Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications
Abstract
1. Introduction
2. Protein Quality
2.1. The Importance of Proteins and Amino Acids for Human Nutrition and Health
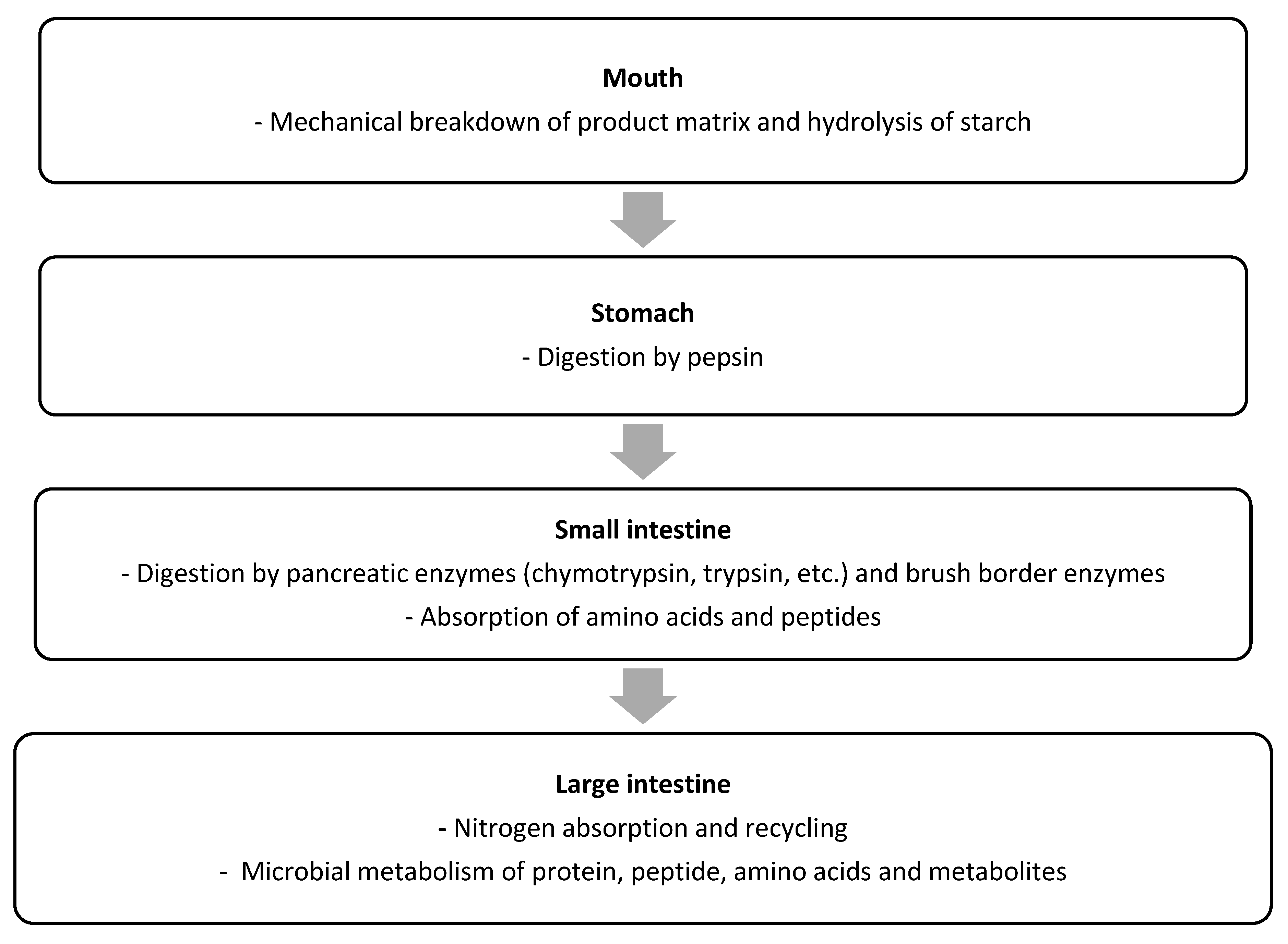
2.2. Protein Digestion and Absorption by Humans
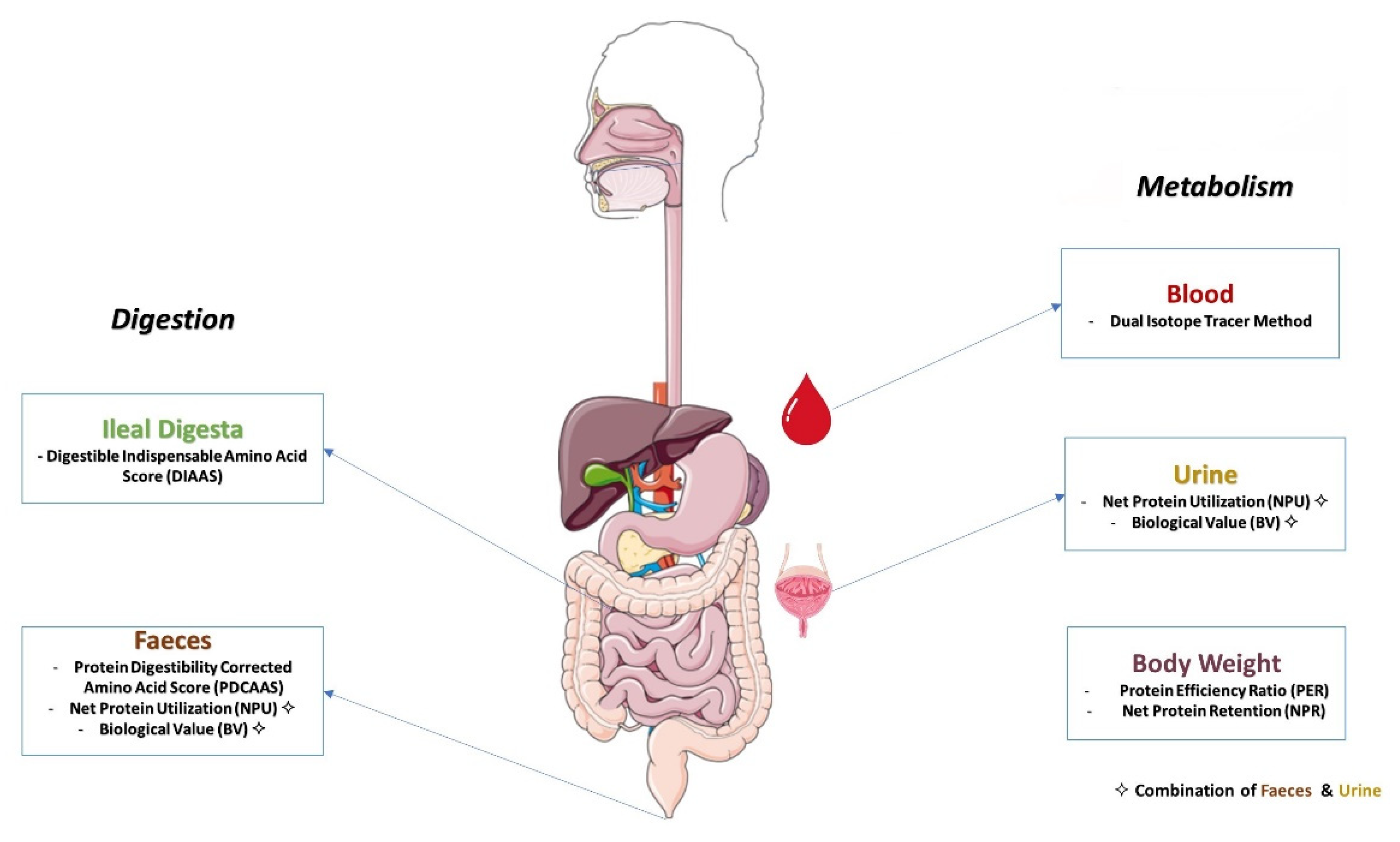
3. Protein Quality Measurement
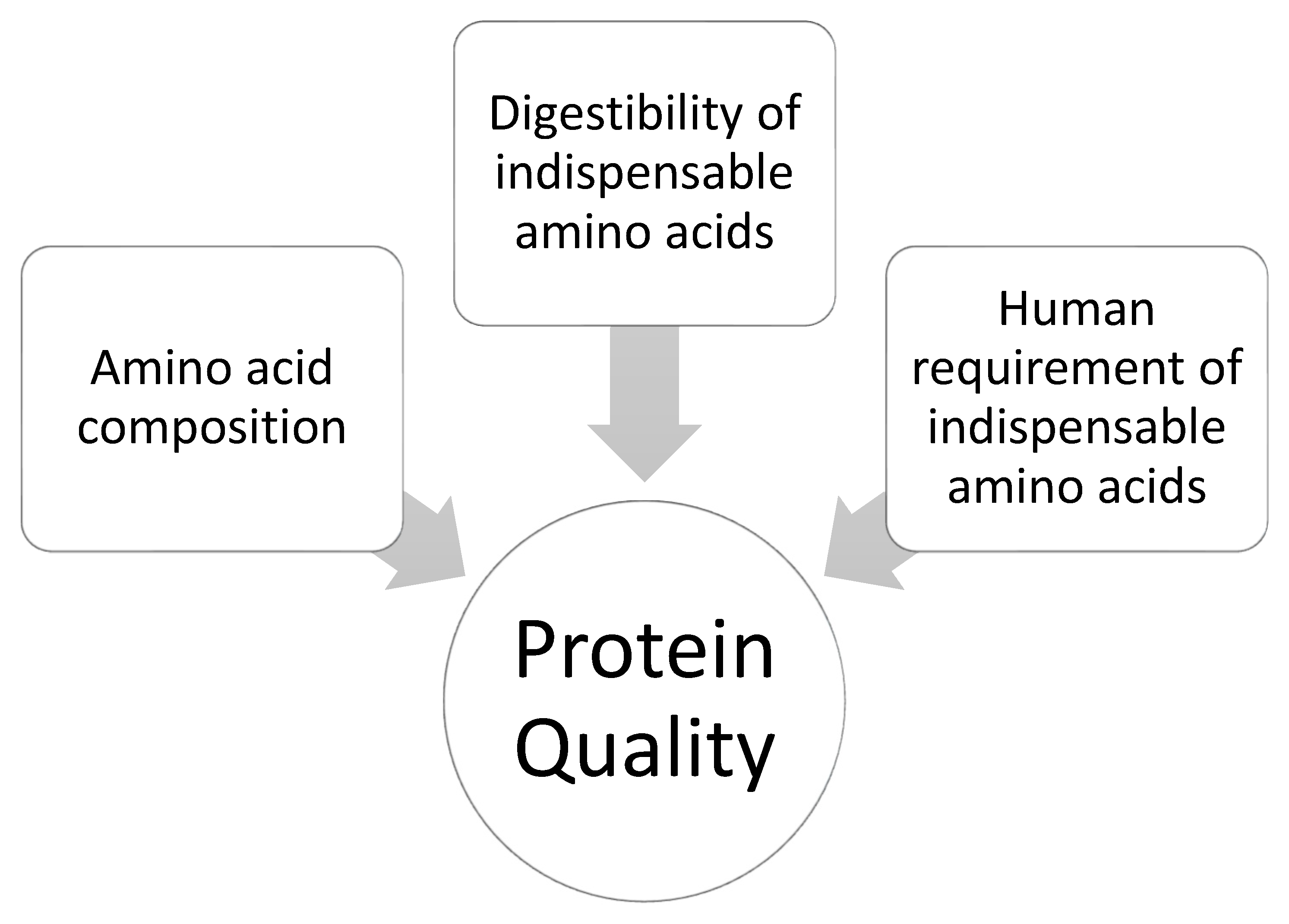
3.1. Defining Protein Quality
3.2. Methods for Determining Protein Quality Based on Amino Acid Digestibility
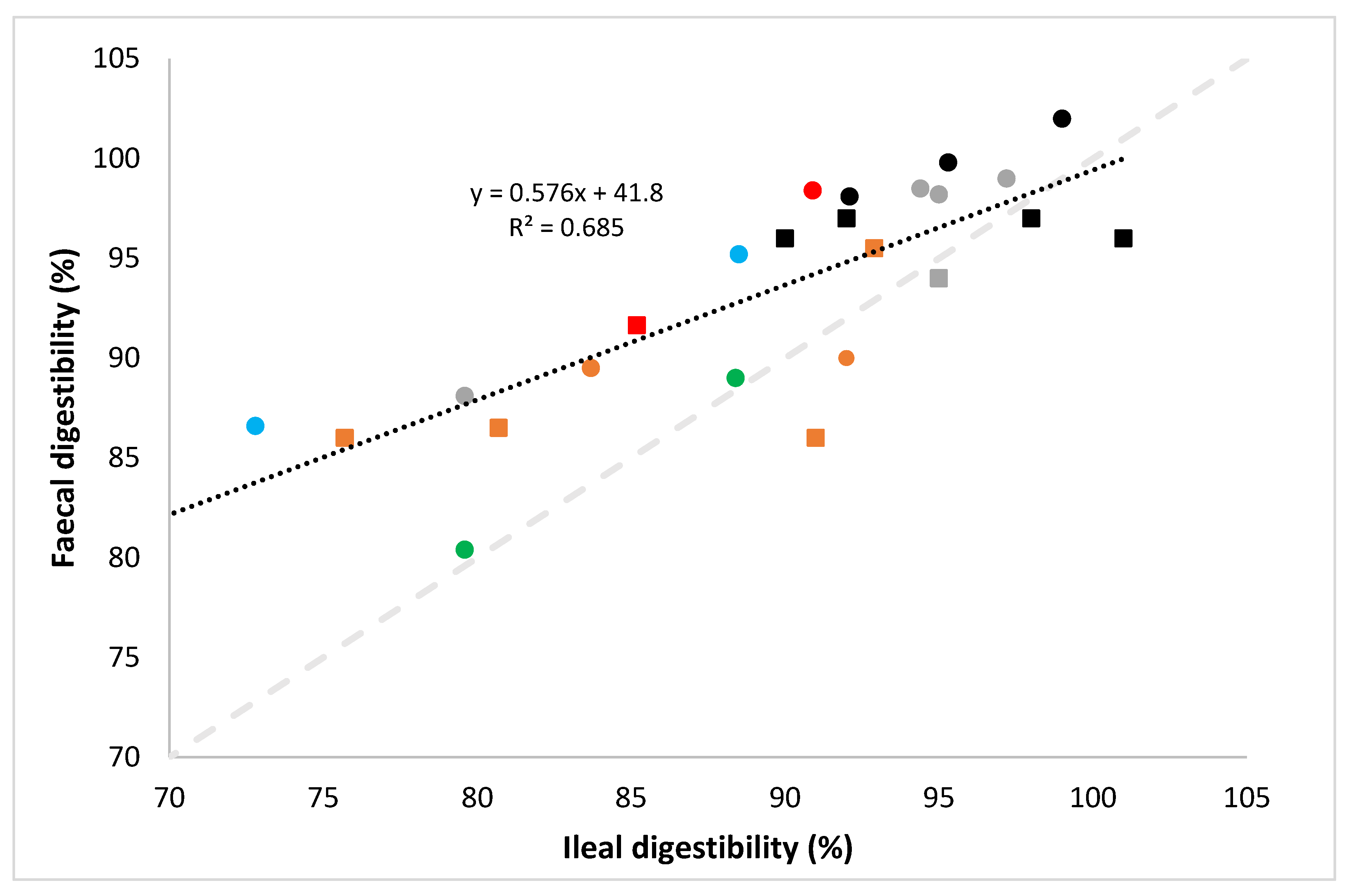
- digestibility in the PDCAAS method is not determined at the ileal but at the faecal level in test species, and
- digestibility in the PDCAAS method is determined at a protein level, and not at the individual amino acid level, and the protein digestibility factor is subsequently applied to every individual IAA.
- the determination of faecal rather than ileal digestibility in the PDCAAS method, despite the fact that it is established that amino acids absorbed past the terminal ileum do not contribute to protein metabolism and that faecal nitrogen levels may be affected by nitrogen metabolism of gut microbiota [28];
- the fact that digestibility in the PDCAAS method is determined on a protein basis, rather than on an individual amino acid basis, despite the fact that it is known that digestibility values between amino acids in protein sources vary widely [30];
- the truncation of protein quality scores at 100% in the PDCAAS method not allowing to consider complementarity of different protein sources on an amino acid basis (for further explanation see Section 6).
3.3. Methods for Determining Protein Quality Based on Growth Studies
3.4. Methods for Determining Protein Quality and Protein Digestibility In Vitro
4. Protein Quality Data from DIAAS Measurements: Interpretation and Application
5. Influence of Food Processing and Preparation on Protein Quality
5.1. Influence of Processing-Induced Modifications in Amino Acids on Protein Quality
5.2. Influence of Processing-Induced Changes in Protein Digestibility on Protein Quality
6. Complementarity of Different Protein Sources
