Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk
Abstract
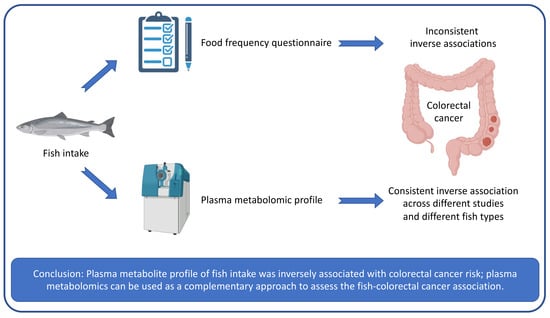
:1. Introduction
2. Methods
Study Population
3. Dietary Assessment
4. Metabolomics Measurement
5. Nondietary Covariates
Statistical Analyses
6. Results
7. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Norat, T.; Vieira, A.R. World Cancer Research Fund International Systematic Literature Review: The Associations between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2017. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018: Diet, Nutrition, Physical Activity and Colorectal Cancer. Available online: http://dietandcancerreport.org (accessed on 17 January 2022).
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer: Food groups and colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegria-Lertxundi, I.; Aguirre, C.; Bujanda, L.; Fernández, F.J.; Polo, F.; Ordovás, J.M.; Etxezarraga, M.C.; Zabalza, I.; Larzabal, M.; Portillo, I.; et al. Food groups, diet quality and colorectal cancer risk in the Basque Country. World J. Gastroenterol. 2020, 26, 4108–4125. [Google Scholar] [CrossRef] [PubMed]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.-C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e6. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.A.; Rinaldi, S.; Ferrari, P.; Carayol, M.; Achaintre, D.; Scalbert, A.; Cross, A.J.; Gunter, M.J.; Fensom, G.K.; Appleby, P.N.; et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015, 102, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zou, L.; Su, J.; Tai, E.S.; Whitton, C.; Van Dam, R.M.; Ong, C.N. Meat and Seafood Consumption in Relation to Plasma Metabolic Profiles in a Chinese Population: A Combined Untargeted and Targeted Metabolomics Study. Nutrients 2017, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Cuparencu, C.; Rinnan, A.; Dragsted, L.O. Combined Markers to Assess Meat Intake—Human Metabolomic Studies of Discovery and Validation. Mol. Nutr. Food Res. 2019, 63, 1900106. [Google Scholar] [CrossRef]
- Mazzilli, K.M.; McClain, K.M.; Lipworth, L.; Playdon, M.C.; Sampson, J.N.; Clish, C.B.; Gerszten, R.E.; Freedman, N.D.; Moore, S.C. Identification of 102 Correlations between Serum Metabolites and Habitual Diet in a Metabolomics Study of the Prostate, Lung, Colorectal, and Ovarian Cancer Trial. J. Nutr. 2020, 150, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Giovannucci, E.L.; Willett, W.C.; Colditz, G.A.; Ascherio, A.; Rosner, B.; Stampfer, M.J. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991, 338, 464–468. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Colditz, G.; Hunter, D.J.; Spiegelman, D.; Barbieri, R.L.; Speizer, F.E. Plasma Sex Steroid Hormone Levels and Risk of Breast Cancer in Postmenopausal Women. J. Natl. Cancer Inst. 1998, 90, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.F.; Spiegelman, D.; Yu, J.; Rifai, N.; Hotamisligil, G.S.; Rimm, E.B. Plasma leptin concentrations and four-year weight gain among US men. Int. J. Obes. 2001, 25, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rexrode, K.M.; Lee, I.-M.; Cook, N.R.; Hennekens, C.H.; Buring, J.E. Baseline Characteristics of Participants in the Women’s Health Study. J. Women's Health Gender-Based Med. 2000, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Buring, J.E.; Manson, J.E.; Giovannucci, E.L.; Moorthy, M.V.; Zhang, S.; Lee, I.M.; Lin, J.H. Circulating Vitamin D Levels and Risk of Colorectal Cancer in Women. Cancer Prev. Res. 2015, 8, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared with Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am. J. Epidemiol. 2018, 187, 1051–1063. [Google Scholar] [CrossRef] [Green Version]
