Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Water Extract of C. tinctoria and Single Flavonoids Substance
2.2. Animals and Experimental Design
2.3. ITT
2.4. GTT
2.5. Biochemical Analysis
2.6. Hemoglobin A1c Analysis
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Components of the Extract of C. tinctoria
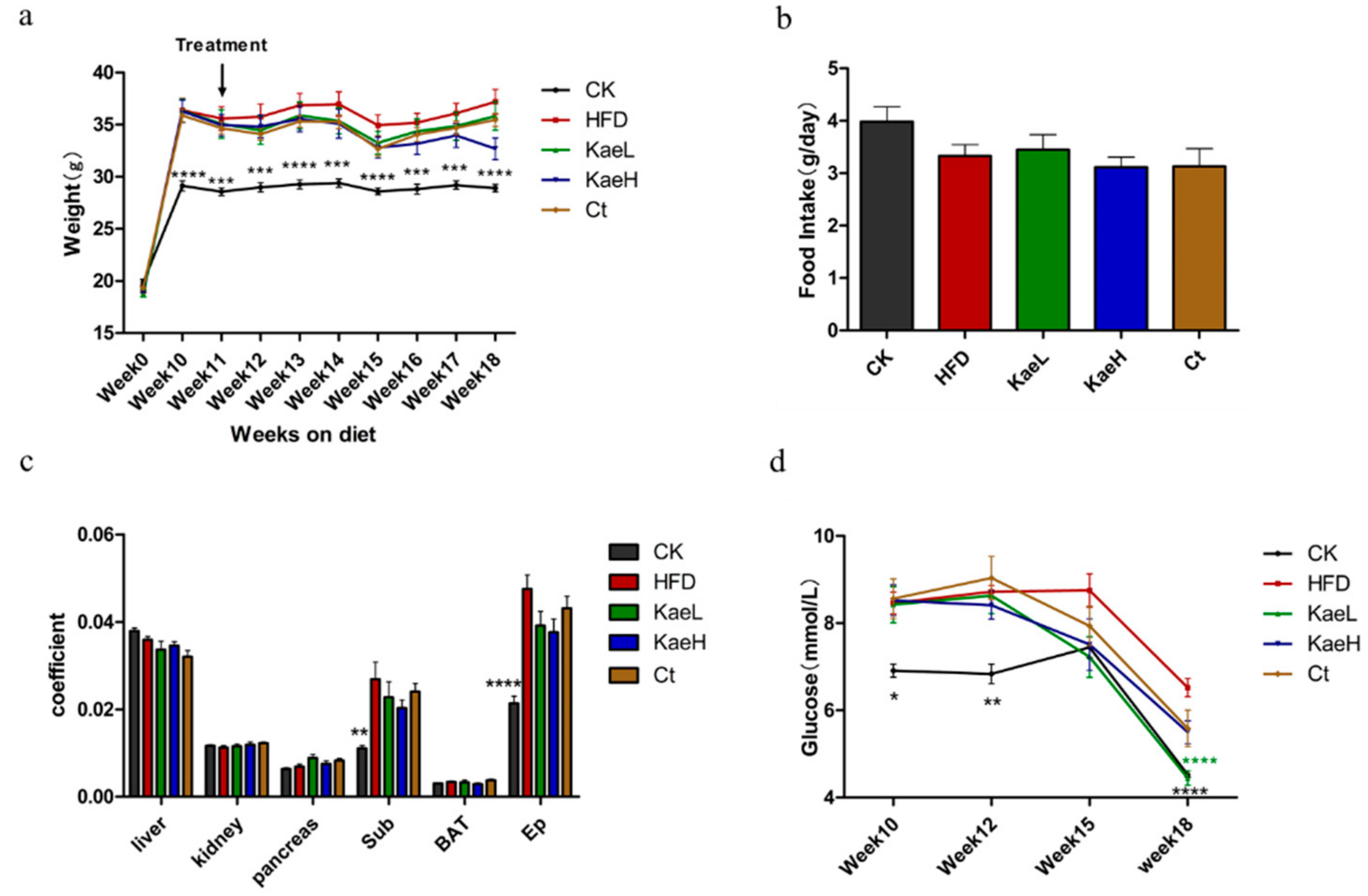
3.2. C. tinctoria and Kaempferol Reduced Body Weight and Improved Obesity in DIO Mice
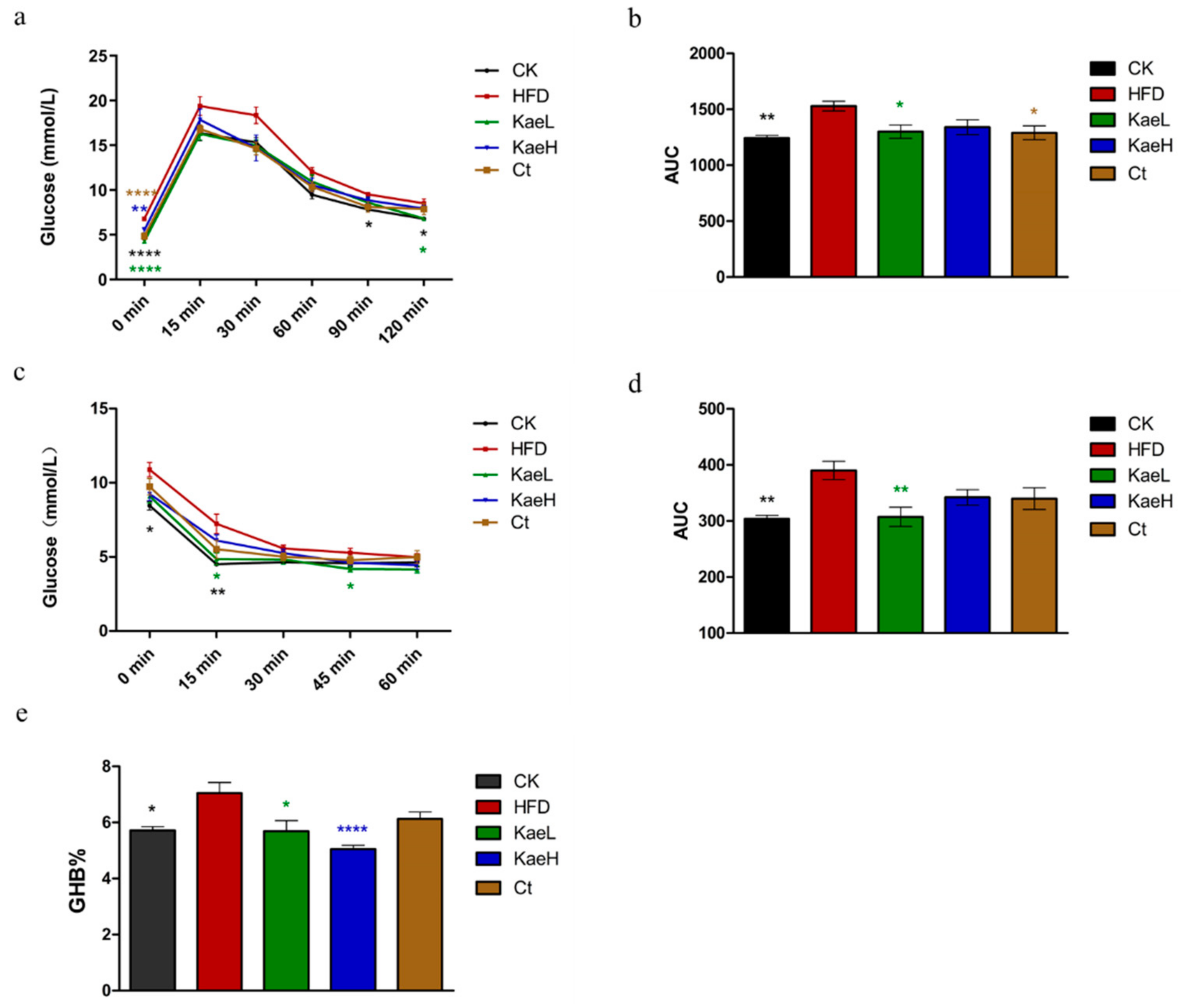
3.3. C. tinctoria and Kaempferol Ameliorated Glucose Metabolism in DIO Mice
3.4. C. tinctoria and Kaempferol Improve Obesity without Effect on the Liver and Kidney in DIO Mice
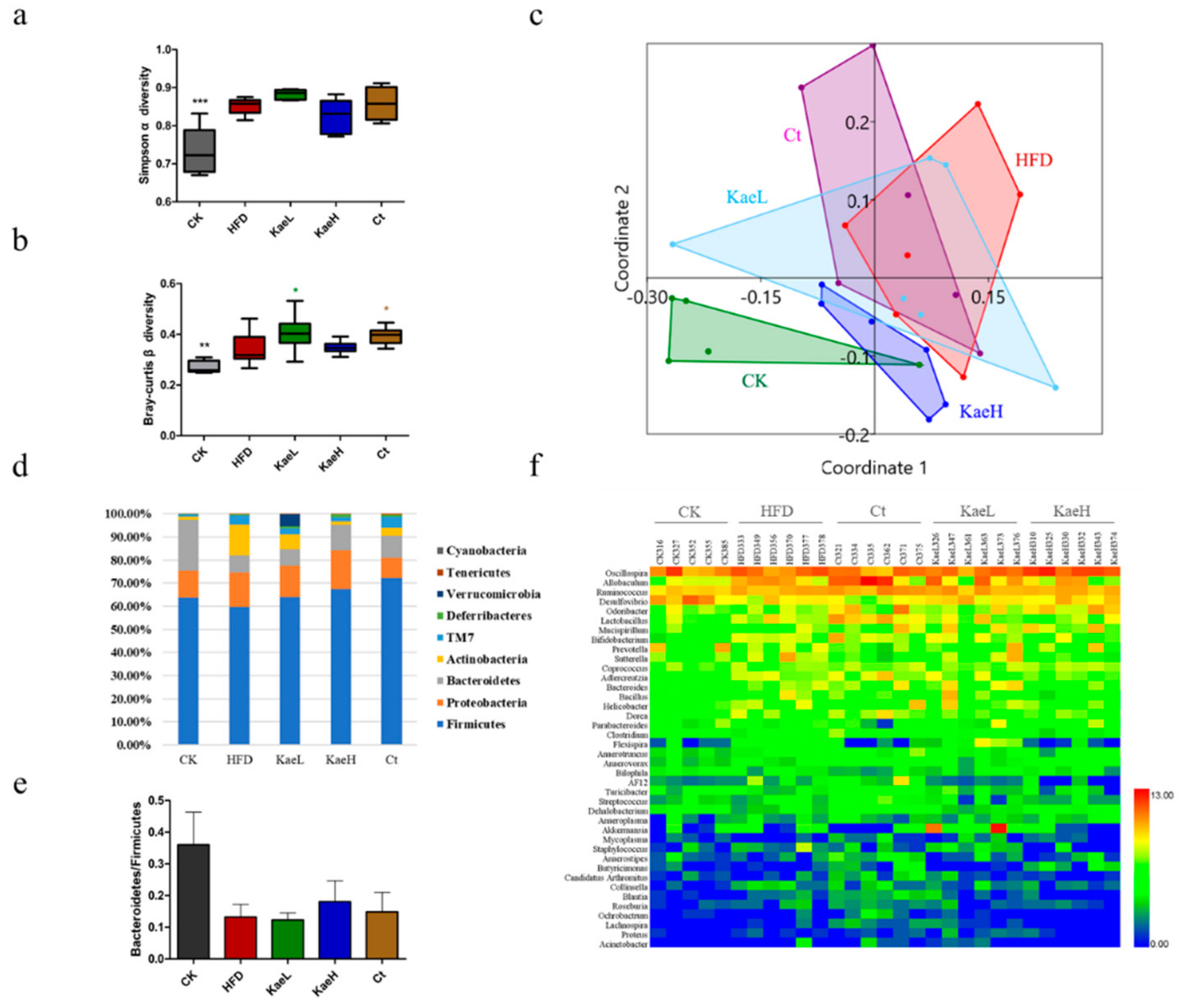
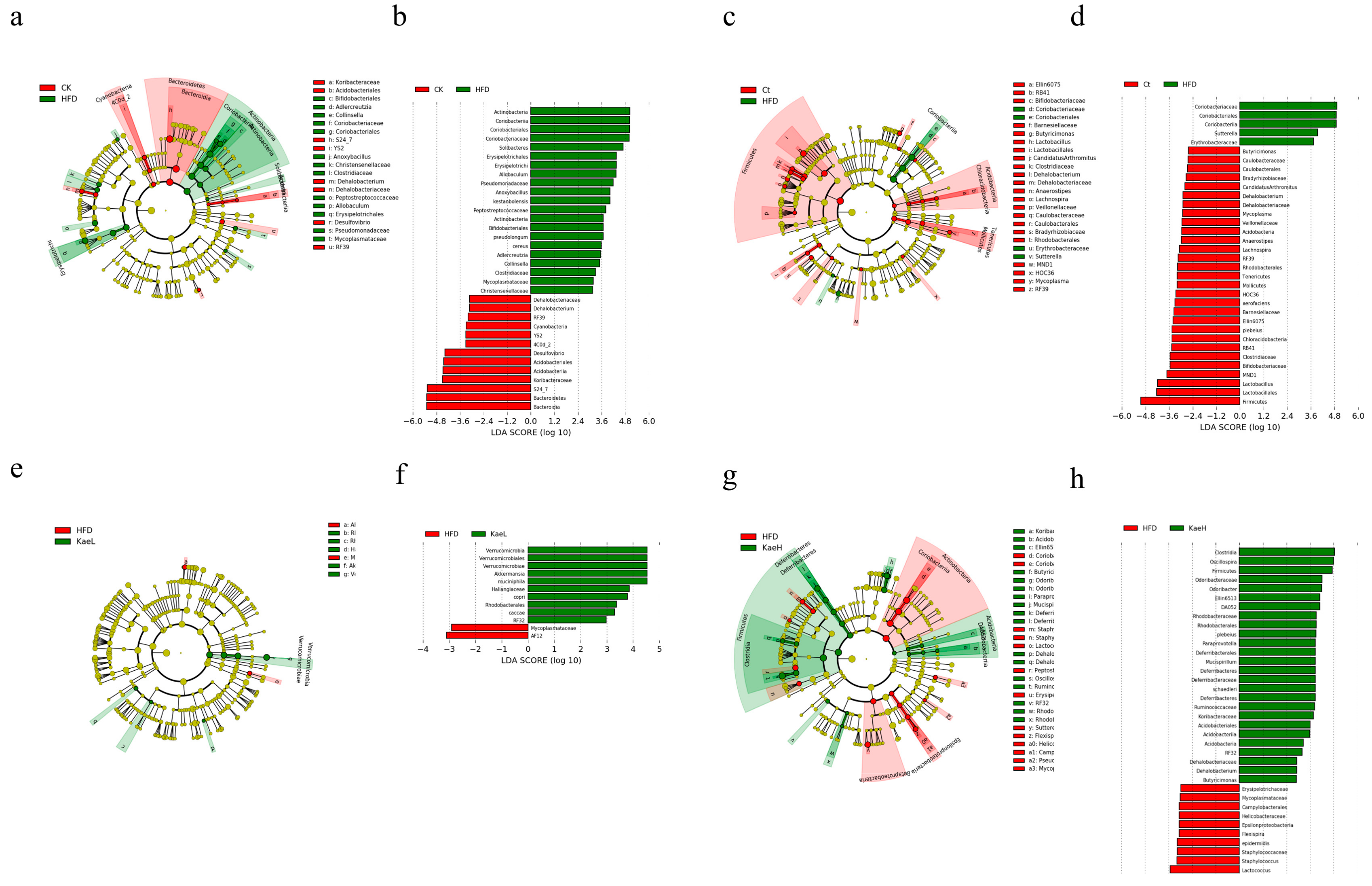
3.5. Effect of C. tinctoria and Kaempferol on Gut Microbe in DIO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Said, M.M.; Sandra, S.; Jaime, L.; Ignacio, B. Systems and WBANs for Controlling Obesity. J. Healthc. Eng. 2018, 2018, 2340276. [Google Scholar] [CrossRef]
- Polsky, S.; Ellis, S.L. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulgaron, E.R.; Delamater, A.M. Obesity and Type 2 Diabetes in Children: Epidemiology and Treatment. Curr. Diabetes Rep. 2014, 14, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Shen, J.; Hu, M.; Tan, W.; Ding, J.; Xiao, P. Traditional uses, phytochemistry, pharmacology, and toxicology of Coreopsis tinctoria Nutt.: A review. J. Ethnopharmacol. 2020, 269, 113690. [Google Scholar] [CrossRef]