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks i n 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- World Health Organization Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO: Genova, Italy, 2003; Volume 916.
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. a global review of food-based dietary guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Timotijevic, L.; Barnett, J.; Shepherd, R.; Lähteenmäki, L.; Raats, M.M. A review of consumer awareness, understanding and use of food-based dietary guidelines. Br. J. Nutr. 2011, 106, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Springmann, M.; Spajic, L.; Clark, M.A.; Poore, J.; Herforth, A.; Webb, P.; Rayner, M.; Scarborough, P. The healthiness and sustainability of national and global food based dietary guidelines: Modelling study. BMJ 2020, 370, m2322. [Google Scholar] [CrossRef] [PubMed]
- van de Kamp, M.E.; van Dooren, C.; Hollander, A.; Geurts, M.; Brink, E.J.; van Rossum, C.; Biesbroek, S.; de Valk, E.; Toxopeus, I.B.; Temme, E.H.M. Healthy diets with reduced environmental impact?—The greenhouse gas emissions of various diets adhering to the Dutch food based dietary guidelines. Food Res. Int. 2018, 104, 14–24. [Google Scholar] [CrossRef] [PubMed]
- The Eat-Lancet Commission; EAT-Lancet Commission Healthy Diets from Planet. Food Planet Health. 2019. [Google Scholar]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A comprehensive review of the benefits of and the barriers to the switch to a plant-based diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Lassen, A.D.; Christensen, L.M.; Trolle, E. Development of a Danish adapted healthy plant-based diet Based on the EAT-Lancet reference diet. Nutrients 2020, 12, 738. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef]
- Searchinger, T.; Hanson, C.; Ranganathan, J.; Lipinski, B.; Waite, R.; Winterbottom, R.; Dinshaw, A.; Heimlich, R.; Boval, M.; Chemineau, P.; et al. Creating a Sustainable Food Future. A Menu of Solutions to Sustainably Feed More Than 9 Billion People by 2050. World Resources Report 2013–14: Interim Findings; World Resources Institute: Washington DC, USA, 2014; ISBN 978-1-56973-817-7. [Google Scholar]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 deficiency is prevalent among Czech vegans who do not use Vitamin B12 supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef]
