Understanding Sex Differences in Childhood Undernutrition: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results: Potential Explanatory Factors for Sex Differences
3.1. Maternal and Newborn Factors
3.2. Endocrine/Immune Factors
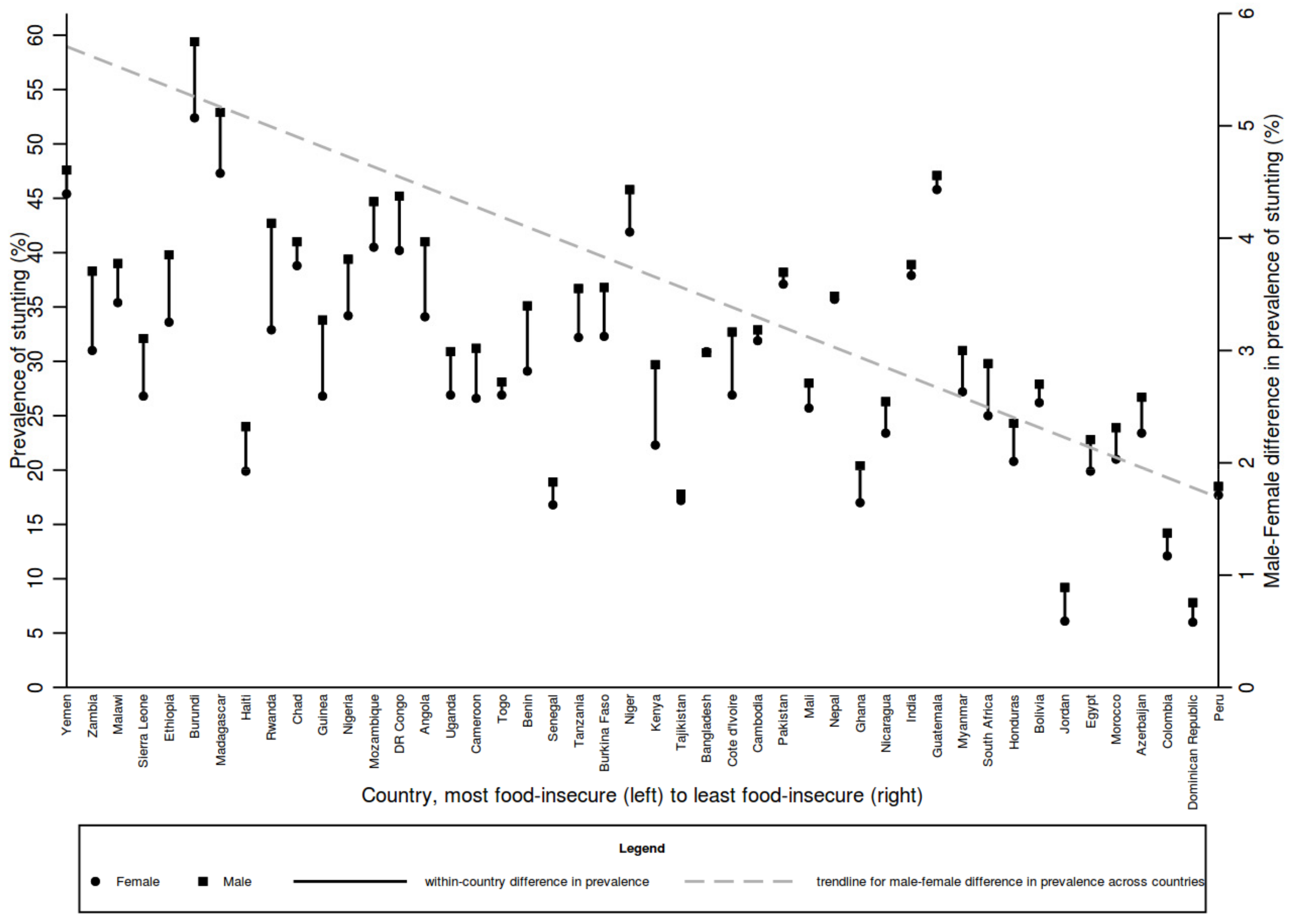
3.3. Age
3.4. Evolutionary Explanations
3.5. Infant and Young Child Feeding and Care Practices
3.6. Sex and Socioeconomic Status
3.7. Gender Perceptions
3.8. Indicators of Undernutrition
4. Possible Implications of Sex Differences for Undernutrition Programming and Policy
4.1. Sex Differences in Treatment Outcomes and Mortality Implications
4.2. The Policy Environment
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurstans, S.; Opondo, C.; Seal, A.; Wells, J.; Khara, T.; Dolan, C.; Briend, A.; Myatt, M.; Garenne, M.; Sear, R.; et al. Boys are more likely to be undernourished than girls: A systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob. Health 2020, 5, e004030. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.; Benjamin-Chung, J.; Colford, J.M., Jr.; Coyle, J.; van der Laan, M.J.; Hubbard, E.; Dayal, S.; Malenica, I.; Hejazi, N.; Sofrygin, O.; et al. Causes and consequences of child growth failure in low- and middle-income countries. MedRxiv 2020. [Google Scholar] [CrossRef]
- Khara, T.; Mwangome, M.; Ngari, M.; Dolan, C. Children concurrently wasted and stunted: A meta-analysis of prevalence data of children 6–59 months from 84 countries. Matern. Child Nutr. 2017, 14, e12516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng-Amoako, G.A.O.; Sunday Karamagi, C.A.; Nangendo, J.; Okiring, J.; Kiirya, Y.; Aryeetey, R.; Mupere, E.; Myatt, M.; Briend, A.; Kalyango, J.N.; et al. Factors associated with concurrent wasting and stunting among children 6-59 months in Karamoja, Uganda. Matern. Child Nutr. 2020, 17, e13074. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Schoenbuchner, S.; Pietzsch, S.; Dolan, C.; Lelijveld, N.; Briend, A. Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch. Public Health 2018, 76, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Obeng-Amoako, G.A.O.; Myatt, M.; Conkle, J.; Muwaga, B.K.; Aryeetey, R.; Okwi, A.L.; Okullo, I.; Mupere, E.; Wamani, H.; Briend, A.; et al. Concurrently wasted and stunted children 6-59 months in Karamoja, Uganda: Prevalence and case detection. Matern. Child Nutr. 2020, 16, e13000. [Google Scholar] [CrossRef] [Green Version]
- Garenne, M.; Thurstans, S.; Briend, A.; Dolan, C.; Khara, T.; Myatt, M.; Seal, A.; Wells, J.C. Changing sex differences in undernutrition of African children: Findings from Demographic and Health Surveys. J. Biosoc. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Isanaka, S.; Hitchings, M.D.; Berthé, F.; Briend, A.; Grais, R.F. Linear growth faltering and the role of weight attainment: Prospective analysis of young children recovering from severe wasting in Niger. Matern. Child Nutr. 2019, 15, e12817. [Google Scholar] [CrossRef]
- Imam, A.; Hassan-Hanga, F.; Sallahdeen, A.; Farouket, Z.L. A cross-sectional study of prevalence and risk factors for stunting among under-fives attending acute malnutrition treatment programmes in north-western Nigeria: Should these programmes be adapted to also manage stunting? Int. Health 2020, 13, 262–271. [Google Scholar] [CrossRef]
