A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities
Abstract
1. Introduction
2. Risk Factors of Cancer-Related Fatigue
2.1. Biological and Genetic Risk Factors
2.2. Biobehavioral Risk Factors
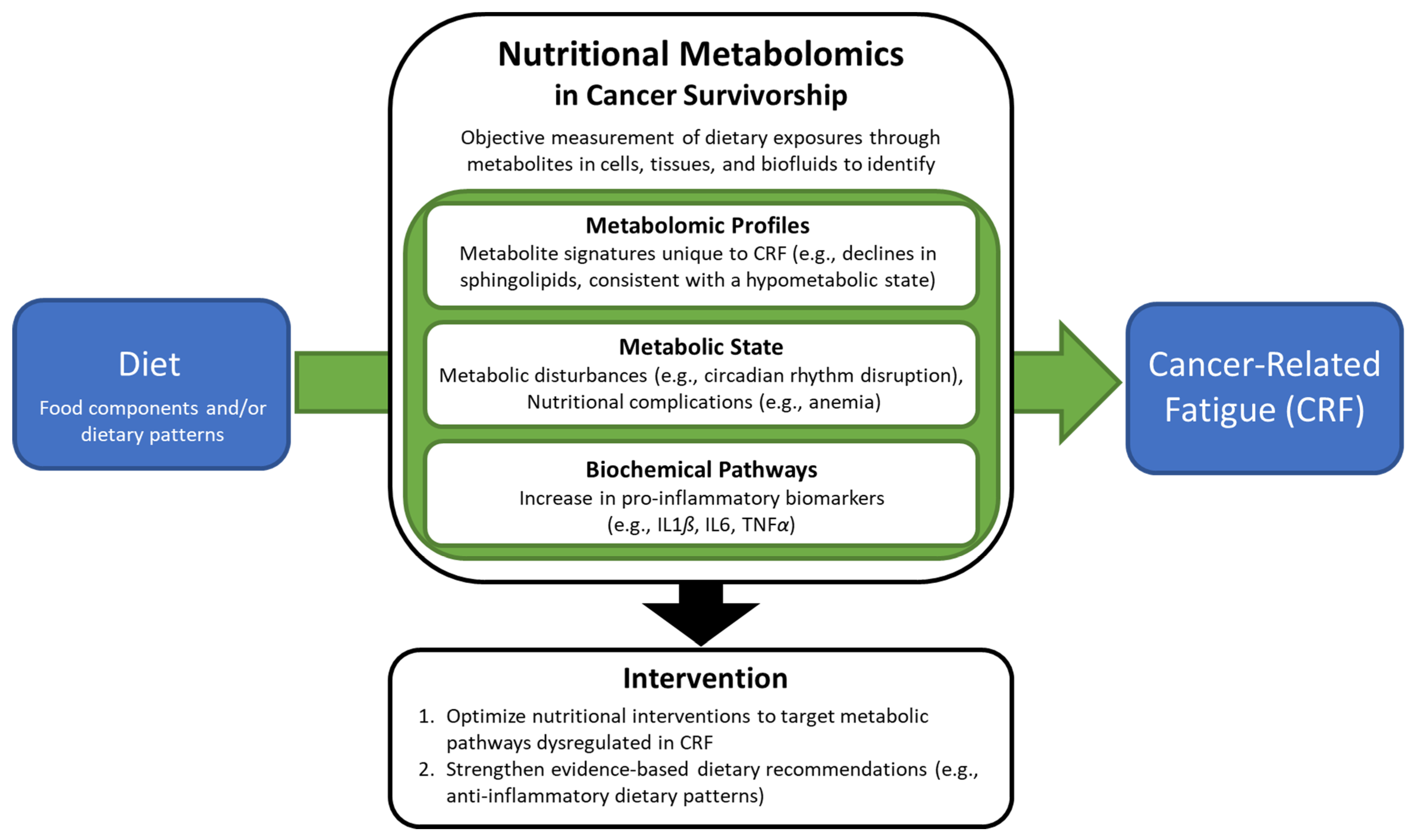
3. Metabolomic Studies of Cancer-Related Fatigue
4. Dietary Recommendations for Cancer-Related Fatigue
5. Challenges of Nutritional Metabolomics in Cancer-Related Fatigue
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Cancer Society. Survivorship: During and After Treatment. Available online: https://www.cancer.org/treatment/survivorship-during-and-after-treatment.html (accessed on 17 March 2022).