- Groufh-Jacobsen, S.; Hess, S.Y.; Aakre, I.; Folven Gjengedal, E.L.; Blandhoel Pettersen, K.; Henjum, S. Vegans, vegetarians and pescatarians are at risk of iodine deficiency in Norway. Nutrients 2020, 12, 3555. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and mineral status in a vegan diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Madsen, M.T.B.; Jørgensen, N.R.; Cohen, A.S.; Hansen, T.; Vestergaard, H.; Pedersen, O.; Allin, K.H. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur. J. Clin. Nutr. 2018, 72, 1046–1054. [Google Scholar] [CrossRef]
- Weaver, C.M.; Plawecki, K.L. Dietary calcium: Adequacy of a vegetarian diet. Am. J. Clin. Nutr. 1994, 59, 1238S–1241S. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Craig, W.J.; Baines, S.K. Zinc and vegetarian diets. Med. J. Aust. 2013, 199, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Baum, J.I.; Starck, C.; Moughan, P.J. Factors contributing to the selection of dietary protein food sources. Clin. Nutr. 2018, 37, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J. Population protein intakes and food sustainability indices: The metrics matter. Glob. Food Sec. 2021, 29, 100548. [Google Scholar] [CrossRef]
- Moughan, P.J.; Wolfe, R.R. Determination of dietary amino acid digestibility in humans. J. Nutr. 2019, 149, 2101–2109. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- Ertl, P.; Knaus, W.; Zollitsch, W. An approach to including protein quality when assessing the net contribution of livestock to human food supply. Animal 2016, 10, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Berardy, A.; Johnston, C.S.; Plukis, A.; Vizcaino, M.; Wharton, C. Integrating protein quality and quantity with environmental impacts in life cycle assessment. Sustainability 2019, 11, 2747. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products and Allergies (NDA), N. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- FAO Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; FAO: Auckland, New Zealand, 2013.
