Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Participant: P
2.2.2. Intervention: I
2.2.3. Comparison: C
2.2.4. Outcome: O
2.2.5. Study Design: S
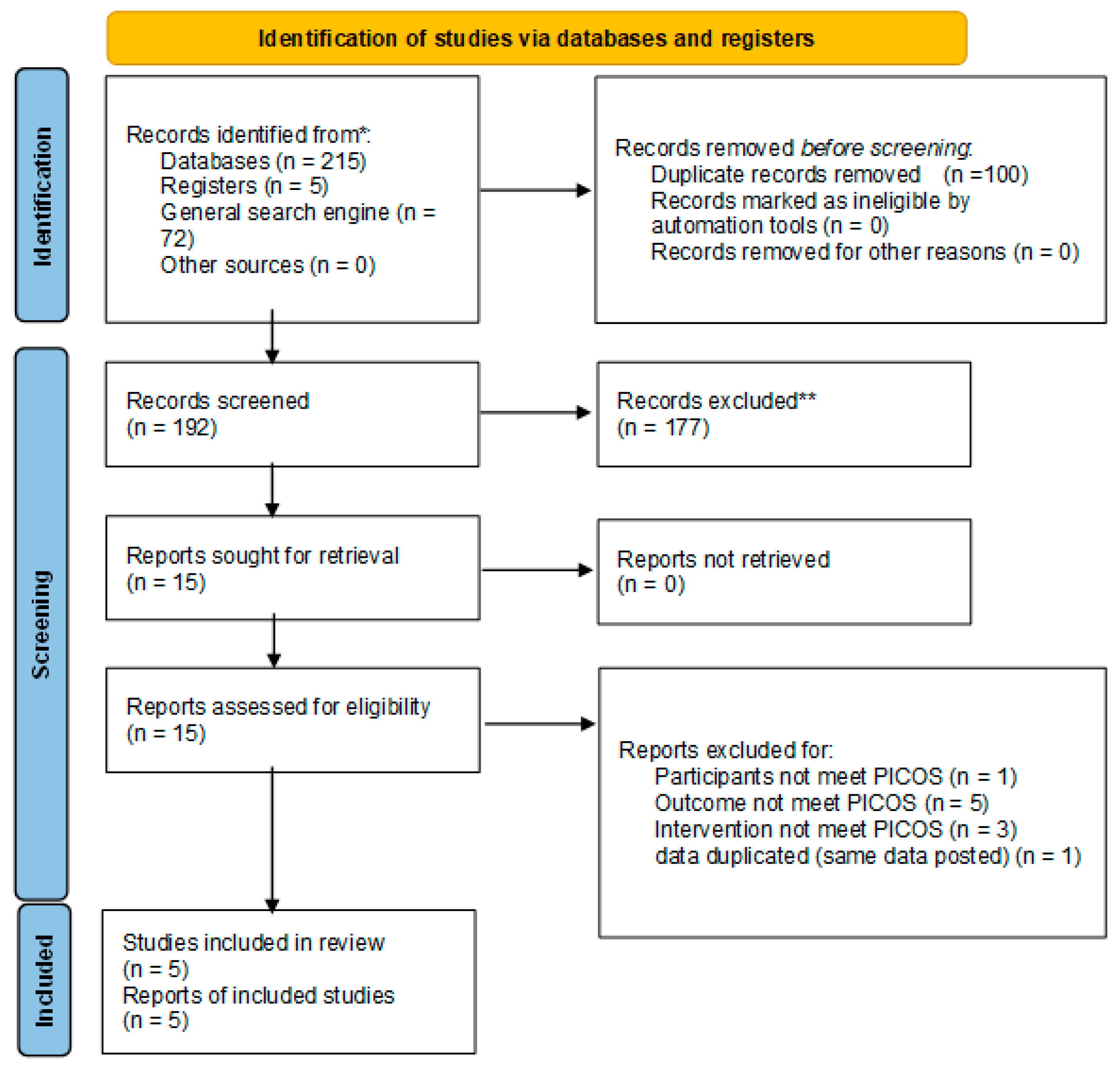
2.3. Method of Literature Search
2.3.1. Search Databases and Clinical Trial Registration Databases
2.3.2. Study Selection
- Reports on mixed results using other effective materials except PGPs or ginsenoside.