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.M.; Irwin, M.; Hinds, P.; Miller, A.; Berger, A.; Jacobsen, P.; Ancoli-Israel, S.; Reeve, B.B.; Mustian, K.; O’Mara, A.; et al. Recommendations for High-Priority Research on Cancer-Related Fatigue in Children and Adults. JNCI J. Natl. Cancer Inst. 2013, 105, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of Cancer-Related Fatigue on the Lives of Patients: New Findings From the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in Breast Cancer Survivors: Occurrence, Correlates, and Impact on Quality of Life. J. Clin. Oncol. 2000, 18, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Breitbart, W.; Cella, D.; A Curt, G.; E Groopman, J.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Scherr, S.L.; Portenoy, R.K. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition. Semin. Hematol. 1997, 34, 4–12. [Google Scholar] [PubMed]
- Stasi, R.; Abriani, L.; Beccaglia, P.; Terzoli, E.; Amadori, S. Cancer-related fatigue: Evolving concepts in evaluation and treatment. Cancer 2003, 98, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of side effects during and post-treatment in breast cancer survivors. Breast J. 2017, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F.; Mock, V. Assessment and management of cancer-related fatigue in adults. Lancet 2003, 362, 640–650. [Google Scholar] [CrossRef]
- Kessels, E.; Husson, O.; Van der Feltz-Cornelis, C.M. The effect of exercise on cancer-related fatigue in cancer survivors: A systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2018, 14, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- van Waart, H.; van Harten, W.H.; Buffart, L.M.; Sonke, G.S.; Stuiver, M.M.; Aaronson, N.K. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psycho-Oncology 2016, 25, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.M.; Lowe-Strong, A.; Rankin-Watt, J.; Campbell, A.; Gracey, J.H. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psycho-Oncology 2013, 22, 186–194. [Google Scholar] [CrossRef]
- Goedendorp, M.M.; Gielissen, M.F.; Verhagen, C.A.; Bleijenberg, G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst. Rev. 2009, 2009, CD006953. [Google Scholar] [CrossRef]
- Duijts, S.F.A.; Faber, M.M.; Oldenburg, H.S.A.; Van Beurden, M.; Aaronson, N.K. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors-a meta-analysis. Psycho-Oncology 2011, 20, 115–126. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Donovan, K.A.; Vadaparampil, S.T.; Small, B.J. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007, 26, 660–667. [Google Scholar] [CrossRef]
- Orrow, G.R.; Hickok, J.T.; Roscoe, J.A.; Raubertas, R.F.; Andrews, P.L.; Flynn, P.J.; Hynes, H.E.; Banerjee, T.K.; Kirshner, J.J.; King, D.K. Differential Effects of Paroxetine on Fatigue and Depression: A Randomized, Double-Blind Trial From the University of Rochester Cancer Center Community Clinical Oncology Program. J. Clin. Oncol. 2003, 21, 4635–4641. [Google Scholar]
- Stockler, M.R.; O’Connell, R.; Nowak, A.K.; Goldstein, D.; Turner, J.; Wilcken, N.R.; Wyld, D.; Abdi, E.A.; Glasgow, A.; Beale, P.J.; et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: A placebo-controlled double-blind randomised trial. Lancet Oncol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Roscoe, J.A.; Morrow, G.R.; Hickok, J.T.; Mustian, K.M.; Griggs, J.J.; Matteson, S.E.; Bushunow, P.; Qazi, R.; Smith, B. Effect of paroxetine hydrochloride (Paxil®) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res. Treat. 2005, 89, 243–249. [Google Scholar] [CrossRef]