- IoM (Institute of Medicine) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press: Washington, DC, USA, 2005; ISBN 030908525X.
- Joint WHO/FAO/UNU Expert Consultation Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2007; ISBN 9241209356.
- Gilani, G.S.; Lee, N. PROTEIN|Quality. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4847–4854. ISBN 978-0-12-227055-0. [Google Scholar]
- Institute of Medicine (U.S.) Food and Nutrition Board. Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients; National Academy Press: Washington, DC, USA, 1998; Volume 55, ISBN 0309570808. [Google Scholar]
- Wang, D.; Ye, J.; Shi, R.; Zhao, B.; Liu, Z.; Lin, W.; Liu, X. Dietary protein and amino acid restriction: Roles in metabolic health and aging-related diseases. Free Radic. Biol. Med. 2022, 178, 226–242. [Google Scholar] [CrossRef]
- Baum, J.; Børsheim, E.; Allman, B.; Walker, S. Health benefits of dietary protein throughout the life cycle. In The Health Benefits of Foods—Current Knowledge and Further Development; IntechOpen: London, UK, 2020. [Google Scholar]
- Millward, D.J. Identifying recommended dietary allowances for protein and amino acids: A critique of the 2007 WHO/FAO/UNU report. Br. J. Nutr. 2012, 108, s3–s21. [Google Scholar] [CrossRef]
- Pillai, R.R.; Kurpad, A.V. Amino acid requirements in children and the elderly population. Br. J. Nutr. 2012, 108, S44–S49. [Google Scholar] [CrossRef]
- Bröer, S.; Fairweather, S.J. Amino acid transport across the mammalian intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- Singh, H.; Gallier, S. Chapter 2—Processing of Food Structures in the Gastrointestinal Tract and Physiological Responses. In Singh Digestion and Health; Boland, M., Golding, M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 51–81. ISBN 978-0-12-404610-8. [Google Scholar]
- Bornhorst, G.M.; Paul Singh, R. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu. Rev. Food Sci. Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef]
- Protein Digestion, Absorption and Metabolism. Available online: https://med.libretexts.org/@go/page/1869 (accessed on 22 November 2021).
- Moran, E.T. Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity. Anim. Feed Sci. Technol. 2016, 221, 284–303. [Google Scholar] [CrossRef]
- Jahan-Mihan, A.; Luhovyy, B.L.; Khoury, D.; Harvey Anderson, G. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef] [PubMed]
- Heda, R.; Toro, F.; Tombazzi, C.R. Physiology, Pepsin; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- van der Wielen, N.; de Vries, S.; Gerrits, W.; Jannink, K.; Moughan, P.; Mensink, M.; Hendriks, W. Presence of unabsorbed free amino acids at the end of the small intestine of humans and pigs: Potential implications for amino acid bioavailability. Curr. Dev. Nutr. 2021, 5, 530. [Google Scholar] [CrossRef]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Rutherfurd, S.M.; Kim, I.Y.; Moughan, P.J. Protein quality as determined by the digestible indispensable amino acid score: Evaluation of factors underlying the calculation. Nutr. Rev. 2016, 74, 584–599. [Google Scholar] [CrossRef]
- FAO/WHO Protein Quality Evaluation. Report of the Joint FAO/WHO Expert Consultation; Food & Agriculture Org.: Rome, Italy, 1991; Volume 51. [Google Scholar]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is best? J. Sport. Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Kurpad, A.V. Protein: Quality and Sources, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 123–130. ISBN 978-0-12-384885-7. [Google Scholar]
- Millward, D.J.; Jackson, A.A. Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: Implications for recommended protein and amino acid intakes. Public Health Nutr. 2004, 7, 387–405. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2019, 20, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.; Tomé, D.; Moughan, P.; Burlingame, B. Report of a Sub-Committee of the 2011 FAO Consultation on “Protein Quality Evaluation in Human Nutrition” on: The assessment of amino acid digestibility in foods for humans and including a collation of published ileal amino acid digestibility data for. FAO Expert 2012, 2012, 58. [Google Scholar]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO/UNU. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization (WHO): Geneva, Switzerland, 1985. [Google Scholar]
- Schaafsma, G. The protein digestibility–Corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef]
- Devi, S.; Varkey, A.; Sheshshayee, M.S.; Preston, T.; Kurpad, A.V. Measurement of protein digestibility in humans by a dual-tracer method. Am. J. Clin. Nutr. 2018, 107, 984–991. [Google Scholar] [CrossRef]
- van der Wielen, N.; Khodorova, N.V.; Gerrits, W.J.J.; Gaudichon, C.; Calvez, J.; Tomé, D.; Mensink, M. Blood 15N:13C enrichment ratios are proportional to the ingested quantity of protein with the dual-tracer approach for determining amino acid bioavailability in humans. J. Nutr. 2020, 150, 2346–2352. [Google Scholar] [CrossRef]
- Food and Agriculture Organization(FAO). Protein Quality Assessment in Follow-Up Formula for Young Children and Ready to Use Therapeutic Foods: Report of the FAO Expert Working Group; Food and Agriculture Organization(FAO): Rome, Italy, 2017; ISBN 9789251311202. [Google Scholar]