- Jiang, B.; Le, L.; Wan, W.; Zhai, W.; Hu, K.; Xu, L.; Xiao, P. The Flower Tea Coreopsis tinctoria Increases Insulin Sensitivity and Regulates Hepatic Metabolism in Rats Fed a High-Fat Diet. Endocrinology 2015, 156, 2006–2018. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, D.; Cheng, X.; Chen, Y.; Duan, M.; Wei, L. The Preliminary Screening of Antihypertension Activity Effective Parts for Hotan Coreopsis tinctoria Nutt. J. Yunnan Univ. Tradit. Chin. Med. 2016, 39, 8–10. [Google Scholar]
- Li, Y.; Yang, P.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, L.Y. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, Y.; Liu, J.; Li, H.; Li, A.; Hong, L.; Cui, G.; Sun, R.; Wulasihan, M.; Sun, J.; et al. Coreopsis tinctoria Modulates Lipid Metabolism by Decreasing Low-Density Lipoprotein and Improving Gut Microbiota. Cell. Physiol. Bio-Chem. 2018, 48, 1060–1074. [Google Scholar] [CrossRef]
- Jiang, B.; Lv, Q.; Wan, W.; Le, L.; Xu, L.; Hu, K.; Xiao, P. Transcriptome analysis reveals the mechanism of the effect of flower tea Coreopsis tinctoria on hepatic insulin resistance. Food Funct. 2018, 9, 5607–5620. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ran, Z.; Zhang, Y.; Song, Z.; Wang, L.; Yao, L.; Zhang, M.; Xin, J.; Mao, X. Marein ameliorates diabetic nephropathy by inhibiting renal sodium glucose transporter 2 and activating the AMPK signaling pathway in db/db mice and high glucose-treated HK-2 cells. Biomed. Pharmacother. 2020, 131, 110684. [Google Scholar] [CrossRef]
