2 | 1.41 ± 2.15 | 1.63 ± 2.22 | −0.780 | 0.435 |
| Dairy 2 | 2.88 ± 2.20 | 2.67 ± 2.07 | −0.740 | 0.459 |
| Seed and nuts 2 | 1.53 ± 2.06 | 1.32 ± 2.12 | −1.096 | 0.273 |
| Fish and seafood 2 | 3.19 ± 2.18 | 3.43 ± 2.13 | −0.869 | 0.385 |
| Poultry 2 | 1.26 ± 2.17 | 1.08 ± 2.05 | −0.481 | 0.631 |
| Red meat 2 | 3.12 ± 1.81 | 2.84 ± 1.98 | −0.691 | 0.490 |
| Added sugars 2 | 4.81 ± 0.82 | 4.87 ± 0.73 | −0.266 | 0.790 |
| Cooking oils 2 | 9.86 ± 0.51 | 9.73 ± 0.81 | −1.059 | 0.289 |
| Alcohol 2 | 5.00 ± 0.00 | 5.00 ± 0.00 | 0.000 | 1.000 |
| Sodium 2 | 6.53 ± 2.10 | 6.03 ± 1.78 | −1.863 | 0.062 |
| Variables | r | p-Value | Variables | r | p-Value |
|---|---|---|---|---|---|
| Energy 1 | −0.219 | 0.108 | Potassium 1 | −0.461 | <0.001 |
| Protein 1 | −0.347 | 0.009 | Iron 1 | −0.349 | 0.009 |
| Dietary fiber 1 | −0.331 | 0.014 | Zinc 1 | −0.371 | 0.005 |
| Vitamin A 1 | −0.274 | 0.043 | Selenium 1 | −0.389 | 0.003 |
| Vitamin B2 2 | −0.276 | 0.042 | Manganese 2 | −0.292 | 0.031 |
| Niacin 1 | −0.321 | 0.017 | Tryptophan 1 | −0.233 | 0.091 |
| Calcium 1 | −0.353 | 0.008 | Total CHEI score 1 | −0.402 | 0.002 |
| Phosphorus 1 | −0.447 | 0.001 | Fruits 2 | −0.002 | 0.991 |
| Variables | CES-D Score | |||
|---|---|---|---|---|
| R2 | β | 95%CI | p-Value | |
| Chao index | 0.721 | −0.348 | −0.061, −0.021 | <0.001 |
| Shannon index | 0.710 | −0.334 | −5.619, −1.709 | <0.001 |
| Simpson index | 0.678 | 0.260 | 4.610, 25.499 | 0.006 |
| PC1 | 0.639 | −0.175 | −10.898, 1.020 | 0.102 |
| Bacteroidetes | 0.637 | −0.153 | −0.198, 0.024 | 0.121 |
| Variables | Total Effect (c) | Direct Effect (c’) | Indirect Effect (ab) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variable and Mediators | β | SE | CI | β | SE | CI | β | SE | CI |
| Total CHEI score | −0.228 | 0.102 | −0.433, −0.023 | ||||||
| via Chao | −0.109 | 0.098 | −0.306, 0.088 | −0.119 | 0.063 | −0.249, −0.004 | |||
| via Shannon | −0.121 | 0.099 | −0.320, 0.079 | −0.108 | 0.061 | −0.230, 0.004 | |||
| via Simpson | −0.139 | 0.106 | −0.352, 0.074 | −0.090 | 0.048 | −0.200, −0.008 | |||
| Calcium | −0.006 | 0.003 | −0.011, −0.0004 | ||||||
| via Chao | −0.003 | 0.002 | −0.008, 0.002 | −0.003 | 0.002 | −0.006, 0.0001 | |||
| via Shannon | −0.003 | 0.003 | −0.008, 0.002 | −0.002 | 0.002 | −0.006, 0.0001 | |||
| Phosphorus | −0.006 | 0.002 | −0.011, −0.0007 | ||||||
| via Chao | −0.003 | 0.002 | −0.008, 0.001 | −0.002 | 0.002 | −0.006, 0.0002 | |||
| Selenium | −0.086 | 0.031 | −0.149, −0.023 | ||||||
| via Shannon | −0.055 | 0.031 | −0.116, 0.007 | −0.031 | 0.017 | −0.063, 0.003 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitiniyazi, G.; Cao, X.; Chen, Y.; Zhang, R.; Liu, Y.; Li, Z.; Gu, D.; Li, T.; Xia, S. Impact of Gut Microbiota on the Association between Diet and Depressive Symptoms in Breast Cancer. Nutrients 2022, 14, 1186. https://doi.org/10.3390/nu14061186
Maitiniyazi G, Cao X, Chen Y, Zhang R, Liu Y, Li Z, Gu D, Li T, Xia S. Impact of Gut Microbiota on the Association between Diet and Depressive Symptoms in Breast Cancer. Nutrients. 2022; 14(6):1186. https://doi.org/10.3390/nu14061186
Chicago/Turabian StyleMaitiniyazi, Gusonghan, Xiaoyun Cao, Yue Chen, Rong Zhang, Yuan Liu, Ziyuan Li, Danfeng Gu, Tong Li, and Shufang Xia. 2022. "Impact of Gut Microbiota on the Association between Diet and Depressive Symptoms in Breast Cancer" Nutrients 14, no. 6: 1186. https://doi.org/10.3390/nu14061186
APA StyleMaitiniyazi, G., Cao, X., Chen, Y., Zhang, R., Liu, Y., Li, Z., Gu, D., Li, T., & Xia, S. (2022). Impact of Gut Microbiota on the Association between Diet and Depressive Symptoms in Breast Cancer. Nutrients, 14(6), 1186. https://doi.org/10.3390/nu14061186