- Berenson, J.R.; Yellin, O.; Shamasunder, H.K.; Chen, C.-S.; Charu, V.; Woliver, T.B.; Sanani, S.; Schlutz, M.; Nassir, Y.; Swift, R.A.; et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support. Care Cancer 2014, 23, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, P.; Morrow, G.R.; Roscoe, J.A.; Heckler, C.; Mohile, S.; Janelsins, M.; Peppone, L.; Hemstad, A.; Esparaz, B.T.; Hopkins, J.O. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: A University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010, 116, 3513–3520. [Google Scholar] [PubMed]
- Spathis, A.; Fife, K.; Blackhall, F.; Dutton, S.; Bahadori, R.; Wharton, R.; O’Brien, M.; Stone, P.; Benepal, T.; Bates, N.; et al. Modafinil for the Treatment of Fatigue in Lung Cancer: Results of a Placebo-Controlled, Double-Blind, Randomized Trial. J. Clin. Oncol. 2014, 32, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zhang, Z.; Yu, X.; Zhao, J.; Qiu, F.; Huang, J. Psychotropic drugs for the management of cancer-related fatigue: A systematic review and meta-analysis. Eur. J. Cancer Care 2016, 25, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Sheng, P.; Jin, H.; He, H.; Qi, E.; Chen, W.; Dong, Y.; Hou, L. Effect of Methylphenidate in Patients with Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e84391. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Yennurajalingam, S.; Palmer, J.L.; Perez-Cruz, P.E.; Frisbee-Hume, S.; Allo, J.A.; Williams, J.L.; Cohen, M.Z. Methylphenidate and/or a Nursing Telephone Intervention for Fatigue in Patients With Advanced Cancer: A Randomized, Placebo-Controlled, Phase II Trial. J. Clin. Oncol. 2013, 31, 2421–2427. [Google Scholar] [CrossRef]
- Moraska, A.R.; Sood, A.; Dakhil, S.R.; Sloan, J.A.; Barton, D.; Atherton, P.J.; Suh, J.J.; Griffin, P.C.; Johnson, D.B.; Ali, A.; et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Long-Acting Methylphenidate for Cancer-Related Fatigue: North Central Cancer Treatment Group NCCTG-N05C7 Trial. J. Clin. Oncol. 2010, 28, 3673–3679. [Google Scholar] [CrossRef]
- Breitbart, W.; Alici, Y. Psychostimulants for Cancer-Related Fatigue. J. Natl. Compr. Cancer Netw. 2010, 8, 933–942. [Google Scholar] [CrossRef]
- Begley, S.; Rose, K.; O’Connor, M. The use of corticosteroids in reducing cancer-related fatigue: Assessing the evidence for clinical practice. Int. J. Palliat. Nurs. 2016, 22, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; A Castillo, D.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Feunang, Y.D.; Dorrestein, P.C.; O Dragsted, L.; Draper, J.; et al. Perspective: Dietary Biomarkers of Intake and Exposure—Exploration with Omics Approaches. Adv. Nutr. Int. Rev. J. 2019, 11, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.E.; Boushey, C.J.; Shvetsov, Y.B.; Ettienne, R.; Reedy, J.; Wilkens, L.R.; Le Marchand, L.; Henderson, B.E.; Kolonel, L.N. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: The Dietary Patterns Methods Project. Am. J. Clin. Nutr. 2015, 101, 587–597. [Google Scholar] [CrossRef]
- National Cancer Institute. Metabolomics. Available online: https://dceg.cancer.gov/research/how-we-study/metabolomics (accessed on 24 February 2022).