- Obeng-Amoako, G.A.O.; Wamani, H.; Conkle, J.; Aryeetey, R.; Nangendo, J.; Mupere, E.; Kalyango, J.N.; Myatt, M.; Briend, A.; Karamagi, C.A.S. Concurrently wasted and stunted 6–59 months children admitted to the outpatient therapeutic feeding programme in Karamoja, Uganda: Prevalence, characteristics, treatment outcomes and response. PLoS ONE. 2020, 15, e0230480. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Rosati, A.; Sarti, R.D.; Cruciani, L.; Cutuli, A.M. Does fetal sex affect pregnancy outcome? Gend. Med. 2007, 4, 19–30. [Google Scholar] [CrossRef]
- Broere-Brown, Z.A.; Adank, M.C.; Benschop, L.; Tielemans, M.; Muka, T.; Gonçalves, R.; Bramer, W.M.; Schoufour, J.D.; Voortman, T.; Steegers, E.A.P.; et al. Fetal sex and maternal pregnancy outcomes: A systematic review and meta-analysis. Biol. Sex Differ. 2020, 11, 26. [Google Scholar] [CrossRef]
- Marino, M.; Masella, R.; Bulzomi, P.; Campesi, I.; Malorni, W.; Franconi, F. Nutrition and human health from a sex–gender perspective. Mol. Asp. Med. 2011, 32, 1–70. [Google Scholar] [CrossRef]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 34–38. [Google Scholar] [CrossRef]
- Townsel, C.D.; Emmer, S.F.; Campbell, W.A.; Hussain, N. Gender Differences in Respiratory Morbidity and Mortality of Preterm Neonates. Front. Pediatr. 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Hutt, C. Sex Differences in Human Development. Hum. Dev. 1972, 15, 153–170. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. The Risk of Maternal Nutritional Depletion and Poor Outcomes Increases in Early or Closely Spaced Pregnancies. J. Nutr. 2003, 133, 1732S–1736S. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Ahmed, T.; Black, R.E.; Cousens, S.; Dewey, K.G.; Giugliani, E.R.J.; Haider, B.A.; Kirkwood, B.R.; Morris, S.S.; Sachdev, H.P.S.; et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008, 371, 417–440. [Google Scholar] [CrossRef]
- GNR. 2020 Global Nutrition Report: Action on Equity to End Malnutrition; Development Initiatives: Bristol, UK, 2020. [Google Scholar]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haig, D. Genetic Conflicts in Human Pregnancy. Q. Rev. Biol. 1993, 68, 495–532. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Natural selection and sex differences in morbidity and mortality in early life. J. Theor. Biol. 2000, 202, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, J.F. Ultrasound evidence of sexual difference in fetal size in first trimester. Br. Med. J. 1980, 281, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J. Boys live dangerously in the womb. Am. J. Hum. Biol. 2009, 22, 330–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, J.O.; De Paredes, B.; Wagner, M.; De Navarro, L.; Suescun, J.; Christiansen, N.; Herrera, M.G. Nutritional supplementation and the outcome of pregnancy. I. Birth weight. Am. J. Clin. Nutr. 1979, 32, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macaulay, S.; Munthali, R.J.; Dunger, D.B.; Norris, S.A. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabet. Med. 2018, 35, 1425–1433. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Prioreschi, A.; Kehoe, S.H.; Ward, K.A.; Norris, S.A. A maternal “mixed, high sugar” dietary pattern is associated with fetal growth. Matern. Child Nutr. 2020, 16, e12912. [Google Scholar] [CrossRef] [Green Version]