- Guillin, F.M.; Gaudichon, C.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Airinei, G.; Khodorova, N.; Benamouzig, R.; Pomport, P.-H.; Martin, J.; Calvez, J. Real ileal amino acid digestibility of pea protein compared to casein in healthy humans: A randomized trial. Am. J. Clin. Nutr. 2021, 115, 353–363. [Google Scholar] [CrossRef]
- Calvez, J.; Benoit, S.; Piedcoq, J.; Khodorova, N.; Azzout-Marniche, D.; Tomé, D.; Benamouzig, R.; Airinei, G.; Gaudichon, C. Very low ileal nitrogen and amino acid digestibility of zein compared to whey protein isolate in healthy volunteers. Am. J. Clin. Nutr. 2021, 113, 70–82. [Google Scholar] [CrossRef]
- Deglaire, A.; Bos, C.; Tomé, D.; Moughan, P.J. Ileal digestibility of dietary protein in the growing pig and adult human. Br. J. Nutr. 2009, 102, 1752–1759. [Google Scholar] [CrossRef]
- Gaudichon, C.; Calvez, J. Determinants of amino acid bioavailability from ingested protein in relation to gut health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.K.; Weisell, R.; Albert, J.; Tomé, D.; Kurpad, A.V.; Uauy, R. Research approaches and methods for evaluating the protein quality of human foods proposed by an fao expert working group in 2014. J. Nutr. 2016, 146, 929–932. [Google Scholar] [CrossRef] [PubMed]
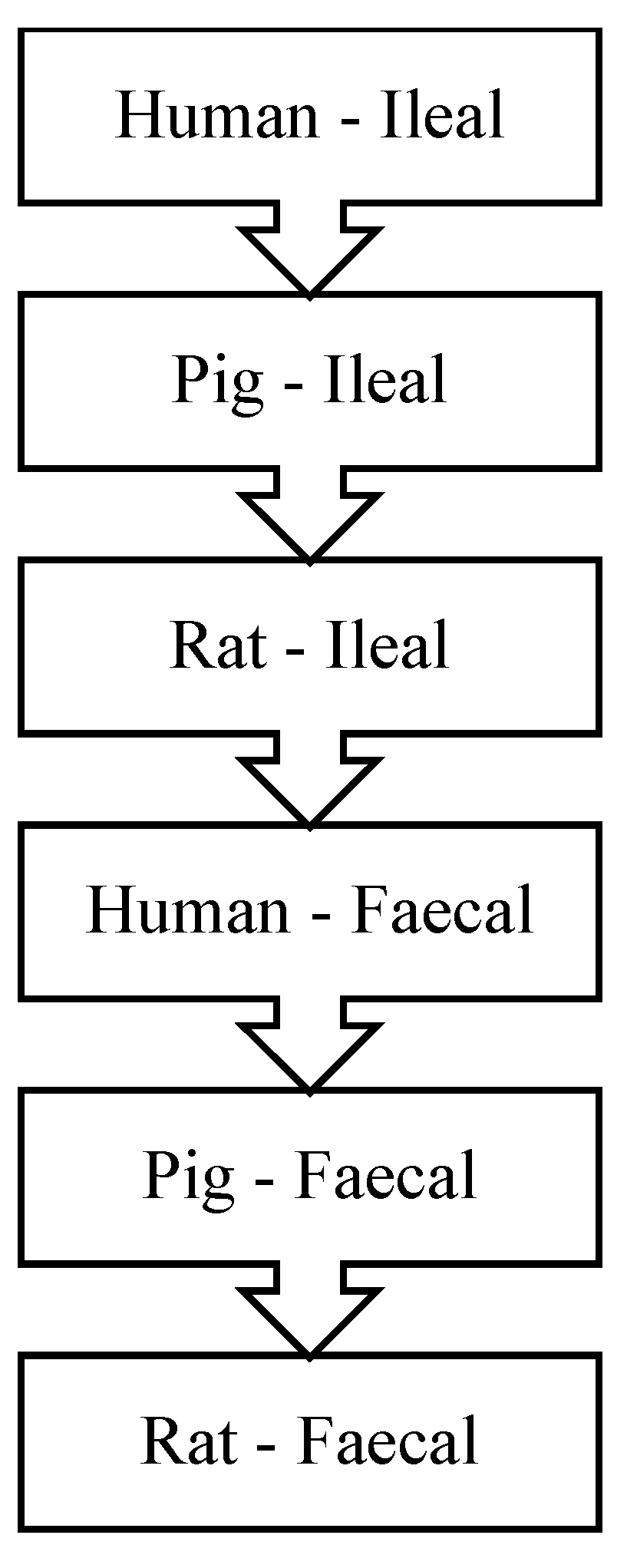
- Deglaire, A.; Moughan, P.J. Animal models for determining amino acid digestibility in humans—A review. Br. J. Nutr. 2012, 108, S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.M.; Moughan, P.J.; Wilson, M.N.; Maher, K.; Tasman-Jones, C. Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man. Br. J. Nutr. 1994, 71, 29–42. [Google Scholar] [CrossRef]
- Hendriks, W.H.; Van Baal, J.; Bosch, G. Ileal and faecal protein digestibility measurement in humans and other non-ruminants—A comparative species view. Br. J. Nutr. 2012, 108. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef]
- Nitrayová, S.; Brestenský, M.; Patráš, P. Comparison of two methods of protein quality evaluation in rice, rye and barley as food protein sources in human nutrition. Potravin. Slovak J. Food Sci. 2018, 12, 762–766. [Google Scholar] [CrossRef][Green Version]
- Bailey, H.M.; Stein, H.H. Raw and roasted pistachio nuts (Pistacia vera L.) are ‘good’ sources of protein based on their digestible indispensable amino acid score as determined in pigs. J. Sci. Food Agric. 2020, 100, 3878–3885. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Bains, K.; Moughan, P.J. Available lysine and digestible amino acid contents of proteinaceous foods of India. Br. J. Nutr. 2012, 108, S59–S68. [Google Scholar] [CrossRef]
- Bailey, H.M.; Mathai, J.K.; Berg, E.P.; Stein, H.H. Most meat products have digestible indispensable amino acid scores that are greater than 100, but processing may increase or reduce protein quality. Br. J. Nutr. 2020, 124, 1–9. [Google Scholar] [CrossRef]
- Fanelli, N.S.; Bailey, H.M.; Guardiola, L.V.; Stein, H.H. Values for digestible indispensable amino acid score (DIAAS) determined in pigs are greater for milk than for breakfast cereals, but DIAAS values for individual ingredients are additive in combined meals. J. Nutr. 2021, 151, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, S.M.; Montoya, C.A.; Scholten, P.T.; Rutherfurd, S.M.; Moughan, P.J. Cooking conditions affect the true ileal digestible amino acid content and digestible indispensable amino acid score (DIAAS) of bovine meat as determined in pigs. J. Nutr. 2018, 148, 1564–1569. [Google Scholar] [CrossRef]
- Bailey, H.M. Digestible Indispensable Amino Acid Scores for Meat Products. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2018. [Google Scholar]
- Cervantes-Pahm, S.K.; Liu, Y.; Stein, H.H. Digestible indispensable amino acid score and digestible amino acids in eight cereal grains. Br. J. Nutr. 2014, 111, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Han, F.; Wang, Y.; Fan, L.; Song, G.; Chen, X.; Jiang, P.; Miao, H.; Han, Y. Digestible indispensable amino acid scores of nine cooked cereal grains. Br. J. Nutr. 2019, 121, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Franczyk, A.; Zimoch-Korzycka, A.; Appah, P.; Utioh, A.; Neufeld, J.; House, J.D. Impact of processing on the protein quality of pinto bean (Phaseolus vulgaris) and buckwheat (Fagopyrum esculentum Moench) flours and blends, as determined by in vitro and in vivo methodologies. J. Agric. Food Chem. 2017, 65, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef]
- Rieder, A.; Afseth, N.K.; Böcker, U.; Knutsen, S.H.; Kirkhus, B.; Mæhre, H.K.; Ballance, S.; Wubshet, S.G. Improved estimation of in vitro protein digestibility of different foods using size exclusion chromatography. Food Chem. 2021, 358. [Google Scholar] [CrossRef]
- Heo, J.M.; Kiarie, E.; Kahindi, R.K.; Maiti, P.; Woyengo, T.A.; Nyachoti, C.M. Standardized ileal amino acid digestibility in egg from hyperimmunized hens fed to weaned pigs. J. Anim. Sci. 2012, 90, 239–241. [Google Scholar] [CrossRef]
- Han, F.; Moughan, P.J.; Li, J.; Pang, S. Digestible indispensable amino acid scores (DIAAS) of six cooked CHINESE pulses. Nutrients 2020, 12, 3831. [Google Scholar] [CrossRef]