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Giovannucci, E.L. An Empirical Dietary Inflammatory Pattern Score Enhances Prediction of Circulating Inflammatory Biomarkers in Adults. J. Nutr. 2017, 147, 1567–1577. [Google Scholar] [CrossRef]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Barsevick, A.; Geneqol, C.; Frost, M.; Zwinderman, A.; Hall, P.; Halyard, M. I’m so tired: Biological and genetic mechanisms of cancer-related fatigue. Qual. Life Res. 2010, 19, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of Cancer-Related Fatigue. Oncologist 2007, 12, 22–34. [Google Scholar] [CrossRef]
- Bender, C.M.; Sereika, S.M.; Brufsky, A.M.; Ryan, C.; Vogel, V.G.; Rastogi, P.; Cohen, S.; Casillo, F.E.; Berga, S.L. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause 2007, 14, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.; Aziz, N. Altered Cortisol Response to Psychologic Stress in Breast Cancer Survivors with Persistent Fatigue. Psychosom. Med. 2005, 67, 277–280. [Google Scholar] [CrossRef]
- Agteresch, H.J.; Dagnelie, P.C.; Van Der Gaast, A.; Stijnen, T.; Wilson, J.H.P. Randomized Clinical Trial of Adenosine 5′-Triphosphate in Patients With Advanced Non-Small-Cell Lung Cancer. J. Natl. Cancer Inst. 2000, 92, 321–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orre, I.J.; Murison, R.; Dahl, A.A.; Ueland, T.; Aukrust, P.; Fosså, S.D. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav. Immun. 2009, 23, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.; Park, J.Y.; Permuth-Wey, J.; Rincon, M.A.; Phillips, K.M.; Small, B.J.; Jacobsen, P.B. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: Preliminary findings. Brain Behav. Immun. 2012, 26, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dodd, M.; Lee, K.; West, C.; Paul, S.M.; Cooper, B.A.; Wara, W.; Swift, P.S.; Dunn, L.B.; Aouizerat, B.E. Preliminary Evidence of an Association Between a Functional Interleukin-6 Polymorphism and Fatigue and Sleep Disturbance in Oncology Patients and Their Family Caregivers. J. Pain Symptom Manag. 2010, 40, 531–544. [Google Scholar] [CrossRef]
- Aouizerat, B.E.; Dodd, M.; Lee, K.; West, C.; Paul, S.M.; Cooper, B.A.; Wara, W.; Swift, P.; Dunn, L.B.; Miaskowski, C. Preliminary Evidence of a Genetic Association Between Tumor Necrosis Factor Alpha and the Severity of Sleep Disturbance and Morning Fatigue. Biol. Res. Nurs. 2009, 11, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Collado-Hidalgo, A.; Bower, J.E.; Ganz, P.A.; Irwin, M.; Cole, S.W. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav. Immun. 2008, 22, 1197–1200. [Google Scholar] [CrossRef]
- Kurzrock, R. The role of cytokines in cancer-related fatigue. Cancer 2001, 92, 1684–1688. [Google Scholar] [CrossRef]
- Cella, D. Quality of life and clinical decisions in chemotherapy-induced anemia. Oncologist 2006, 20, 25–28. [Google Scholar]
- Wang, X.S. Pathophysiology of Cancer-Related Fatigue. Clin. J. Oncol. Nurs. 2008, 12, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Goforth, H.W. Long-term and Short-term Effects of Insomnia in Cancer and Effective Interventions. Cancer J. 2014, 20, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Colacino, J.; Cornellier, M.L.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.; Knutson, K. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef]
- Stern, J.H.; Grant, A.S.; Thomson, C.A.; Tinker, L.; Hale, L.; Brennan, K.M.; Woods, N.F.; Chen, Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity 2014, 22, E55–E61. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Zeitzer, J.M.; Conrad, A.; Giese-Davis, J.; Mustian, K.M.; Popek, V.; Nga, K.; Spiegel, D. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J. Clin. Sleep Med. 2008, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Ose, J.; Lin, T.; Warby, C.A.; Gigic, B.; Steindorf, K.; Schrotz-King, P.; Abbenhardt-Martin, C.; Zielske, L.; Boehm, J.; et al. Inflammation- and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur. J. Cancer Care 2019, 28, e13055. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Bennett, J.A.; Nail, L.; Schwartz, A. Strength, Physical Activity, and Age Predict Fatigue in Older Breast Cancer Survivors. Oncol. Nurs. Forum 2008, 35, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mustian, K.M.; Peppone, L.J.; Palesh, O.G.; Janelsins, M.C.; Mohile, S.G.; Purnell, J.Q.; Darling, T.V. Exercise and Cancer-related Fatigue. US Oncol. 2009, 5, 20–23. [Google Scholar] [CrossRef]