- Prioreschi, A.; Wrottesley, S.V.; Said-Mohamed, R.; Nyati, L.; Newell, M.-L.; Norris, S.A. Understanding how maternal social and biological factors are related to fetal growth in an urban South African cohort. J. Dev. Orig. Health Dis. 2020, 12, 79–87. [Google Scholar] [CrossRef]
- Saville, N.M.; Harris-Fry, H.; Marphatia, A.; Reid, A.; Cortina-Borja, M.; Manandhar, D.S.; Wells, J.C. Differences in maternal and early child nutritional status by offspring sex in lowland Nepal. Am. J. Hum. Biol. 2021, e23637. [Google Scholar] [CrossRef]
- Amosu, A.M.; Atulomah, N.O.; Olanrewaju, M.F.; Akintunde, T.I.; Babalola, A.O.; Akinnuga, A.M.; Ojezele, M.O. Retrospective study of some factors influencing delivery of low birth weight babies in Ibadan, Oyo state, Nigeria. Sci. Res. Essays 2011, 6, 236–240. [Google Scholar]
- Vu, H.D.; Dickinson, C.; Kandasamy, Y. Sex Difference in Mortality for Premature and Low Birth Weight Neonates: A Systematic Review. Am. J. Perinatol. 2018, 35, 707–715. [Google Scholar]
- Navarro, A.D. Female eco-stability and severe malnutrition in children: Evidence from humanitarian aid interventions of Action Against Hunger in African, Asian and Latin American countries. Nutricion Clinica y Dietetica Hospitalaria 2018, 34, 127–134. [Google Scholar] [CrossRef]
- Tamini, R.M.; Lagiou, P.; Mucci, L.A.; Chung-Cheng Hsieh, C.C.; Hans-Olov Adami, H.O.; Trichopoulos, D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ 2003, 326, 1245–1246. [Google Scholar] [CrossRef] [Green Version]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Garenne, M.; Lafon, M. Sexist diseases. Perspect. Biol. Med. 1998, 41, 176–189. [Google Scholar] [CrossRef]
- Butterworth, M.; McClellan, B.; Aklansmith, M. Influence of Sex on Immunoglobulin Levels. Nature 1967, 214, 1224–1225. [Google Scholar] [CrossRef]
- Fish, E. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Tomkins, A.; Watson, F. Malnutrition and Infection. A review, Andrew Tomkins and Fiona Watson. United Nations Administrative Committee on Coordination/Sub Committee on Nutrition, 1989. 136 pp. Copies available from: Dr. J. B. Mason, Technical Secretary ACC/SCN, c/o WHO, 20 Avenue Appia, CH-1211 Geneva 27, Switzerland. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 446. [Google Scholar]
- Rodriguez-Morales, A.; Bolivar-Mejía, A.; Alarcón-Olave, C.; Calvo-Betancourt, L. Encyclopedia of Food and Health. Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 98–103. [Google Scholar] [CrossRef]
- Hack, M.B.; Schluchter, M.; Cartar, L.; Rahman, M.; Cuttler, L.; Borawski, E. Growth of Very Low Birth Weight Infants to Age 20 Years. Pediatrics 2003, 112, e30–e38. [Google Scholar] [CrossRef] [Green Version]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Garenne, M. Demographic evidence of sex differences in vulnerability to infectious diseases. J. Infect. Dis. 2015, 211, 331–332. [Google Scholar] [CrossRef] [Green Version]