- The PICOS criteria is not met.
- The study is an in vitro or animal (except human) in vivo test.
2.4. Data Collection and Meta-Analysis
2.4.1. Data Collection
2.4.2. Data Items
2.4.3. Data Synthesis and Meta-Analysis
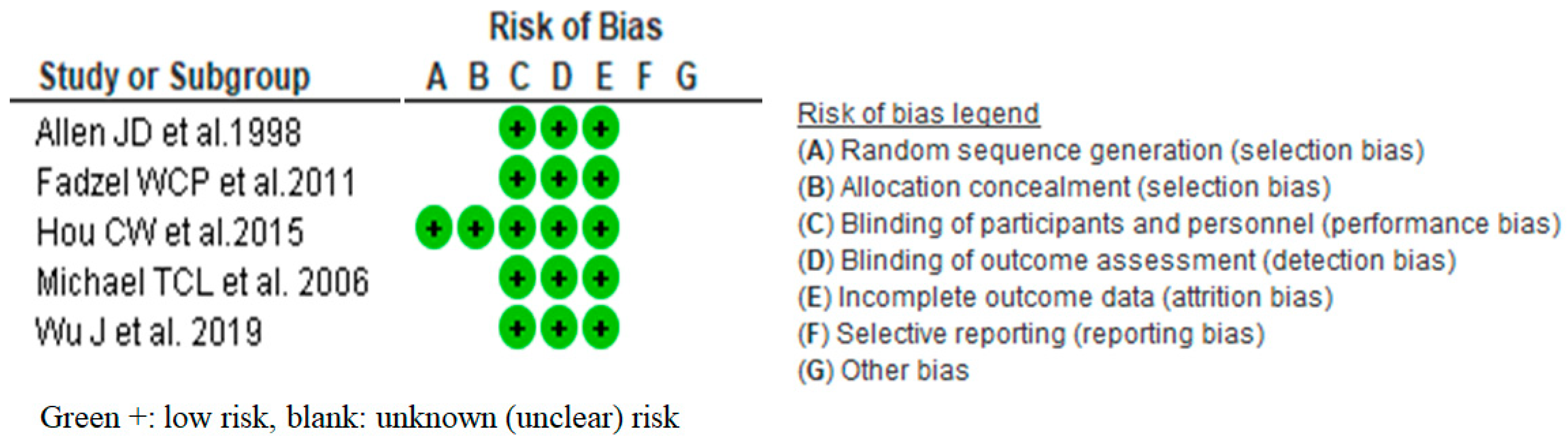
2.4.4. Risk of Bias of Individual Study
2.4.5. Risk of Bias of Across Studies
2.4.6. Additional Analysis
3. Results
3.1. Study Selection and Description of Included Studies
3.2. Risk of Bias of Included Studies
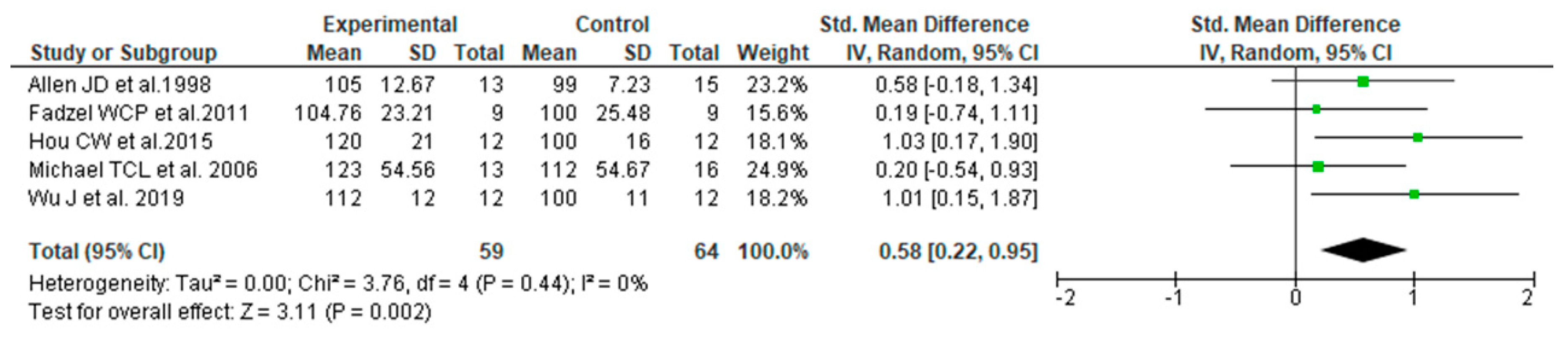
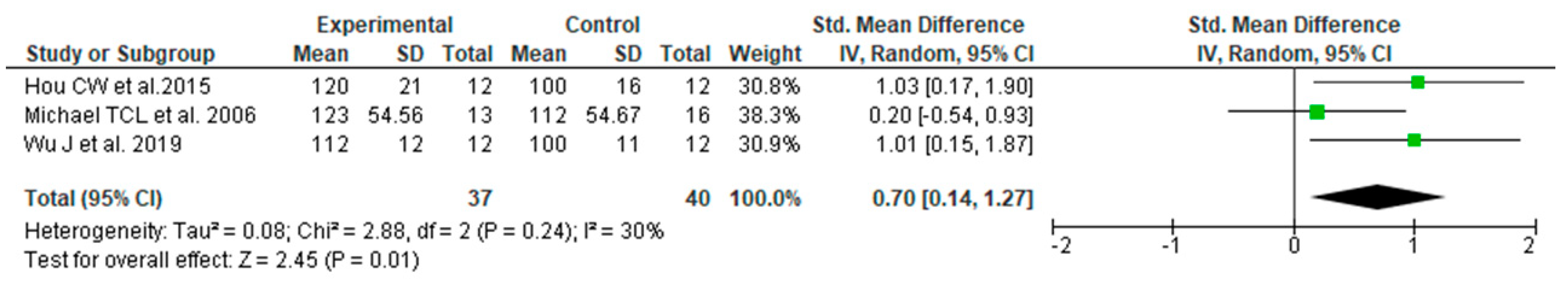
3.3. Data Synthesis Based on the Results of Literature Search and Meta-Analysis
3.4. Additional Analyses
3.4.1. Additional Analysis for Ginsenoside Contents
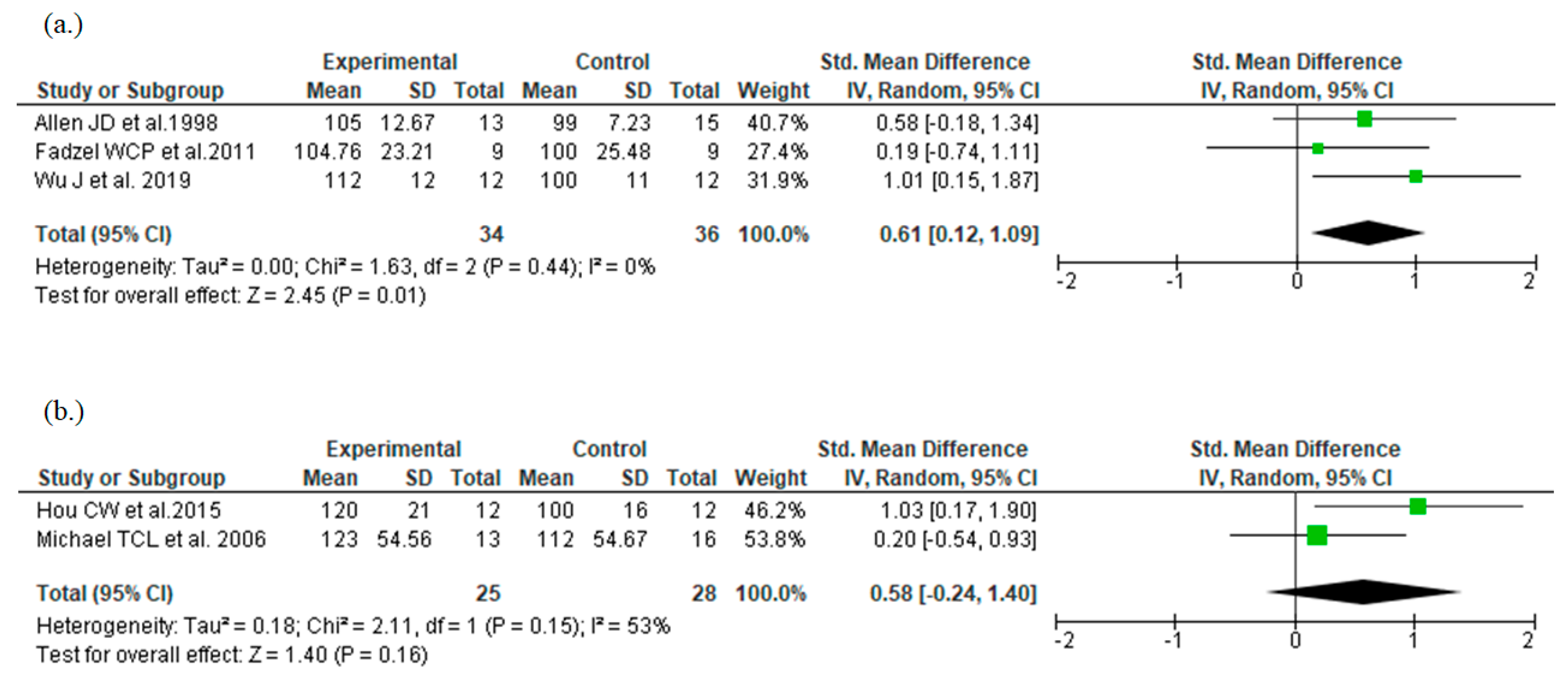
3.4.2. Additional Analyses for Study Duration
3.5. Quality of Evidence
4. Discussion
4.1. Ginseng, Ginsenosides, and Their Regulations
4.2. Effects of PGP Ingestion
4.3. Effects of Ginsenoside Rg1
4.4. More Verification of Items
4.4.1. Study Design
4.4.2. Ginsenosides Other Than Rg1