- Begmatov, N.; Li, J.; Bobakulov, K.; Numonov, S.; Aisa, H.A. The chemical components of Coreopsis tinctoria Nutt. and their antioxidant, antidiabetic and antibacterial activities. Nat. Prod. Res. 2018, 34, 1772–1776. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Xue, J.; Liu, J.; Chen, X. Muhuyati Wulasihan Flavonoids furom Coreopsis tinctoria adjust lipid metabolism in hyperlipidemia animals by down-regulating adipose differentiation-related protein. Lipids Health Dis. 2014, 13, 193. [Google Scholar] [CrossRef] [Green Version]
- Lan, S.; Lin, J.; Zheng, N. Evaluation of the antioxidant activity of Coreopsis tinctoria nuff. and optimisation of isolation by response surface methodology. Acta Pharm. 2014, 64, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.; Pepper, T.; Shipman, K. HbA1c: A review of non-glycaemic variables. J. Clin. Pathol. 2019, 72, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson, R.; Ignell, C.; Shaat, N.; Berntorp, K. HbA1c as a predictor of diabetes after gestational diabetes mellitus. Prim. Care Diabetes 2017, 11, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shi, S.; Zhao, M.; Chai, X.; Tu, P. Coreosides A–D, C14-polyacetylene glycosides from the capitula of Coreopsis tinctoria and its anti-inflammatory activity against COX-2. Fitoterapia 2013, 87, 93–97. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Zheng, W.; Zhang, Y.; Du, J.; Dong, C.; Tao, N. Structural characterization of a polysaccharide from Coreopsis tinctoria Nutt. and its function to modify myeloid derived suppressor cells. Int. J. Biol. Macromol. 