- Garenne, M.; Myatt, M.; Khara, T.; Dolan, C.; Briend, A. Concurrent wasting and stunting among under-five children in Niakhar, Senegal. Matern. Child Nutr. 2019, 15, e12736. [Google Scholar] [CrossRef] [Green Version]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Lord, G. Role of Leptin in Immunology. Nutr. Rev. 2002, 60 (Suppl. 10), S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.; Petri, W.A., Jr. Leptin Regulation of Immune Responses. Trends Mol. Med. 2016, 22, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, D.; Leger, J.; Levy-Marchal, C.; Oury, J.F.; Czernichow, P. Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. J. Clin. Endocrinol. Metab. 1998, 83, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; ElZalabany, M.; Salama, M.; Ansari, B. Serum leptin concentrations during severe protein-energy malnutrition: Correlation with growth parameters and endocrine function. Metabolism 2000, 49, 819–825. [Google Scholar] [CrossRef]
- Bellone, S.; Rapa, A.; Petri, A.; Zavallone, A.; Strigini, L.; Chiorboli, E.; Ciardi, L.; Aguzzi, A.; Bona, G. Leptin levels as function of age, gender, auxological and hormonal parameters in 202 healthy neonates at birth and during the first month of life. J. Endocrinol. Investig. 2004, 27, 18–23. [Google Scholar] [CrossRef]
- Matsuda, J.; Yokota, I.; Iida, M.; Murakami, T.; Naito, E.; Ito, M.; Shima, K.; Kuroda, Y. Serum leptin concentration in cord blood: Relationship to birth weight and gender. J. Clin. Endocrinol. Metab. 1997, 82, 1642–1644. [Google Scholar] [CrossRef]
- Bartz, S.; Mody, A.; Hornik, C.; Bain, J.R.; Muehlbauer, M.; Kiyimba, T.; Kiboneka, E.; Stevens, R.; Bartlett, J.; Peter, J.S.; et al. Severe Acute Malnutrition in Childhood: Hormonal and Metabolic Status at Presentation, Response to Treatment, and Predictors of Mortality. J. Clin. Endocrinol. Metab. 2014, 99, 2128–2137. [Google Scholar] [CrossRef] [Green Version]
- Kerr, D.S.; Stevens, M.C.; Robinson, H.M. Fasting metabolism in infants. I. Effect of severe undernutrition on energy and protein utilization. Metabolism 1978, 27, 411–435. [Google Scholar]
- Adair, L.S.; Guilkey, D.K. Age-specific determinants of stunting in Filipino children. J. Nutr. 1997, 127, 314–320. [Google Scholar] [CrossRef]
- Bork, K.A.; Diallo, A. Boys Are More Stunted than Girls from Early Infancy to 3 Years of Age in Rural Senegal. J. Nutr. 2017, 147, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Moestue, H. Can anthropometry measure gender discrimination? An analysis using WHO standards to assess the growth of Bangladeshi children. Public Health Nutr. 2009, 12, 1085–1091. [Google Scholar]
- Costa, J.C.; Blumenberg, C.; Victora, C. Growth patterns by sex and age among under-5 children from 87 low-income and middle-income countries. BMJ Glob. Health 2021, 6, e007152. [Google Scholar] [CrossRef]
- Kraemer, S. The fragile male. Br. Med. J. 2000, 321, 1609–1612. [Google Scholar] [CrossRef]
- Xirocostas, Z.A.; Everingham, S.E.; Moles, A.T. The sex with the reduced sex chromosome dies earlier: A comparison across the tree of life. Biol. Lett. 2020, 16, 20190867. [Google Scholar] [CrossRef] [Green Version]