4.5. Limitations of This Study
4.6. Safety Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGP | Panax genus plant |
| SMD | standardized mean difference |
| PICOS | participant, intervention, comparison, and outcomes |
| SD | standard deviation |
| CI | confidence interval |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| RCT | randomized controlled trial |
| RevMan 5 | Review Manager 5.3 |
| GRADE | grading of recommendations assessment, development and evaluations |
| VO2max | maximal oxygen uptake |
| IL | interleukin |
| TNFα | tumor necrosis factor α |
| mRNA | messenger ribonucleic acid |
| PGC-1 | peroxisome proliferator-activated receptor gamma coactivator-1 |
| IKs channel | intermediate-conductance calcium-activated potassium channel |
| eNOS | endothelial nitric oxide synthase |
| AMSTER | a measurement tool to assess systematic reviews |
| PI3 kinase/Akt | Phosphoinositide 3-kinase/RAC-beta serine/threonine-protein kinase |
References
- Eliason, B.C.; Kruger, J.; Mark, D.; Rasmann, D.N. Dietary supplement users: Demographics, product use and medical system interaction. J. Am. Board Fam. Pract. 1997, 10, 265–271. [Google Scholar] [PubMed]
- Harnack, L.J.; Rydell, S.A.; Stang, J. Prevalence of use of herbal products by adults in the Minneapolis/St Paul, Minn, Metropolitan area. Mayo Clin. Proc. 2001, 76, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.-H.; So, S.-H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.V.; Kim, J.; Myung, S.K.; Cho, Y.A. Efficacy of Ginseng Supplements on Fatigue and Physical Performance: A Meta-analysis. J. Korean Med. Sci. 2016, 31, 1879–1886. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions—Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://handbook-5-1.cochrane.org/ (accessed on 2 August 2021).
- Julian, P.T.H.; Sally, G.; Cochrane Handbook for Systematic Reviews of Interventions. Chapter 12: Interpreting Results and Drawing Conclusions. Available online: https://handbook-5-1.cochrane.org/chapter_12/12_interpreting_results_and_drawing_conclusions.htm/ (accessed on 2 August 2021).