- Al-Shaar, L.; Yuan, C.; Rosner, B.; Dean, S.B.; Ivey, K.L.; Clowry, C.M.; Sampson, L.A.; Barnett, J.B.; Rood, J.; Harnack, L.J.; et al. Reproducibility and Validity of a Semi-quantitative Food Frequency Questionnaire in Men Assessed by Multiple Methods. Am. J. Epidemiol. V. 2020, 190, 1122–1132. [Google Scholar] [CrossRef]
- Feskanich, D.; Rimm, E.B.; Giovannucci, E.L.; Colditz, G.A.; Stampfer, M.J.; Litin, L.B.; Willett, W.C. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1993, 93, 790–796. [Google Scholar] [CrossRef]
- Cao, Y.; Strate, L.L.; Keeley, B.R.; Tam, I.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Meat intake and risk of diverticulitis among men. Gut 2018, 67, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Paynter, N.P.; Balasubramanian, R.; Giulianini, F.; Wang, D.D.; Tinker, L.F.; Gopal, S.; Deik, A.A.; Bullock, K.; Pierce, K.A.; Scott, J.; et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018, 137, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.K.; Clish, C.B.; Kraft, P.; Wu, C.; Souza, A.L.; Deik, A.A.; Tworoger, S.S.; Wolpin, B.M. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clin. Chem. 2013, 59, 1657–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.-L.; Rosenthal, R.; Rubin, D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992, 111, 172. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Pastor, J.M.; Salvador, M.; Argandoña, M.; Bernal, V.; Reina-Bueno, M.; Csonka, L.N.; Iborra, J.L.; Vargas, C.; Nieto, J.J.; Cánovas, M. Ectoines in cell stress protection: Uses and biotechnological production. Biotechnol. Adv. 2010, 28, 782–801. [Google Scholar] [CrossRef]
- Ding, J.; Dai, R.; Yang, L.; He, C.; Xu, K.; Liu, S.; Zhao, W.; Xiao, L.; Luo, L.; Zhang, Y.; et al. Inheritance and Establishment of Gut Microbiota in Chickens. Front. Microbiol. 2017, 8, 1967. [Google Scholar] [CrossRef]
- Bowen, B.P.; Northen, T.R. Dealing with the unknown: Metabolomics and Metabolite Atlases. J. Am. Soc. Mass Spectrom. 2010, 21, 1471–1476. [Google Scholar] [CrossRef] [Green Version]
- Gibney, M.J.; Walsh, M.; Brennan, L.; Roche, H.M.; German, B.; van Ommen, B. Metabolomics in human nutrition: Opportunities and challenges. Am. J. Clin. Nutr. 2005, 82, 497–503. [Google Scholar] [CrossRef]
- Shoaie, S.; Ghaffari, P.; Kovatcheva-Datchary, P.; Mardinoglu, A.; Sen, P.; Pujos-Guillot, E.; de Wouters, T.; Juste, C.; Rizkalla, S.; Chilloux, J.; et al. Quantifying Diet-Induced Metabolic Changes of the Human Gut Microbiome. Cell Metab. 2015, 22, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, H. Intake of arachidonic acid-containing lipids in adult humans: Dietary surveys and clinical trials. Lipids Health Dis. 2019, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed Meat and Colorectal Cancer: A Review of Epidemiologic and Experimental Evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Carr, P.R.; Walter, V.; Brenner, H.; Hoffmeister, M. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int. J. Cancer 2016, 138, 293–302. [Google Scholar] [CrossRef]