- Song, S. Does famine influence sex ratio at birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc. Biol. Sci. 2012, 279, 2883–2890. [Google Scholar]
- Cronk, L. Boy or girl: Gender preferences from a Darwinian point of view. Reprod. BioMed. Online 2007, 15, 23–32. [Google Scholar] [CrossRef]
- Gibson, M.A.; Mace, R. Strong mothers bear more sons in rural Ethiopia. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270 (Suppl. 1), S108–S109. [Google Scholar] [CrossRef] [Green Version]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- da Costa, T.H.; Haisma, H.; Wells, J.C.K.; Mander, A.P.; Whitehead, R.G.; Bluck, L.J.C. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J. Nutr. 2010, 140, 2227–2232. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E.; Knott, C.D.; Conklin-Brittain, N. Infant sex predicts breast milk energy content. Am. J. Hum. Biol. 2010, 22, 50–54. [Google Scholar] [CrossRef]
- Quinn, E.A. No evidence for sex biases in milk macronutrients, energy, or breastfeeding frequency in a sample of filipino mothers. Am. J. Phys. Anthropol. 2013, 152, 209–216. [Google Scholar] [CrossRef]
- Galante, L.; Milan, A.M.; Reynolds, C.M.; Cameron-Smith, D.; Vickers, M.H.; Pundir, S. Sex-Specific Human Milk Composition: The Role of Infant Sex in Determining Early Life Nutrition. Nutrients 2018, 10, 1194. [Google Scholar] [CrossRef] [Green Version]
- Libster, R.; Hortoneda, J.B.; Laham, F.R.; Casellas, J.M.; Israele, V.; Polack, N.R.; Delgado, M.F.; Klein, M.I.; Polack, F.P. Breastfeeding Prevents Severe Disease in Full Term Female Infants With Acute Respiratory Infection. Pediatr. Infect. Dis. J. 2009, 28, 131–134. [Google Scholar] [CrossRef]
- Tumilowicz, A.; Habicht, J.-P.; Pelto, G.; Pelletier, D.L. Gender perceptions predict sex differences in growth patterns of indigenous Guatemalan infants and young children. Am. J. Clin. Nutr. 2015, 102, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Wamani, H.; Åstrøm, A.N.; Peterson, S.; Tumwine, J.K.; Tylleskär, T. Boys are more stunted than girls in Sub-Saharan Africa: A meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Cruz, L.M.G.; Azpeitia, G.G.; Súarez, D.R.; Rodríguez, A.S.; Ferrer, J.F.L.; Serra-Majem, L. Factors Associated with Stunting among Children Aged 0 to 59 Months from the Central Region of Mozambique. Nutrients 2017, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Akombi, B.J.; Agho, K.E.; Hall, J.J.; Wali, N.; Renzaho, A.M.N.; Merom, D. Stunting, Wasting and Underweight in Sub-Saharan Africa: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espo, M.; Kulmala, T.; Maleta, K.; Cullinan, T.; Salin, M.-L.; Ashorn, P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2007, 91, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Gewa, C.A.; Yandell, N. Undernutrition among Kenyan children: Contribution of child, maternal and household factors. Public Health Nutr. 2012, 15, 1029–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Addressing Sex and Gender in Epidemic-Prone Infectious Diseases; WHO: Geneva, Switzerland, 2007.