- Abelilla, J.J.; Liu, Y.; Stein, H.H. Digestible indispensable amino acid score (DIAAS) and protein digestibility corrected amino acid score (PDCAAS) in oat protein concentrate measured in 20- to 30-kilogram pigs. J. Sci. Food Agric. 2018, 98, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.M.; Stein, H.H. Can the digestible indispensable amino acid score methodology decrease protein malnutrition. Anim. Front. 2019, 9, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, W.D.; Marinangeli, C.P.F.; Cargo-Froom, C.; Franczyk, A.; House, J.D.; Elango, R.; Columbus, D.A.; Kiarie, E.; Rogers, M.; Shoveller, A.K. Comparison of methodologies used to define the protein quality of human foods and support regulatory claims. Appl. Physiol. Nutr. Metab. 2020, 45, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Grandison, A.S. Postharvest handling and preparation of foods for processing. Food Process. Handb. 2011, 1–30. [Google Scholar] [CrossRef]
- Aluko, R.E. 15—Food protein-derived peptides: Production, isolation, and purification. In Proteins in Food Processing; Woodhead Publishing: Sawston, UK, 2018; pp. 389–412. ISBN 978-0-08-100722-7. [Google Scholar]
- Fabbri, A.D.T.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastron. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; Tar’an, B.; House, J.D. Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci. Nutr. 2020, 8, 2950–2958. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Finot, P.A. Food processing and storage as a determinant of protein and amino acid availability. Experientia Suppl. 1983, 44, 135–156. [Google Scholar] [CrossRef]
- Rérat, A.; Calmes, R.; Vaissade, P.; Finot, P.-A.A. Nutritional and metabolic consequences of the early Maillard reaction of heat treated milk in the pig. Significance for man. Eur. J. Nutr. 2002, 41, 1–11. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Butré, C.I.; Wierenga, P.A.; Bruininx, E.M.A.M.A.M.; Gruppen, H.; Hendriks, W.H.; van der Poel, A.F.B.B. Apparent ileal digestibility of Maillard reaction products in growing pigs. PLoS ONE 2018, 13, e0199499. [Google Scholar] [CrossRef]
- Nyakayiru, J.; van Lieshout, G.A.A.; Trommelen, J.; van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; van Loon, L.J.C. The glycation level of milk protein strongly modulates post-prandial lysine availability in humans. Br. J. Nutr. 2020, 123, 545–552. [Google Scholar] [CrossRef]
- Zenker, H.E.; Van Lieshout, G.A.A.; Van Gool, M.P.; Bragt, M.C.E.; Hettinga, K.A. Lysine blockage of milk proteins in infant formula impairs overall protein digestibility and peptide release. Food Funct. 2020, 11, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Moughan, P.J. Digestible reactive lysine in selected milk-based products. J. Dairy Sci. 2005, 88, 40–48. [Google Scholar] [CrossRef]
- Torbatinejad, N.M.; Rutherfurd, S.M.; Moughan, P.J. Total and reactive lysine contents in selected cereal-based food products. J. Agric. Food Chem. 2005, 53, 4454–4458. [Google Scholar] [CrossRef] [PubMed]
- Erbersdobler, H.F.; Hupe, A. Determination of lysine damage and calculation of lysine bio-availability in several processed foods. Z. ErnAhrungswiss. 1991, 30, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Nutrition|Effects of food processing. Encycl. Grain Sci. 2004, 328–340. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J. Agric. Food Chem. 1999, 47, 1295–1319. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Geypens, B.; Luypaerts, A.; Hiele, M.; Ghoos, Y.; Rutgeerts, P. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J. Nutr. 1998, 128, 1716–1722. [Google Scholar] [CrossRef]
- Kaewtapee, C.; Eklund, M.; Wiltafsky, M.; Piepho, H.-P.P.; Mosenthin, R.; Rosenfelder, P. Influence of wet heating and autoclaving on chemical composition and standardized ileal crude protein and amino acid digestibility in full-fat soybeans for pigs1,2. J. Anim. Sci. 2017, 95, 779–788. [Google Scholar] [CrossRef]
- El-gasim, A.Y.A.; Abdalla, A.A. Effect of domestic processing methods on chemical composition, in vitro digestibility of protein and starch and functional properties of Bambara groundnut (Voandzeia subterranea) Seed. Res. J. Agric. Biol. Sci. 2007, 3, 24–34. [Google Scholar]
- Correia, I.; Nunes, A.; Barros, A.S.; Delgadillo, I. Comparison of the effects induced by different processing methods on sorghum proteins. J. Cereal Sci. 2010, 51, 146–151. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Kaewtapee, C.; Mosenthin, R.; Nenning, S.; Wiltafsky, M.; Schäffler, M.; Eklund, M.; Rosenfelder-Kuon, P. Standardized ileal digestibility of amino acids in European soya bean and rapeseed products fed to growing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e695–e705. [Google Scholar] [CrossRef]
- Llopart, E.E.; Drago, S.R.; De Greef, D.M.; Torres, R.L.; González, R.J. Effects of extrusion conditions on physical and nutritional properties of extruded whole grain red sorghum (Sorghum spp.). Int. J. Food Sci. Nutr. 2014, 65, 34–41. [Google Scholar] [CrossRef]
- Salgó, A.; Ganzler, K.; Jécsai, J. Simple enzymic methods for prediction of plant protein digestibility. Amin. Acid Compos. Biol. Value Cereal Proteins 1985, 311–323. [Google Scholar] [CrossRef]