| NHS, NHSII, and HPFS | WHS (Included in the Nested Case-Control Analysis of CRC) | ||||
|---|---|---|---|---|---|
| Overall (n = 5269) | Included in the Nested Case-Control Analysis of CRC | ||||
| Cases (n = 559) | Controls (n = 559) | Cases (n = 266) | Controls (n = 266) | ||
| Age at blood draw, years | 53 (9) | 61 (8) | 61 (8) | 59 (8) | 59 (8) |
| Female, % | 90 | 65 | 65 | 100 | 100 |
| White race, % | 98 | 97 | 99 | 98 | 98 |
| BMI, kg/m2 | 25.4 (4.9) | 25.9 (4.4) | 25.5 (4.2) | 26.7 (5.5) | 26.2 (5.0) |
| Participants selected as cases in sub-studies, % | 50 | 100 | 0 | 100 | 0 |
| Fasting at blood collection, % | 69 | 65 | 65 | 73 | 73 |
| Multivitamin use, % | 68 | 62 | 64 | 29 | 28 |
| Regular aspirin use, % | 42 | 47 | 49 | 10 | 13 |
| Endoscopy, % | 28 | 43 | 47 | 4 a | 3 a |
| Family history of CRC, % | 11 | 18 | 14 | 14 | 11 |
| Smoking, % | |||||
| Never | 54 | 45 | 46 | 45 | 54 |
| Former | 36 | 45 | 46 | 43 | 36 |
| Current | 10 | 10 | 8 | 12 | 10 |
| Physical activity, MET-hours/week | 18.4 (22.2) | 19.9 (21.6) | 21.2 (24.7) | 14.4 (20.8) | 16.5 (27.6) |
| Alternate Healthy Eating Index b | 24.7 (6.6) | 25.3 (6.7) | 25.5 (7.0) | / | / |
| Assigned to aspirin group (for WHS), % | / | / | / | 43 | 49 |
| Assigned to vitamin E group (for WHS), % | / | / | / | 45 | 50 |
| Dietary intake | |||||
| Total energy intake, kcal/day | 1830 (506) | 1873 (552) | 1910 (543) | 1692 (530) | 1752 (538) |
| Alcohol intake, g/day | 5.8 (10.4) | 8.7 (14.5) | 8.4 (12.9) | 5.0 (9.9) | 4.2 (7.6) |
| Total red meat intake, servings/week | 6.6 (4.2) | 7.1 (5.2) | 6.8 (4.4) | 6.1 (5.2) | 6.5 (5.4) |
| Unprocessed red meat, servings/week | 4.9 (3.2) | 5.1 (3.8) | 5.0 (3.3) | 4.7 (3.7) | 5.2 (3.9) |
| Processed red meat, servings/week | 1.6 (1.8) | 2.0 (2.3) | 1.7 (1.9) | 1.3 (1.8) | 1.3 (1.8) |
| Poultry, servings/week | 4.3 (2.6) | 4.2 (2.9) | 4.2 (2.3) | 2.7 (1.8) | 2.8 (1.8) |
| Total fish, servings/week | 1.8 (1.6) | 2.0 (1.5) | 2.3 (2.1) | 1.5 (1.4) | 1.5 (1.4) |
| Dark meat fish, servings/week | 0.3 (0.5) | 0.3 (0.5) | 0.4 (0.6) | 0.2 (0.3) | 0.2 (0.4) |
| Canned tuna fish, servings/week | 0.9 (0.9) | 0.9 (0.9) | 1.1 (1.4) | 0.7 (0.8) | 0.7 (0.9) |
| NHS, NHSII, HPFS (n = 5269) | WHS (n = 532) | ||||
|---|---|---|---|---|---|
| Number of Known Metabolites in the Metabolite Profile Score | r (95% CI) | Number of Known Metabolites Available in the Score Calculation | r (95% CI) | ||
| Training a (n = 3688) | Testing (n = 1581) | ||||
| Total red meat | 53 | 0.44 (0.41, 0.46) | 0.46 (0.42, 0.49) | 50 | 0.33 (0.25, 0.40) |
| Unprocessed red meat | 55 | 0.40 (0.38, 0.43) | 0.42 (0.38, 0.46) | 55 | 0.36 (0.28, 0.43) |
| Processed red meat | 36 | 0.32 (0.29, 0.35) | 0.33 (0.29, 0.38) | 34 | 0.19 (0.12, 0.28) |
| Poultry | 7 | 0.21 (0.18, 0.24) | 0.18 (0.14, 0.23) | 6 | 0.12 (0.04, 0.20) |
| Total fish | 18 | 0.40 (0.38, 0.43) | 0.39 (0.35, 0.43) | 18 | 0.31 (0.23, 0.38) |
| Dark meat fish | 27 | 0.42 (0.40, 0.45) | 0.42 (0.38, 0.46) | 25 | 0.32 (0.24, 0.39) |
| Canned tuna fish | 11 | 0.22 (0.19, 0.25) | 0.20 (0.15, 0.25) | 11 | 0.14 (0.06, 0.22) |
| Dietary Intake | Metabolite Profile Score | |||||
|---|---|---|---|---|---|---|
| NHS/HPFS (n = 1118) | WHS (n = 532) | Pooled | NHS/HPFS (n = 1118) | WHS (n = 532) | Pooled | |
| Total red meat | ||||||