2019, 126, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, C.; Fu, W.; Zhang, H.; Lao, Y.; Zhou, H.; Tan, H.; Xu, H. Screening of the active fractions from the Coreopsis tinctoria Nutt. Flower on diabetic endothelial protection and determination of the underlying mechanism. J. Ethnopharmacol. 2020, 253, 112645. [Google Scholar] [CrossRef]
- Hoppe, W.; Schramm, H.J.; Sturm, M.; Hunsmann, N.; Gaßmann, J. Accumulation of Unusual Phenylpropanoids in Transformed and Non-Transformed Root Cultures of Coreopsis tinctoria. Z. Nat. C 1989, 44, 573–577. [Google Scholar]
- Liu, Y.; Du, D.; Liang, Y.; Xin, G.; Huang, B.; Huang, W. Novel polyacetylenes from Coreopsis tinctoria Nutt. J. Asian Nat. Prod. Res. 2015, 17, 744–749. [Google Scholar] [CrossRef]
- Jing, S.; Chai, W.; Guo, G.; Zhang, X.; Dai, J.; Yan, L. Comparison of antioxidant and antiproliferative activity between Kunlun Chrysanthemum flowers polysaccharides (KCCP) and fraction PII separated by column chromatography. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2016, 1019, 169–177. [Google Scholar] [CrossRef]
- Torres-Villarreal, D.; Camacho, A.; Castro, H.; Ortiz-Lopez, R.; de la Garza, A.L. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J. Physiol. Biochem. 2019, 75, 83–88. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. BioMed Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef]