- Peppone, L.J.; Rickles, A.S.; Janelsins, M.C.; Insalaco, M.R.; Skinner, K.A. The Association Between Breast Cancer Prognostic Indicators and Serum 25-OH Vitamin D Levels. Ann. Surg. Oncol. 2012, 19, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.-W.; Fabian, C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Walters, E.; Varshney, S.; Johnson, C. Deficiencies of micronutrients, altered bowel function, and quality of life during late follow-up after pancreaticoduodenectomy for malignancy. Pancreatology 2002, 2, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2009, 119, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef]
- Feng, L.R.; Barb, J.J.; Allen, H.; Regan, J.; Saligan, L. Steroid Hormone Biosynthesis Metabolism Is Associated with Fatigue Related to Androgen Deprivation Therapy for Prostate Cancer. Front. Cell Dev. Biol. 2021, 9, 642307. [Google Scholar] [CrossRef]
- Feng, L.R.; Barb, J.J.; Regan, J.; Saligan, L.N. Plasma metabolomic profile associated with fatigue in cancer patients. Cancer Med. 2021, 10, 1623–1633. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Kober, K.M.; Kuo, C.-H.; Yeh, K.-H.; Kuo, T.-C.; Tseng, Y.J.; Miaskowski, C.; Liang, J.-T.; Shun, S.-C. A Pilot Study of Metabolomic Pathways Associated With Fatigue in Survivors of Colorectal Cancer. Biol. Res. Nurs. 2021, 23, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Sok, P.; Taylor, O.; Woodhouse, J.P.; Bernhardt, M.B.; Raghubar, K.P.; Kahalley, L.S.; Lupo, P.J.; Hockenberry, M.J.; Scheurer, M.E. Cerebrospinal Fluid Metabolomic Profiles Associated With Fatigue During Treatment for Pediatric Acute Lymphoblastic Leukemia. J. Pain Symptom Manag. 2021, 61, 464–473. [Google Scholar] [CrossRef]
- Lyon, D.E.; Starkweather, A.; Yao, Y.; Garrett, T.; Kelly, D.L.; Menzies, V.; Dereziński, P.; Datta, S.; Kumar, S.; Jackson-Cook, C. Pilot Study of Metabolomics and Psychoneurological Symptoms in Women with Early Stage Breast Cancer. Biol. Res. Nurs. 2017, 20, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Ando, H.; Ishizuka, K.; Kitagawa, R.; Okubo, S.; Saito, H.; Kokubu, S.; Miyazaki, A.; Ikejima, K.; Shiina, S.; et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229772. [Google Scholar] [CrossRef] [PubMed]
- American Institute for Cancer Research. Cancer Survivors. Available online: https://www.wcrf.org/dietandcancer/cancer-survivors/ (accessed on 2 February 2022).
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.M.; Weiss, J.M.; Kind, A.J.H.; Robbins, J. Senescent Swallowing: Impact, Strategies, and Interventions. Nutr. Clin. Pract. 2009, 24, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Avlund, K.; Schultz-Larsen, K.; Christiansen, N.; Holm-Pedersen, P. Number of Teeth and Fatigue in Older Adults. J. Am. Geriatr. Soc. 2011, 59, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Elia, M. The Malnutrition Advisory Group consensus guidelines for the detection and management of malnutrition in the community. Nutr. Bull. 2001, 26, 81–83. [Google Scholar] [CrossRef]
- Robien, K.; Demark-Wahnefried, W.; Rock, C.L. Evidence-Based Nutrition Guidelines for Cancer Survivors: Current Guidelines, Knowledge Gaps, and Future Research Directions. J. Am. Diet. Assoc. 2011, 111, 368–375. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA A Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2013, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the Association of Diet and Persistent Cancer-Related Fatigue: A Pilot Study. Oncol. Nurs. Forum 2013, 40, E41–E49. [Google Scholar] [CrossRef]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Q.; Kang, X.; Song, Y.; Zhao, W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: A cross-sectional study. BMC Cancer 2010, 10, 453. [Google Scholar] [CrossRef]
- Kenkhuis, M.F.; van Duinjoven, F.J.B.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Bruekink, S.O.; Janssen-Heijen, M.L.; Keulen, E.T.P.; Mols, F.; Weijenber, M.P.; Bours, M.J.L. Longitudinal associations of fiber, vegetable, and fruit intake with quality of life and fatigue in colorectal cancer survivors up to 24 months post-treatment. Am. J. Clin. Nutr. 2022, 3, 822–832. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Romaguera, D.; Mitrou, P.; Reedy, J.; Bender, A.; Brockton, N.T. Further Guidance in Implementing the Standardized 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Score. Cancer Epidemiol. Biomark. Prev. 2020, 29, 889–894. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. Available online: www.DietaryGuidelines.gov (accessed on 24 February 2022).