- Hou, C.-W.; Lee, S.-D.; Kao, C.-L.; Cheng, I.-S.; Lin, Y.-N.; Chuang, S.-J.; Chen, C.-Y.; Ivy, J.L.; Kuo, C.-H. Improved inflammatory balance of human skeletal muscle during exercise after supplementations of the ginseng-based steroid Rg1. PLoS ONE 2015, 10, e0116387. [Google Scholar] [CrossRef]
- Wu, J.; Saovieng, S.; Cheng, I.-S.; Liu, T.; Hong, S.; Lin, C.-Y.; Su, I.-C.; Huang, C.-Y.; Kuo, C.-H. Ginsenoside Rg1 supplementation clears senescence-associated β-galactosidase in exercising human skeletal muscle. J. Ginseng Res. 2019, 43, 580–588. [Google Scholar] [CrossRef]
- Fadzel, W.C.P.; Chen, C.K.; Amit, B. Effects of acute supplementation of Panax ginseng on endurance running in a hot & humid environment. Indian J. Med. Res. 2011, 133, 96–102. [Google Scholar]
- Liang, M.T.; Podolka, T.D.; Chuang, W.J. Panax notoginseng supplementation enhances physical performance during endurance exercise. J. Strength Cond. Res. 2005, 19, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; McLung, J.; Nelson, A.G.; Welsch, M. Ginseng supplementation does not enhance healthy young adults’ peak aerobic exercise performance. J. Am. Coll. Nutr. 1998, 17, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Angelova, N.; Kong, H.-W.; Van Der Heijden, R.; Yang, S.-Y.; Choi, Y.H.; Kim, H.K.; Wang, M.; Hankemeier, T.; Van Der Greef, J.; Xu, G.; et al. Recent methodology in the phytochemical analysis of ginseng. Phytochem. Anal. 2008, 19, 2–16. [Google Scholar] [CrossRef]
- Xiu, Y.; Li, X.; Sun, X.; Dan, X.; Rui, M.; Huanxi, Z.; Shuying, L. Simultaneous determination and difference evaluation of 14 ginsenosides in Panax ginseng roots cultivated in different areas and ages by high-performance liquid chromatography coupled with triple quadrupole mass spectrometer in the multiple reaction–monitoring mode combined with multivariate statistical analysis. J. Ginseng Res. 2019, 43, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Soldati, F.; Tanaka, O. Panax ginseng: Relation between age of plant and content of ginsenosides. Planta Med. 1984, 50, 351–352. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Li, J.; Zhang, H.; Ding, L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 664–668. [Google Scholar] [CrossRef]
- Li, X.G.; Yan, Y.Z.; Jin, X.J.; Yong, K.K.; Md, R.U.; Yeon, B.K.; Hanhong, B.; Young, C.K.; Sang, W.L.; Sang, U.P. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012, 9, 679–683. [Google Scholar] [CrossRef]
- Soldati, F.; Sticher, O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2nd communication. Planta Med. 1980, 39, 348–357. [Google Scholar] [CrossRef]
- Kang, O.J.; Kim, J.S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng C. A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Zhao, L.; Zhang, Z.H.; Tang, Z.H. Profiling of ginsenosides in the two medicinal Panax herbs based on ultra-performance liquid chromatography-electrospray ionization-mass spectrometry. Springerplus 2016, 5, 1770. [Google Scholar] [CrossRef]
- Wu, W.; Sun, L.; Zhang, Z.; Guo, Y.; Liu, S. Profiling and multivariate statistical analysis of Panax ginseng based on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 141–150. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, S.H.; Lee, Y.S.; Doo, J.C.; Bo, R.C.; Geum, S.K.; Nam, I.B.; Dae, Y.L. Mass spectrometry based profiling and imaging of various ginsenosides from Panax ginseng roots at different ages. Int. J. Mol. Sci. 2017, 18, 1114. [Google Scholar] [CrossRef]