- Dias, T.; Bronze, M.R.; Houghton, P.J.; Mota-Filipe, H.; Paulo, A. The flavonoid-rich fraction of Coreopsis tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats. J. Ethnopharmacol. 2010, 132, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Jin, M.; Qin, N.; Qiao, W.; Yue, X.; Duan, H.; Niu, W. Flavonoid derivative exerts an antidiabetic effect via AMPK activation in diet-induced obesity mice. Nat. Prod. Res. 2016, 30, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yu, L.; Zhang, Y.; Feng, L.; Kong, S.; Tan, H.; Xu, H.; Huang, C. Extracts of Coreopsis tinctoria Nutt. Flower Exhibit Antidiabetic Effects via the Inhibition of α-Glucosidase Activity. J. Diabetes Res. 2016, 2016, 1564748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodrígue, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Nitert, M.D. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichette, J.; Fynn-Sackey, N.; Gagnon, J. Hydrogen Sulfide and Sulfate Prebiotic Stimulates the Secretion of GLP-1 and Improves Glycemia in Male Mice. Endocrinology 2017, 158, 3416–3425. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]

| Component | Retention Time (min) | Peak Area (mV·min) | Adduct Type |
|---|---|---|---|
| Eriodictyol | 4.57 | 4.42 × 107 | [M+H]+ |
| Kaempferol | 5.04 | 8.54 × 106 | [M+H]+ |
| Glycine-Betaine | 0.96 | 7.23 × 106 | [M+H]+ |
| N-Fructosyl isoleucine | 1.78 | 7.02 × 106 | [M+H]+ |
| Taxifolin | 4.62 | 2.94 × 106 | [M-H]- |
| Luteolin | 5.83 | 2.63 × 106 | [M+H]+ |
| Anileridine | 6.32 | 2.04 × 106 | [M+H]+ |
| 3,5-Dicaffeoylquininic acid | 4.55 | 1.66 × 106 | [M-H]- |
| 4-Methoxycinnamic acid | 6.03 | 1.66 × 106 | [M-H]- |