- American Diabetes Association. Available online: https://www.diabetesfoodhub.org/articles/what-is-the-diabetes-plate-method.html#:~:text=The%20Diabetes%20Plate%20Method%20is,you%20need%20is%20a%20plate! (accessed on 24 February 2022).
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Robb, S.W.; Hébert, J.R.; Huang, H.; Ebell, M.H. Greater adherence to a Mediterranean diet is associated with lower prevalence of colorectal adenomas in men of all races. Nutr. Res. 2017, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- A Whalen, K.; McCullough, M.L.; Flanders, W.D.; Hartman, T.J.; Judd, S.; Bostick, R.M. Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with Biomarkers of Inflammation and Oxidative Balance in Adults. J. Nutr. 2016, 146, 1217–1226. [Google Scholar] [CrossRef]
- Masino, S.A.; Ruskin, D.N. Ketogenic Diets and Pain. J. Child Neurol. 2013, 28, 993–1001. [Google Scholar] [CrossRef]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Soleymani, T.; Gower, B.A. Favorable Effects of a Ketogenic Diet on Physical Function, Perceived Energy, and Food Cravings in Women with Ovarian or Endometrial Cancer: A Randomized, Controlled Trial. Nutrients 2018, 10, 1187. [Google Scholar] [CrossRef]
- Harmon, B.E.; Carter, M.; Hurley, T.G.; Shivappa, N.; Teas, J.; Hebert, J.R. Nutrient Composition and Anti-inflammatory Potential of a Prescribed Macrobiotic Diet. Nutr. Cancer 2015, 67, 933–940. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.-H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the gut microbiome and fatigue in cancer patients. Sci. Rep. 2021, 11, 5847. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Breedveld-Peters, J.J.L.; Koole, J.L.; Müller-Schulte, E.; van der Linden, B.W.A.; Windhausen, C.; Bours, M.J.L.; van Roekel, E.H.; Weijenberg, M.P. Colorectal cancers survivors’ adherence to lifestyle recommendations and cross-sectional associations with health-related quality of life. Br. J. Nutr. 2018, 120, 188–197. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. [Google Scholar] [CrossRef]
- Lei, Y.-Y.; Ho, S.C.; Cheng, A.; Kwok, C.; Lee, C.-K.I.; Cheung, K.L.; Lee, R.; Loong, H.H.; He, Y.-Q.; Yeo, W. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Guideline Is Associated with Better Health-Related Quality of Life among Chinese Patients with Breast Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 275–285. [Google Scholar] [CrossRef]
- Baguley, B.J.; Skinner, T.L.; Wright, O. Nutrition therapy for the management of cancer-related fatigue and quality of life: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef]
- Zainordin, N.H.; Talib, R.A.; Shahril, M.R.; Sulaiman, S.; Karim, N.A. Dietary Changes and Its Impact on Quality of Life among Malay Breast and Gynaecological Cancer Survivors in Malaysia. Asian Pac. J. Cancer Prev. 2020, 21, 3689–3696. [Google Scholar] [CrossRef]
- Song, S.; Youn, J.; Lee, Y.J.; Kang, M.; Hyun, T.; Song, Y.; Lee, J.E. Dietary supplement use among cancer survivors and the general population: A nation-wide cross-sectional study. BMC Cancer 2017, 17, 891. [Google Scholar] [CrossRef] [PubMed]
- Koole, J.L.; Bours, M.J.L.; Geijsen, A.J.M.R.; Gigic, B.; Ulvik, A.; E Kok, D.; Brezina, S.; Ose, J.; Baierl, A.; Böhm, J.; et al. Circulating B-vitamin biomarkers and B-vitamin supplement use in relation to quality of life in patients with colorectal cancer: Results from the FOCUS consortium. Am. J. Clin. Nutr. 2021, 113, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Koole, J.L.; Bours, M.J.; Breedveld-Peters, J.J.; van Roekel, E.H.; Breukink, S.O.; Janssen-Heijnen, M.L.; Vogelaar, F.J.; Aquarius, M.; Keulen, E.; Stoot, J.; et al. Is dietary supplement use longitudinally associated with fatigue in stage I–III colorectal cancer survivors? Clin. Nutr. 2020, 39, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, A.S.; Culakova, E.; Kleckner, I.R.; Belcher, E.K.; Demark-Wahnefried, W.; Parker, E.A.; Padula, G.D.A.; Ontko, M.; Janelsins, M.C.; Mustian, K.M.; et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients 2021, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Lapidari, P.; Djehal, N.; Havas, J.; Gbenou, A.; Martin, E.; Charles, C.; Dauchy, S.; Pistilli, B.; Cadeau, C.; Bertaut, A.; et al. Determinants of use of oral complementary-alternative medicine among women with early breast cancer: A focus on cancer-related fatigue. Breast Cancer Res. Treat. 