- Hu, C.; Wei, H.; Kong, H.; Bouwman, J.; Gonzalez-Covarrubias, V.; van der Heijden, R.; Reijmers, T.H.; Bao, X.; Verheij, E.R.; Hankemeier, T.; et al. Linking biological activity with herbal constituents by systems biology-based approaches: Effects of Panax ginseng in type 2 diabetic Goto-Kakizaki rats. Mol. Biosyst. 2011, 7, 3094–3103. [Google Scholar] [CrossRef]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Li, S.L.; Zhang, H.; Yun, W.; Zhi, L.Z.; Shi, L.C.; Hong, X.X. Holistic quality evaluation of commercial white and red ginseng using a UPLC QTOF-MS/MS-based metabolomics approach. J. Pharm. Biomed. Anal. 2012, 62, 258–273. [Google Scholar] [CrossRef]
- Meyer, A.; Shibata, S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001, 16, S28–S37. [Google Scholar]
- Kitagawa, I.; Taniyama, T.; Shibuya, H.; Noda, T.; Yoshikawa, M. Chemical studies on crude drug processing. V. On the constituents of ginseng radix rubra (2): Comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi 1987, 107, 495–505. (In Japanese) [Google Scholar] [CrossRef]
- Kitagawa, I.; Yoshikawa, M.; Yoshihara, T.; Hayashi, T.; Taniyama, T. Chemical studies of crude drugs (1). Constituents of Ginseng radix rubra. Yakugaku Zasshi 1983, 103, 612–622. (In Japanese) [Google Scholar] [CrossRef]
- Kite, G.C.; Howes, M.R.; Leon, C.J.; Simmonds, M.S.J. Liquid chromatography/mass spectrometry of malonyl-ginsenosides in the authentication of ginseng. Rapid Commun. Mass Spectrom. 2003, 17, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Seung, K.L.; Nak, D.K.; Man, K.P.; Chong, K.K.; Jeong, H.P. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-F.; Gao, Y.; Xu, S.-Y.; Liu, H.; Xue, X.; Zhang, Y.; Zhang, H.; Liu, M.-N.; Xiong, H.; Lin, R.-C.; et al. Remarkable impact of steam temperature on ginsenosides transformation from.m fresh ginseng to red ginseng. J. Ginseng Res. 2018, 42, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, J.Y.; Ko, S.K. Changes in the contents of prosapogenin in Red ginseng (Panax ginseng) depending on the extracting conditions. J. Ginseng Res. 2016, 40, 86–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, E.O.; Cha, K.H.; Lee, E.H.; Sang, M.K.; Sang, W.C.; Cheol, H.P.; Byung, H.U. Bioavailability of ginsenosides from white and red ginsengs in the simulated digestion model. J. Agric. Food Chem. 2014, 62, 10055–10063. [Google Scholar] [CrossRef]
- So, S.-H.; Lee, J.W.; Kim, Y.-S.; Hyun, S.H.; Han, C.-K. Red ginseng monograph. J. Ginseng Res. 2018, 42, 549–561. [Google Scholar] [CrossRef]
- ISO 19610:2017. Traditional Chinese Medicine—General Requirements for Industrial Manufacturing Process of Red Ginseng (Panax ginseng C.A. Meyer); ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Lee, J.W.; Ji, S.-H.; Choi, B.-R.; Choi, D.J.; Lee, Y.-G.; Kim, H.-G.; Kim, G.-S.; Kim, K.; Lee, Y.-H.; Baek, N.-I.; et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef]
- World Health Organization Geneva. Radix Ginseng. In WHO Monographs on Selected Medicinal Plants VOLUME 1; World Health Organization: Pregny-Chambésy, Switzerland, 1999; Volume 1, pp. 168–182. [Google Scholar]
- European Medicines Agency. Ginseng Radix European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/herbal/ginseng-radix (accessed on 2 August 2021).