| Caffeoyl quinic acid | 3.37 | 1.46 × 106 | [M-H]- |
| Coumaric acid | 6.27 | 1.46 × 106 | [M+H-H2O]+ |
| Quercetin-3-O-glucoside | 4.46 | 1.45 × 106 | [M+H]+ |
| Eriodictyol-7-O-glucoside | 3.90 | 1.32 × 106 | [M+H]+ |
| N,N-Dimethyldodecylamine N-oxide | 6.62 | 1.29 × 106 | [M+H]+ |
| Luteolin-4’-O-glucoside | 4.90 | 1.24 × 106 | [M+H]+ |
| Luteolin-7-O-glucoside | 4.33 | 1.03 × 106 | [M+H]+ |
| isookanin-7-O-glucoside | 4.25 | 8.47 × 105 | [M-H]- |
| Naringenin | 6.37 | 7.43 × 105 | [M+H]+ |
| Quercetin-3-O-glucoside; PlaSMA ID-1264 | 4.27 | 3.39 × 105 | [M-H]- |
| Caffeic acid | 4.06 | 3.28 × 105 | [M+H]+ |
| Hyperoside | 4.93 | 2.70 × 105 | [M+H]+ |
| Naringenin-7-O-glucoside | 5.16 | 2.66 × 105 | [M+H]+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Yang, M.; Xu, J.; Hu, Y.; Gao, R.; Huang, K.; He, X. Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1160. https://doi.org/10.3390/nu14061160
Zhang F, Yang M, Xu J, Hu Y, Gao R, Huang K, He X. Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet. Nutrients. 2022; 14(6):1160. https://doi.org/10.3390/nu14061160
Chicago/Turabian StyleZhang, Feng, Minglan Yang, Jia Xu, Yanzhou Hu, Ruxin Gao, Kunlun Huang, and Xiaoyun He. 2022. "Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet" Nutrients 14, no. 6: 1160. https://doi.org/10.3390/nu14061160
APA StyleZhang, F., Yang, M., Xu, J., Hu, Y., Gao, R., Huang, K., & He, X. (2022). Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet. Nutrients, 14(6), 1160. https://doi.org/10.3390/nu14061160