| Basic model a | 1.09 (0.97, 1.24) | 0.94 (0.80, 1.10) | 1.03 (0.94, 1.14) | 1.07 (0.95, 1.21) | 1.16 (0.97, 1.39) | 1.10 (0.99, 1.21) |
| Multivariable model b | 1.16 (0.99, 1.35) | 0.95 (0.77, 1.16) | 1.07 (0.95, 1.21) | 1.02 (0.89, 1.16) | 1.14 (0.95, 1.37) | 1.06 (0.95, 1.18) |
| Unprocessed red meat | ||||||
| Basic model a | 1.03 (0.91, 1.16) | 0.91 (0.77, 1.08) | 0.99 (0.89, 1.09) | 1.05 (0.93, 1.18) | 1.13 (0.95, 1.35) | 1.08 (0.97, 1.19) |
| Multivariable model b | 1.05 (0.91, 1.21) | 0.91 (0.74, 1.12) | 1.00 (0.89, 1.13) | 1.00 (0.87, 1.14) | 1.12 (0.93, 1.34) | 1.04 (0.93, 1.15) |
| Processed red meat | ||||||
| Basic model a | 1.18 (1.04, 1.34) | 1.01 (0.85, 1.18) | 1.11 (1.00, 1.23) | 1.12 (0.99, 1.27) | 1.18 (0.98, 1.41) | 1.14 (1.03, 1.26) |
| Multivariable model b | 1.23 (1.06, 1.42) | 1.03 (0.85, 1.25) | 1.15 (1.03, 1.29) | 1.07 (0.93, 1.22) | 1.16 (0.95, 1.40) | 1.10 (0.98, 1.22) |
| Poultry | ||||||
| Basic model a | 1.02 (0.91, 1.15) | 0.91 (0.77, 1.08) | 0.99 (0.90, 1.09) | 0.99 (0.88, 1.12) | 0.87 (0.73, 1.04) | 0.95 (0.86, 1.05) |
| Multivariable model b | 1.07 (0.94, 1.22) | 0.94 (0.78, 1.12) | 1.02 (0.92, 1.14) | 0.99 (0.87, 1.12) | 0.86 (0.72, 1.04) | 0.94 (0.85, 1.05) |
| Total fish | ||||||
| Basic model a | 0.82 (0.71, 0.95) | 1.00 (0.84, 1.19) | 0.89 (0.79, 0.99) | 0.87 (0.76, 0.98) | 0.85 (0.71, 1.02) | 0.86 (0.78, 0.95) |
| Multivariable model b | 0.84 (0.72, 0.98) | 1.06 (0.88, 1.29) | 0.92 (0.82, 1.04) | 0.87 (0.77, 0.99) | 0.84 (0.70, 1.02) | 0.86 (0.77, 0.96) |
| Dark meat fish | ||||||
| Basic model a | 0.96 (0.85, 1.09) | 0.86 (0.72, 1.03) | 0.93 (0.84, 1.03) | 0.86 (0.76, 0.98) | 0.85 (0.71, 1.02) | 0.86 (0.78, 0.95) |
| Multivariable model b | 0.98 (0.86, 1.11) | 0.86 (0.71, 1.04) | 0.94 (0.85, 1.05) | 0.87 (0.76, 0.99) | 0.84 (0.70, 1.03) | 0.86 (0.77, 0.96) |
| Canned tuna fish | ||||||
| Basic model a | 0.80 (0.69, 0.93) | 0.98 (0.83, 1.17) | 0.87 (0.78, 0.98) | 0.87 (0.77, 0.99) | 0.87 (0.72, 1.04) | 0.87 (0.79, 0.96) |
| Multivariable model b | 0.82 (0.70, 0.95) | 1.00 (0.83, 1.20) | 0.89 (0.79, 1.00) | 0.88 (0.77, 1.00) | 0.87 (0.72, 1.05) | 0.87 (0.78, 0.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chandler, P.D.; Zeleznik, O.A.; Wu, K.; Wu, Y.; Yin, K.; Song, R.; Avila-Pacheco, J.; Clish, C.B.; Meyerhardt, J.A.; et al. Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk. Nutrients 2022, 14, 978. https://doi.org/10.3390/nu14050978
Wang F, Chandler PD, Zeleznik OA, Wu K, Wu Y, Yin K, Song R, Avila-Pacheco J, Clish CB, Meyerhardt JA, et al. Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk. Nutrients. 2022; 14(5):978. https://doi.org/10.3390/nu14050978
Chicago/Turabian StyleWang, Fenglei, Paulette D. Chandler, Oana A. Zeleznik, Kana Wu, You Wu, Kanhua Yin, Rui Song, Julian Avila-Pacheco, Clary B. Clish, Jeffrey A. Meyerhardt, and et al. 2022. "Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk" Nutrients 14, no. 5: 978. https://doi.org/10.3390/nu14050978
APA StyleWang, F., Chandler, P. D., Zeleznik, O. A., Wu, K., Wu, Y., Yin, K., Song, R., Avila-Pacheco, J., Clish, C. B., Meyerhardt, J. A., Zhang, X., Song, M., Ogino, S., Lee, I.-M., Eliassen, A. H., Liang, L., Smith-Warner, S. A., Willett, W. C., & Giovannucci, E. L. (2022). Plasma Metabolite Profiles of Red Meat, Poultry, and Fish Consumption, and Their Associations with Colorectal Cancer Risk. Nutrients, 14(5), 978. https://doi.org/10.3390/nu14050978