- Howley, E.T. Type of activity: Resistance, aerobic and leisure versus occupational physical activity. Med. Sci Sports Exerc. 2001, 33 (Suppl. S6), S364–S369. [Google Scholar] [CrossRef]
- Kudo, K.; Tachikawa, E.; Kashimoto, T.; Takahashi, E. Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Eur. J. Pharmacol. 1998, 341, 139–144. [Google Scholar] [CrossRef]
- Haam, S.; Park, H. Six week swimming followed by acute uptakes of ginsenoside Rg1 may affect aerobic capacity of SD rats. J. Exerc. Nutr. Biochem. 2015, 19, 311–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Y.; Li, J.; Wang, J.; Li, X.; Li, J.; Chu, S.; Li, L.; Chen, N.; Zhang, L. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. Int. Immunopharmacol. 2020, 87, 106805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, W.; Zhang, B.; Yin, Y.; Zhang, J.; Huang, D.; Huang, R.; Li, W.; Li, W. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int. J. Mol. Med. 2017, 40, 1134–1142. [Google Scholar] [CrossRef]
- Yu, S.-H.; Huang, H.-Y.; Korivi, M.; Hsu, M.-F.; Huang, C.-Y.; Hou, C.-W.; Chen, C.-Y.; Kao, C.-L.; Lee, R.-P.; Lee, S.-D.; et al. Oral Rg1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J. Int. Soc. Sports Nutr. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Korivi, M.; Hou, C.-W.; Huang, C.-Y.; Lee, S.-D.; Hsu, M.-F.; Yu, S.-H.; Chen, C.-Y.; Liu, Y.-Y.; Kuo, C.-H. Ginsenoside-Rg1 Protects the Liver against Exhaustive Exercise-Induced Oxidative Stress in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 932165. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.-F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, H.; Cai, N.; Zhou, S.; Zhao, Y.; Chen, X.; Zheng, S.; Si, Q.; Zhang, W. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3β pathway. Mol. Med. Rep. 2016, 14, 2778–2784. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, M.; Zhao, H.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J. Antiobesity Effects of Ginsenoside Rg1 on 3T3-L1 Preadipocytes and High Fat Diet-Induced Obese Mice Mediated by AMPK. Nutrients 2018, 10, 830. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sekiai, S.; Hatakeyama, H.; Koide, M.; Chaweewannakorn, C.; Yaoita, F.; Tan-No, K.; Sasaki, K.; Watanabe, M.; Sugawara, S.; et al. Neutrophils Provide a Favorable IL-1-Mediated Immunometabolic Niche that Primes GLUT4 Translocation and Performance in Skeletal Muscles. Cell Rep. 2018, 23, 2354–2364. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Li, M.; Du, Y.; Duan, S.; Huang, Y.; Lu, Y.; Zhang, J.; Wang, T.; Fu, F. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget 2017, 8, 55384–55393. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Y.; Wang, Y.; Yu, C.-L.; Zhai, F.-G.; Guan, L.-X. Neuroprotective Effect of the Ginsenoside Rg1 on Cerebral Ischemic Injury In Vivo and In Vitro Is Mediated by PPARγ-Regulated Antioxidative and Anti-Inflammatory Pathways. Evid. Based Complement. Altern. Med. 2017, 2017, 7842082. [Google Scholar] [CrossRef]
- Li, J.-B.; Zhang, R.; Han, X.; Piao, C.-L. Ginsenoside Rg1 inhibits dietary-induced obesity and improves obesity-related glucose metabolic disorders. Braz. J. Med. Biol. Res. 2018, 51, e7139. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, Y.-J.; Song, J.-H.; Chei, S.; Lee, B.-Y. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J. Ginseng. Res. 2019, 43, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Nakata, R.; Inoue, H.; Shimizu, M.; Jun, I.; Sato, R. Role of AMPK and PPARγ1 in exercise-induced lipoprotein lipase in skeletal muscle. Am. J. Physiol. Metab. 2014, 306, E1085–E1092. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Chan, M.C.; Arany, Z. The many roles of PGC-1α in muscle—Recent developments. Metabolism 2014, 63, 441–451. [Google Scholar] [CrossRef]
- Furukawa, T.; Bai, C.-X.; Kaihara, A.; Ozaki, E.; Kawano, T.; Nakaya, Y.; Awais, M.; Sato, M.; Umezawa, Y.; Kurokawa, J. Ginsenoside Re, a Main Phytosterol of Panax ginseng, Activates Cardiac Potassium Channels via a Nongenomic Pathway of Sex Hormones. Mol. Pharmacol. 2006, 70, 1916–1924. [Google Scholar] [CrossRef]