- Stein, H.H.; Fuller, M.F.; Moughan, P.J.; Sève, B.; Mosenthin, R.; Jansman, A.J.M.; Fernández, J.A.; de Lange, C.F.M. Definition of apparent, true, and standardized ileal digestibility of amino acids in pigs. Livest. Sci. 2007, 109, 282–285. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Hendriks, W.H.; Bruininx, E.M.A.M.; Gruppen, H.; van der Poel, A.F.B. Protein structural changes during processing of vegetable feed ingredients used in swine diets: Implications for nutritional value. Nutr. Res. Rev. 2016, 29, 126–141. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in Faba and kidney beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- van Lieshout, G.A.A.A.; Lambers, T.T.; Bragt, M.C.E.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–24. [Google Scholar] [CrossRef]
- Dias, D.R.; Abreu, C.M.P.D.; Silvestre, M.P.C.; Schwan, R.F. In vitro protein digestibility of enzymatically pre-treated bean (Phaseolus vulgaris L.) flour using commercial protease and Bacillus sp. protease. Food Sci. Technol. 2010, 30, 94–99. [Google Scholar] [CrossRef]
- Joye, I. Protein digestibility of cereal products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Fastinger, N.D.; Mahan, D.C. Effect of soybean meal particle size on amino acid andenergy digestibility in grower-finisher swine1234. J. Anim. Sci. 2003, 81, 697–704. [Google Scholar] [CrossRef]
- Kim, J.C.; Mullan, B.P.; Heo, J.M.; Hansen, C.F.; Pluske, J.R. Decreasing dietary particle size of lupins increases apparent ileal amino acid digestibility and alters fermentation characteristics in the gastrointestinal tract of pigs. Br. J. Nutr. 2009, 102, 350–360. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.B.L.; van Dijk, J.-W.; de Lange, A.; Kiskini, A.; Kuklinski, M.; Senden, J.M.G.; van Loon, L.J.C. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am. J. Clin. Nutr. 2013, 98, 121–128. [Google Scholar] [CrossRef]
- Aoyama, S.; Kim, H.-K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef]
- Engelking, L.R. Chapter 44—Vitamin A. In Textbook of Veterinary Physiological Chemistry; Academic Press: Boston, MA, USA, 2015; pp. 282–287. ISBN 978-0-12-391909-0. [Google Scholar]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef]
- Groen, B.B.L.; Horstman, A.M.; Hamer, H.M.; de Haan, M.; van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.W.H.; Rasmussen, B.B.; van Loon, L.J.C. Post-prandial protein handling: You are what you just ate. PLoS ONE 2015, 10, e0141582. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.F.; Ross, M.L.; Camera, D.M.; West, D.W.D.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Castellino, P.; DeFronzo, R.A. Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes 1996, 45, 393–399. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.P.; Jäkel, M.; Soeters, P.B. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J. Nutr. 2005, 135, 1080–1087. [Google Scholar] [CrossRef]
- Schutz, Y. Protein turnover, ureagenesis and gluconeogenesis. Int. J. Vitam. Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Ishikawa-Takata, K.; Takimoto, H. Current protein and amino acid intakes among Japanese people: Analysis of the 2012 national health and nutrition survey. Geriatr. Gerontol. Int. 2018, 18, 723–731. [Google Scholar] [CrossRef]
- USDA. What We Eat in America Database 2017–2018. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (accessed on 16 November 2020).
- Tieland, M.; Borgonjen-Van den Berg, K.J.; Van Loon, L.J.C.; De Groot, L.C.P.G.M. Dietary protein intake in dutch elderly people: A focus on protein sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? J. Int. Soc. Sports Nutr. 2018, 15, 1–6. [Google Scholar] [CrossRef]
- van den Borne, J.J.G.C.; Alferink, S.J.J.; Heetkamp, M.J.W.; Jacobs, A.A.A.; Verstegen, M.W.A.; Gerrits, W.J.J. Asynchronous supply of indispensable amino acids reduces protein deposition in milk-fed calves. J. Nutr. 2012, 142, 2075–2082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of ‘nutritionally non-essential amino acids’ for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Moughan, P.J.; Li, J.; Stroebinger, N.; Pang, S. The complementarity of amino acids in cooked pulse/cereal blends and effects on DIAAS. Plants 2021, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Pencharz, P.B.; Elango, R.; Wolfe, R.R. Recent developments in understanding protein needs—How much and what kind should we eat? Appl. Physiol. Nutr. Metab. 2016, 41, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Riddet Institute DELTA Model 1.3 Web. Available online: https://sustainablenutritioninitiative.com/the-delta-model/explore-the-future/ (accessed on 5 November 2021).

 : cut-off for actual requirement as a combination of amino acid content and digestibility of the respective indispensable amino acid; (A; data from [76]), skim milk powder (B; data from [57]), soy flour (C; data from [57]), rice (D; data from [80]), cooked peas (E; data from [87]) and maize (F; data from [80]).
: cut-off for actual requirement as a combination of amino acid content and digestibility of the respective indispensable amino acid; (A; data from [76]), skim milk powder (B; data from [57]), soy flour (C; data from [57]), rice (D; data from [80]), cooked peas (E; data from [87]) and maize (F; data from [80]).
 : cut-off for actual requirement as a combination of amino acid content and digestibility of the respective indispensable amino acid; (A; data from [76]), skim milk powder (B; data from [57]), soy flour (C; data from [57]), rice (D; data from [80]), cooked peas (E; data from [87]) and maize (F; data from [80]).
