High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects—Yes or No?
Abstract
:1. Introduction
2. Vitamin C and Its Mechanisms of Immunomodulation
2.1. Vitamin C and Leukocyte Function
2.2. Immunomodulation and Vitamin C as an Enzyme Cofactor
2.3. Immunomodulation and Vitamin C as a Regulator of Gene Expression
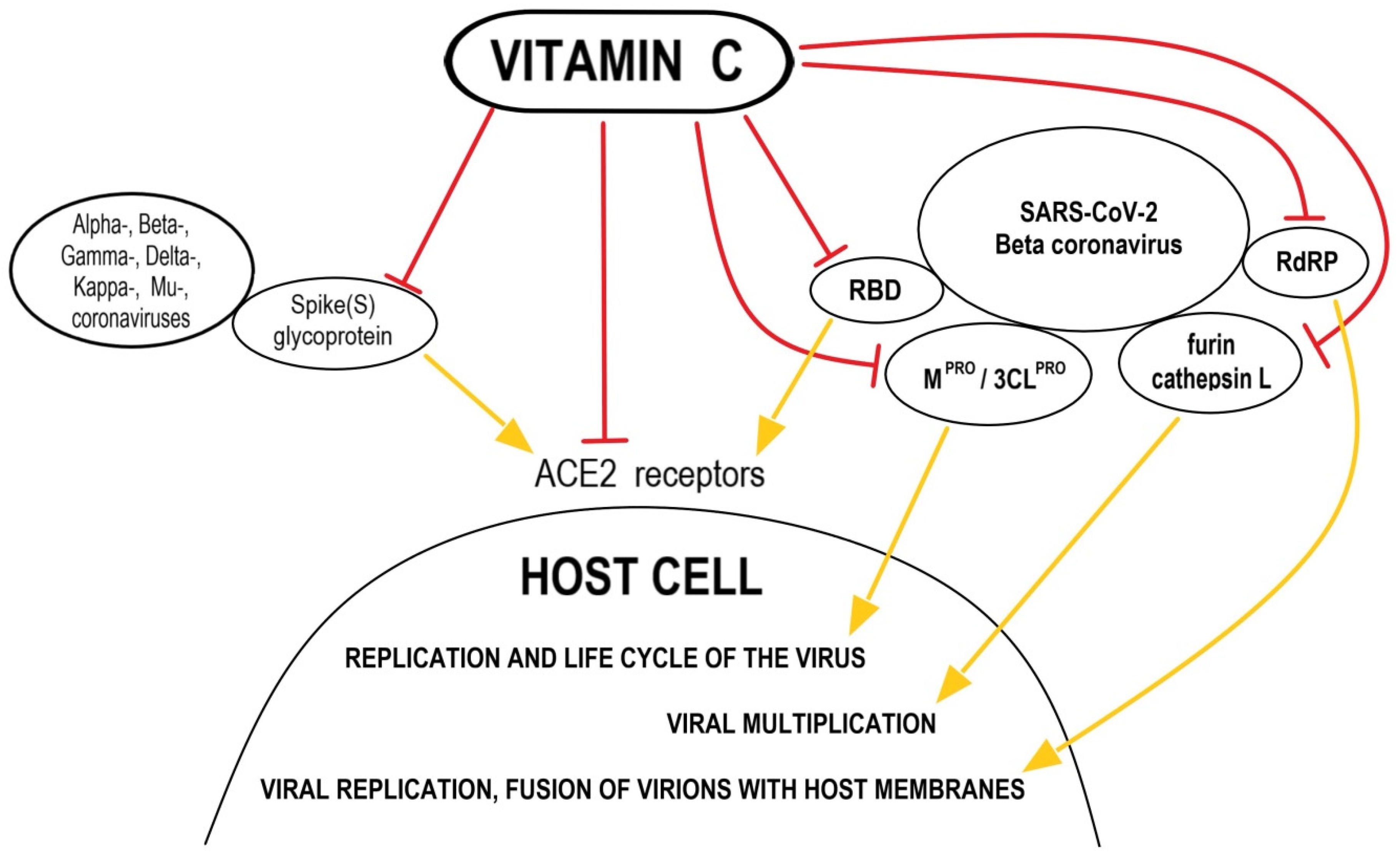
3. Supportive Supplementation—Does High-Dose Vitamin C as a Dietary Supplement Used in Prophylaxis for Anti-SARS-CoV-2 in Healthy Subjects Make Sense?
4. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Dosedĕl, M.; Jirkovský, E.; Macáková, K.; Kujovská Krćmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, L.; Magdalena, R.; Barahona, M.J.; Ramírez, E.; Sanzana, C.; Gutiérrez, J.; Nualart, F. Two distinct faces of vitamin C: AA vs. DHA. Antioxidants 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Rowe, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler III, A.A. The emerging role of vitamin C as a treatment for sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [Green Version]
- Rosetti, C.A.; Real, J.P.; Palma, S.D. High dose of ascorbic acid used in Sars Covid-19 treatment: Scientific and clinical support for its therapeutic implementation. Ars. Pharm. 2020, 61, 145–148. [Google Scholar]
- Canali, R.; Natarelli, L.; Leoni, G.; Azzini, E.; Comitato, R.; Sancak, O.; Barella, L.; Virgili, F. Vitamin C supplementation modulates gene expression in peripheral blood mononuclear cells specifically upon an inflammatory stimulus: A pilot study in healthy subjects. Genes Nutr. 2014, 9, 390. [Google Scholar] [CrossRef] [Green Version]
- Earar, K.; Arbune, M.; Dorobat, C.M.; Rusu-Negraia, M.; Stefanescu, V.; Schipor, O.; Harabor, V.R.; Harabor, A.; Bratu, A.M. Biochemical effects and therapeutic application of vitamin C (C6H8O6) on COVID-19 infection. Rev. Chim. 2020, 71, 473–478. [Google Scholar] [CrossRef]
- Chaghouri, P.; Maalouf, N.; Peters, S.L.; Nowak, P.J.; Peczek, K.; Zasowska-Nowak, A.; Nowicki, M. Two faces of vitamin C in hemodialysis patients: Relation to oxidative stress and inflammation. Nutrients 2021, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Fumeron, C.; Nguyen-Khoa, T.; Saltiel, C.; Kebede, M.; Buisson, C.; Drüeke, T.; Lacour, B.; Massy, Z.A. Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol. Dial. Transplant. 2005, 20, 1874–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, W.H.; Rhea, E.M.; Qu, Z.C.; Hecker, M.R.; May, J.M. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am. J. Physiol. Cell Physiol. 2016, 311, C652–C662. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef] [Green Version]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic acid: Its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef]
- Furuya, A.; Uozaki, M.; Yamasaki, H.; Arakawa, T.; Arita, M.; Koyama, A.H. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int. J. Mol. Med. 2008, 22, 541–545. [Google Scholar]
- Kumar, V.; Kancharla, S.; Kumar Jena, M. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-10. VirusDisease 2021, 32, 29–37. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Ivanov, V.; Ivanova, S.; Rath, M. Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants. Eur. J. Microbiol. Immunol. 2021, 4, 87–94. [Google Scholar] [CrossRef]
- Ivanov, V.; Goc, A.; Ivanova, S.; Niedzwiecki, A.; Rath, M. Inhibition of ACE2 expression by ascorbic acid alone and its combinations with other natural compounds. Inf. Dis. Res. Treat. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Huynh, Q.K.; Wijesinghe, D.S.; Chalfant, C.E.; Brophy, D.F.; Fowler, A.A., III; Natarajan, R. Resolution of sterile inflammation: Role of vitamin C. Mediators Inflamm. 2014, 2014, 173403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C.M. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold Kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C. Vitamin C in pneumonia and sepsis. In Vitamin C. New Biochemical and Functional Insights, 1st ed.; Chen, Q., Vissers, M.C.M., Eds.; CRC Press: Boca Raton, FL, USA; New York, NY, USA; London, UK, 2020; pp. 115–135. [Google Scholar]
- Bozonet, S.M.; Carr, A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, A.L.; Villamor, E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 2007, 65, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Theron, A. Effects of ascorbate on leucocytes: Part III. In vitro and in vivo stimulation of abnormal neutrophil motility by ascorbate. S. Afr. Med. J. 1979, 56, 429–433. [Google Scholar]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3150. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Fan, J. Neutrophil in reverse migration: Role in sepsis. Front. Immunol. 2021, 12, 625. [Google Scholar] [CrossRef]
- Sharma, P.; Raghavan, S.A.V.; Saini, R.; Dikshit, M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc. Biol. 2004, 75, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Ferrón-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.-M.; Bueno, P.; Ferrón, J.-A. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 2009, 153, 224–230. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 610–616. [Google Scholar] [CrossRef]