- Bai, C.-X.; Kurokawa, J.; Tamagawa, M.; Nakaya, H.; Furukawa, T. Nontranscriptional Regulation of Cardiac Repolarization Currents by Testosterone. Circulation 2005, 112, 1701–1710. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, A.G.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
| Database | Search Formula | Last Search Date |
|---|---|---|
| PubMed | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) AND clinical trial | 15 March 2021 |
| PubMed [Mesh] | (ginsenoside): ‘ginsenosides’. (athletic): ‘athletes’, ‘sports’ (muscle): ‘muscles’ (exercise):’exercise therapy’. ‘exercised’. ‘exerciser’. ‘exercisers’. ‘exercising’ (ergogenic): ‘ergogenicity’.’performance-enhancing substances’.’performance-enhancing’.’performance-enhancing substances’. ‘ergogenics’ (clinical trial): ‘clinical trials as topic’ | 15 March 2021 |
| Cochrane library | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) | 15 March 2021 |
| AGRIS | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) AND clinical trial AND (English and Japanese) | 17 March 2021 |
| J-DreamIII (JMEDPlus, JSTPlus) | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) # in Japanese | 16 March 2021 |
| Ichu-shi Web | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) # in Japanese | 15 March 2021 |
| Web of science | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) | 15 March 2021 |
| Scopus | (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) | 15 March 2021 |
| Clinical trial database | search with mixed keywords below; (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) # in English and Japanese | 17 March 2021 |
| General search engine | search with mixed keywords below; (Panax OR ginseng OR ginsenoside) AND (athletic OR muscle OR exercise OR ergogenic aid) # in English and Japanese | 22 March 2021 |
| Study ID | Study Design | |||||||
|---|---|---|---|---|---|---|---|---|
| Crossover/Pararell | Duration | Sample Size | Ingestion Food | Ginsenoside Composition | Exercise | Exercise Intensity | Other Measurement(s) | |
| How C.W. et al., 2015 [12] | crossover | 2 times: night before trial and before trial | 12 male | 5 mg of ginsenoside Rg1 (from P. notoginseng) | clear (95% purity of ginsenoside Rg1) | cycle ergometer | 80% VO2MAX | citrate syntase activity, inflammatory markers |
| Wu J. et al., 2019 [13] | crossover | acute | 12 male | 5 mg of ginsenoside Rg1 (from P. ginseng) | clear (95% purity of ginsenoside Rg1) | cycle ergometer | 80% VO2MAX | leukocyte infiltration in skeletal muscle, inflammatory markers |
| Fadzel W.C.P. et al., 2011 [14] | crossover | acute | 9 male | 200 mg of P. ginseng root extract | unknown (not mentioned) | run treadmill | 70% VO2MAX | heart rate, VO2, core body/skin temperature, blood paramaters |
| Michael T.C.L.. et al., 2006 [15] | pararell | 30 days | EXP: 13 CON: 16 male/female | 1350 mg/day of P. notoginseng extract | partially clear (HPLC analysis of conducted, and some ginsenosides were quantified) | cycle ergometer | 65–70% VO2MAX at start, and added 30 W workload each 5 min | heart rate, VO2peak, skin blood flow |
| Allen J.D. et al., 1998 [16] | pararell | 21 days | EXP: 13 CON: 15 male/female | 200 mg/day of P. ginseng root extract | unknown (not mentioned) | cycle ergometer | Added 50 W workload each 2 min | heart rate, VO2peak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeuchi, S.; Minamida, M.; Nakamura, T.; Konishi, M.; Kamioka, H. Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance. Nutrients 2022, 14, 1185. https://doi.org/10.3390/nu14061185
Ikeuchi S, Minamida M, Nakamura T, Konishi M, Kamioka H. Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance. Nutrients. 2022; 14(6):1185. https://doi.org/10.3390/nu14061185
Chicago/Turabian StyleIkeuchi, Shingo, Mika Minamida, Touma Nakamura, Masatoshi Konishi, and Hiroharu Kamioka. 2022. "Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance" Nutrients 14, no. 6: 1185. https://doi.org/10.3390/nu14061185
APA StyleIkeuchi, S., Minamida, M., Nakamura, T., Konishi, M., & Kamioka, H. (2022). Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance. Nutrients, 14(6), 1185. https://doi.org/10.3390/nu14061185