- Garenne, M. Sex differences in health indicators among children in African DHS surveys. J. Biosoc. Sci. 2003, 35, 601–614. [Google Scholar] [CrossRef]
- Abhishek, S.; Patel, S.K. Gender differentials in feeding practices, health care utilization and nutritional status of children in Northern India. Int. J. Hum. Rights Healthc. 2017, 10, 323–331. [Google Scholar]
- Jayachandran, S.; Kuziemko, I. Why Do Mothers Breastfeed Girls Less than Boys? Evidence and Implications for Child Health in India. Quart. J. Econ. 2011, 126, 1485–1538. [Google Scholar] [CrossRef] [Green Version]
- Bairagi, R. Food crises and female children in rural Bangladesh. Soc. Sci. 1987, 72, 48–51. [Google Scholar]
- Zarulli, V.; Jones, J.B.; Oksuzyan, A.; Lindahl-Jacobsen, R.; Christensen, K.; Vaupel, J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. USA 2018, 115, E832–E840. [Google Scholar] [CrossRef] [Green Version]
- Mark Myatt, T.K.; Collins, S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr. Bull. 2006, 27 (Suppl. 3), S7–S23. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, N.; Myatt, M.; Allafort-Duverger, T.; Balogoun, A.; Ibrahim, A.; Briend, A. Mothers Understand And Can do it (MUAC): A comparison of mothers and community health workers determining mid-upper arm circumference in 103 children aged from 6 months to 5 years. Arch. Public Health 2015, 73, 26. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Grummer-Strawn, L.M. Standard deviation of anthropometric Z-scores as a data quality assessment tool using the 2006 WHO growth standards: A cross country analysis. Bull. World Health Org. 2007, 85, 441–448. [Google Scholar] [CrossRef]
- Tadesse, A.W.; Tadesse, E.; Berhane, Y.; Ekström, E.-C. Comparison of Mid-Upper Arm Circumference and Weight-for-Height to Diagnose Severe Acute Malnutrition: A Study in Southern Ethiopia. Nutrients 2017, 9, 267. [Google Scholar] [CrossRef]
- Rasmussen, J.; Andersen, A.; Fisker, A.B.; Ravn, H.; Sodemann, M.; Rodrigues, A.; Benn, C.S.; PAaby, P. Mid-upper-arm-circumference and mid-upper-arm circumference z-score: The best predictor of mortality? Eur. J. Clin. Nutr. 2012, 66, 998–1003. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Wessells, K.R.; Arnold, C.D.; Prado, E.L.; Abbeddou, S.; Adu-Afarwuah, S.; Ali, H.; Arnold, B.F.; Ashorn, P.; Ashorn, U.; et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child growth: An individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 15S–42S. [Google Scholar] [CrossRef]
- IASC. The Gender Handbook for Humanitarian Action; IASC: Geneva, Switzerland, 2017. [Google Scholar]
- Marphatia, A.A.; Cole, T.J.; Grijalva-Eternod, C.; Wells, J.C.K. Associations of gender inequality with child malnutrition and mortality across 96 countries. Glob. Health Epidemiol. Genom. 2016, 1, e6. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurstans, S.; Opondo, C.; Seal, A.; Wells, J.C.; Khara, T.; Dolan, C.; Briend, A.; Myatt, M.; Garenne, M.; Mertens, A.; et al. Understanding Sex Differences in Childhood Undernutrition: A Narrative Review. Nutrients 2022, 14, 948. https://doi.org/10.3390/nu14050948
Thurstans S, Opondo C, Seal A, Wells JC, Khara T, Dolan C, Briend A, Myatt M, Garenne M, Mertens A, et al. Understanding Sex Differences in Childhood Undernutrition: A Narrative Review. Nutrients. 2022; 14(5):948. https://doi.org/10.3390/nu14050948
Chicago/Turabian StyleThurstans, Susan, Charles Opondo, Andrew Seal, Jonathan C. Wells, Tanya Khara, Carmel Dolan, André Briend, Mark Myatt, Michel Garenne, Andrew Mertens, and et al. 2022. "Understanding Sex Differences in Childhood Undernutrition: A Narrative Review" Nutrients 14, no. 5: 948. https://doi.org/10.3390/nu14050948
APA StyleThurstans, S., Opondo, C., Seal, A., Wells, J. C., Khara, T., Dolan, C., Briend, A., Myatt, M., Garenne, M., Mertens, A., Sear, R., & Kerac, M. (2022). Understanding Sex Differences in Childhood Undernutrition: A Narrative Review. Nutrients, 14(5), 948. https://doi.org/10.3390/nu14050948