- Nytko, K.J.; Maeda, N.; Schläfli, P.; Spielmann, P.; Wenger, R.H.; Stiehl, D.P. Vitamin C is dispensable for oxygen sensing in vivo. Blood 2011, 117, 5485–5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elks, P.M.; van Eeden, F.J.; Dixon, G.; Wang, X.; Reyes-Aldasoro, C.C.; Ingham, P.W.; Whyte, M.K.B.; Walmsley, S.R.; Renshaw, S.A. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Visseres, M.C.M. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: Unique or ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Guz, J.; Oliński, R. The role of vitamin C in epigenetic regulation. Post. Hig. Med. Dosw. 2017, 71, 747–760. [Google Scholar] [CrossRef]
- Härtel, C.; Strunk, T.; Bucsky, P.; Schultz, C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine 2004, 27, 101–106. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żychowska, M.; Grzybkowska, A.; Zasada, M.; Piotrowska, A.; Dworakowska, D.; Czerwińska-Ledwig, O.; Pilch, W.; Antosiewicz, J. Effect of six weeks 1000 mg/day vitamin C supplementation and healthy training in elderly women on genes expression associated with the immune reponse—a randomized controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C supplementation and the common cold-was Linus Pauling right or wrong? Int. J. Vitam. Nutr. Res. 1997, 67, 329–335. [Google Scholar] [PubMed]
- Wise, J. COVID-19: Evidence is lacking to support vitamin D’s role in treatment and prevention. BMJ 2020, 371, m4912. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2021, 32, e2261. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Forouhi, N.G.; Khunti, K. Vitamin D and covid-10. BMJ 2021, 372, n544. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Awano, N.; Fukui, T.; Sasaki, H.; Kyuwa, S. The protective effects of lactoferrin against murine norovirus infection through inhibition of both viral attachment and replication. Biochem. Biophys. Res. Commun. 2013, 434, 791–796. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Medhar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Menshawey, E.; Menshawey, R.; Nabeh, O.A. Shedding light on vitamin D: The shared mechanistic and pathophysiological role between hypovitaminosis D and COVID-19 risk factors and complications. Inflammopharmacology 2021, 29, 1017–1031. [Google Scholar] [CrossRef]
- BourBour, F.; Dahka, S.M.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch. Physiol. Biochem. 2020. [Google Scholar] [CrossRef]
- Lorente, J.A.; Nin, N.; Villa, P.; Vasco, D.; Miguel-Coello, A.B.; Rodriguez, I.; Herrero, R.; Peñuelas, O.; Ruiz-Cabello, J.; Izquierdo-Garcia, J.L. Metabolomic differences between COVID-19 and H1N1 influenza induced ARDS. Crit. Care 2021, 25, 390. [Google Scholar] [CrossRef]
- Feyaerts, A.F.; Luyten, W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-10? Nutrition 2020, 79–80, 110948. [Google Scholar] [CrossRef]
- Hoang, B.X.; Shaw, G.; Fang, W.; Han, B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J. Glob. Antimicrob. Resist. 2020, 23, 256–262. [Google Scholar] [CrossRef]
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H.; de Man, A.M.E. Vitamin C and COVID-19. Front. Med. 2021, 7, 559811. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin C in the treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American Community Hospital Intensive Care Unit in May 2020: A pilot study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef]
- Cheng, R.Z.; Kogan, M.; Davis, D. Ascorbate as prophylaxis and therapy for COVID-19—Update from Shanghai and U.S. medical institutions. Global Adv. Health Med. 2020, 9. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2020, 61, 742–755. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3428. [Google Scholar]
- German Nutrition Society (DGE). New reference values for vitamin C intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Kim, T.K.; Lim, H.R.; Byun, J.S. Vitamin C supplementation reduces the odds of developing a common cold in Republic of Korea Army recruits: Randomised controlled trial. Br. Med. J. Mil. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Commentary: The long history of vitamin C: From prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2021, 12, 659001. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowances. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimer, K.A.; Hart, A.M.; Martin, L.G.; Rubio-Wallace, S. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J. Am. Acad. Nurse Pract. 2009, 21, 295–300. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber-Bzura, B.M. High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects—Yes or No? Nutrients 2022, 14, 979. https://doi.org/10.3390/nu14050979
Gruber-Bzura BM. High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects—Yes or No? Nutrients. 2022; 14(5):979. https://doi.org/10.3390/nu14050979
Chicago/Turabian StyleGruber-Bzura, Beata M. 2022. "High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects—Yes or No?" Nutrients 14, no. 5: 979. https://doi.org/10.3390/nu14050979
APA StyleGruber-Bzura, B. M. (2022). High-Dose Vitamin C Supplementation as a Legitimate Anti-SARS-CoV-2 Prophylaxis in Healthy Subjects—Yes or No? Nutrients, 14(5), 979. https://doi.org/10.3390/nu14050979