2021, 190, 517–529. [Google Scholar] [CrossRef]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Edmands, W.M.; Ferrari, P.; Rothwell, J.; Rinaldi, S.; Slimani, N.; Barupal, D.; Biessy, C.; Jenab, M.; Clavel-Chapelon, F.; Fagherazzi, G.; et al. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am. J. Clin. Nutr. 2015, 102, 905–913. [Google Scholar] [CrossRef]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Huang, W.-Y.; Xiong, X.; Freedman, N.D.; Cross, A.J.; et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef]
- Playdon, M.C.; Sampson, J.N.; Cross, A.J.; Sinha, R.; Guertin, K.; A Moy, K.; Rothman, N.; Irwin, M.L.; Mayne, S.T.; Stolzenberg-Solomon, R.; et al. Comparing metabolite profiles of habitual diet in serum and urine. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; O Dragsted, L.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Ferrari, P.; Carayol, M.; Achaintre, D.; Scalbert, A.; Cross, A.J.; Gunter, M.J.; Fensom, G.K.; Appleby, P.N.; et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015, 102, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Jennings, A.; Mohney, R.P.; Yarand, D.; Mangino, M.; Cassidy, A.; MacGregor, A.; Spector, T.D.; Menni, C. Characterizing Blood Metabolomics Profiles Associated with Self-Reported Food Intakes in Female Twins. PLoS ONE 2016, 11, e0158568. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Ziegler, R.G.; Sampson, J.N.; Stolzenberg-Solomon, R.; Thompson, H.J.; Irwin, M.L.; Mayne, S.T.; Hoover, R.N.; Moore, S. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017, 106, 637–649. [Google Scholar] [CrossRef]
- McGee, E.E.; Kiblawi, R.; Playdon, M.C.; Eliassen, A.H. Nutritional Metabolomics in Cancer Epidemiology: Current Trends, Challenges, and Future Directions. Curr. Nutr. Rep. 2019, 8, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bao, W.; Liu, B.; Caan, B.J.; Lane, D.S.; Millen, A.E.; Simon, M.S.; Thomson, C.A.; Tinker, L.F.; Van Horn, L.V.; et al. Changes in Overall Diet Quality in Relation to Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. J. Acad. Nutr. Diet. 2018, 118, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Trudel-Fitzgerald, C.; Tworoger, S.S.; Poole, E.M.; Zhang, X.; Giovannucci, E.L.; Meyerhardt, J.A.; Kubzansky, L.D. Psychological symptoms and subsequent healthy lifestyle after a colorectal cancer diagnosis. Health Psychol. 2018, 37, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Lampe, J.W.; Tinker, L.F.; Prentice, R.; Hayden, K.M.; Van Horn, L.; Sampson, J.; Stolzenberg-Solomon, R.; Moore, S.C. Objective biomarkers of usual diet: A metabolomics analysis of weighed food intake. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—Critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Brennan, L.; Drevon, C.A.; van Kranen, H.; Manach, C.; Dragsted, L.O.; Roche, H.M.; Andres-Lacueva, C.; Bakker, S.J.L.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the Food Biomarker Alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Gao, Q.; Praticò, G.; Manach, C.; Wishart, D.S.; Scalbert, A.; Feskens, E.J.M. Dietary and health biomarkers—Time for an update. Genes Nutr. 2017, 12, 24. [Google Scholar] [CrossRef]
- Ulaszewska, M.; Garcia-Aloy, M.; Vázquez-Manjarrez, N.; Soria-Florido, M.T.; Llorach, R.; Mattivi, F.; Manach, C. Food intake biomarkers for berries and grapes. Genes Nutr. 2020, 15, 17. [Google Scholar] [CrossRef]
- Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; Manach, C. Biomarkers of intake for tropical fruits. Genes Nutr. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Brandl, B.; Buso, M.E.C.; Skurk, T.; Manach, C. Food intake biomarkers for green leafy vegetables, bulb vegetables, and stem vegetables: A review. Genes Nutr. 2020, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Brandl, B.; Lee, Y.-M.; Hauner, H.; Daniel, H.; Skurk, T. Specificity, Dose Dependency, and Kinetics of Markers of Chicken and Beef Intake Using Targeted Quantitative LC-MS/MS: A Human Intervention Trial. Mol. Nutr. Food Res. 2020, 64, e1900921. [Google Scholar] [CrossRef]
- Ulaszewska, M.; Vázquez-Manjarrez, N.; Garcia-Aloy, M.; Llorach, R.; Mattivi, F.; Dragsted, L.O.; Praticò, G.; Manach, C. Food intake biomarkers for apple, pear, and stone fruit. Genes Nutr. 2018, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Subar, A.F.; George, S.M.; Krebs-Smith, S.M. Extending Methods in Dietary Patterns Research. Nutrients 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Guenther, P.M.; Kirkpatrick, S.; Reedy, J.; Krebs-Smith, S.M.; Buckman, D.W.; Dodd, K.W.; Casavale, K.O.; Carroll, R.J. The Healthy Eating Index-2010 Is a Valid and Reliable Measure of Diet Quality According to the 2010 Dietary Guidelines for Americans. J. Nutr. 2014, 144, 399–407. [Google Scholar] [CrossRef]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.B.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.J.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet Quality Indices and Postmenopausal Breast Cancer Survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2015, 82, 163–173. [Google Scholar] [CrossRef]
- Abung, F.K.; Giovannucci, E.L.; Giulianini, F.; Liang, L.; Chandler, P.; Balasubramanian, R.; E Manson, J.; Feliciano, E.M.C.; Hayden, K.M.; Van Horn, L.; et al. An Empirical Dietary Inflammatory Pattern Score Is Associated with Circulating Inflammatory Biomarkers in a Multi-Ethnic Population of Postmenopausal Women in the United States. J. Nutr. 2018, 148, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Tabung, F.K.; Pernar, C.H.; Wang, W.; Gonzalez-Feliciano, A.G.; Chowdhury-Paulino, I.M.; Clinton, S.K.; Folefac, E.; Song, M.; Kibel, A.S.; et al. Insulinemic and Inflammatory Dietary Patterns and Risk of Prostate Cancer. Eur. Urol. 2021, 79, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Wang, W.; Fung, T.T.; Hu, F.B.; Smith-Warner, S.A.; Chavarro, J.E.; Fuchs, C.S.; Willett, W.C.; Giovannucci, E.L. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br. J. Nutr. 2016, 116, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Pritlove, C.; Capone, G.; Kita, H.; Gladman, S.; Maganti, M.; Jones, J.M. Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors. Nutrients 2020, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowder, S.L.; Playdon, M.C.; Gudenkauf, L.M.; Ose, J.; Gigic, B.; Greathouse, L.; Peoples, A.R.; Sleight, A.G.; Jim, H.S.L.; Figueiredo, J.C. A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities. Nutrients 2022, 14, 1496. https://doi.org/10.3390/nu14071496
Crowder SL, Playdon MC, Gudenkauf LM, Ose J, Gigic B, Greathouse L, Peoples AR, Sleight AG, Jim HSL, Figueiredo JC. A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities. Nutrients. 2022; 14(7):1496. https://doi.org/10.3390/nu14071496
Chicago/Turabian StyleCrowder, Sylvia L., Mary C. Playdon, Lisa M. Gudenkauf, Jennifer Ose, Biljana Gigic, Leigh Greathouse, Anita R. Peoples, Alix G. Sleight, Heather S. L. Jim, and Jane C. Figueiredo. 2022. "A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities" Nutrients 14, no. 7: 1496. https://doi.org/10.3390/nu14071496
APA StyleCrowder, S. L., Playdon, M. C., Gudenkauf, L. M., Ose, J., Gigic, B., Greathouse, L., Peoples, A. R., Sleight, A. G., Jim, H. S. L., & Figueiredo, J. C. (2022). A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities. Nutrients, 14(7), 1496. https://doi.org/10.3390/nu14071496