: cut-off for actual requirement as a combination of amino acid content and digestibility of the respective indispensable amino acid; (A; data from [76]), skim milk powder (B; data from [57]), soy flour (C; data from [57]), rice (D; data from [80]), cooked peas (E; data from [87]) and maize (F; data from [80]).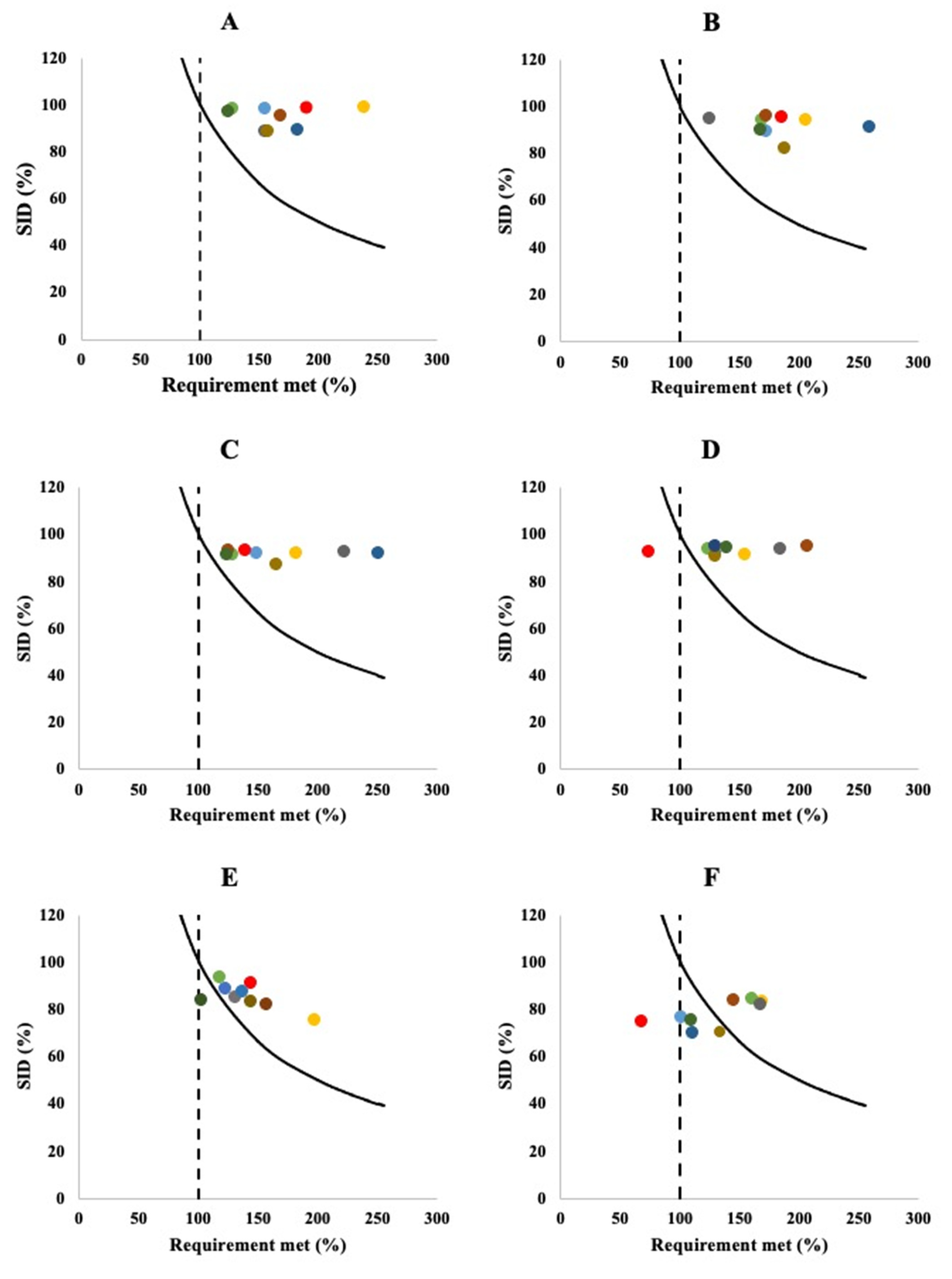

| Age (Years) | His | Ile | Leu | Lys | SAA * | AAA ** | Thr | Trp | Val |
|---|---|---|---|---|---|---|---|---|---|
| 0.5–1 | 22 | 36 | 73 | 64 | 31 | 59 | 34 | 9.5 | 49 |
| 1–2 | 15 | 27 | 54 | 45 | 22 | 40 | 23 | 6.4 | 36 |
| 3–10 | 12 | 23 | 44 | 35 | 18 | 30 | 18 | 4.8 | 29 |
| 11–14 | 12 | 22 | 44 | 35 | 17 | 30 | 18 | 4.8 | 29 |
| 15–18 | 11 | 21 | 42 | 33 | 16 | 28 | 17 | 4.5 | 28 |
| >18 | 10 | 20 | 39 | 30 | 15 | 25 | 15 | 4.0 | 26 |
| Age (Years) | His | Ile | Leu | Lys | SAA * | AAA ** | Thr | Trp | Val |
|---|---|---|---|---|---|---|---|---|---|
| 0–0.5 | 21 | 55 | 96 | 69 | 33 | 94 | 44 | 17 | 55 |
| 0.5–3 | 20 | 32 | 66 | 57 | 27 | 52 | 31 | 8.5 | 43 |
| >3 | 16 | 30 | 61 | 48 | 23 | 41 | 25 | 6.6 | 40 |
| Method | Measurement Principle | Calculations | Refs. |
|---|---|---|---|
| Protein quality methods | |||
| Protein efficiency ratio (PER) | Ratio of weight gain and protein consumed by test group over control (preferred reference protein: casein) | [51] | |
| Net protein ratio (or net protein retention) (NPR) | Difference in weight gain between a test protein group and protein-free diet group per gram of protein consumed by the test protein group. | [58] | |
| Protein digestibility corrected amino acid score (PDCAAS) | Ratio of IAAlim in test protein compared to reference protein corrected for faecal protein digestibility | [32,51,59] | |
| Digestible indispensable amino acid score (DIAAS) | Ratio of IAAlim in test protein compared to reference protein corrected for ileal digestibility of IAAlim | [30,60] | |
| Protein digestibility methods | |||
| True Digestibility (TD) | Percentage of nitrogen observed from protein (food) consumed in the GI tract | [51] | |
| Biological value (BV) | Retained nitrogen over total nitrogen intake, with corrections for faecal and urinary losses. | [58] | |
| Net protein utilization (NPU) | Retained nitrogen over total nitrogen intake, with corrections for faecal and urinary losses. | [32] | |
| Dual isotope tracer method | Compares AA in circular system from intrinsically labelled test protein consumed together with a reference protein with known digestibility labelled differently | [61,62] | |
| Food Item | Food Group | DIAAS Value (%) | IAAlim | SID of IAAlim (%) | Test Species | Protein Reference Pattern | References |
|---|---|---|---|---|---|---|---|
| Dry milk | Dairy | 144 | SAA | 94 | Pig | >3-year-old | [77] |
| Bacon (smoked-cooked) | Pork | 142 | Valine | 95 | Pig | >3-year-old | [79] |
| Milk protein concentrate | Dairy | 141 | SAA | 101 | Pig | >3-year-old | [57] |
| Pork loin (medium) | Pork | 139 | Valine | 95 | Pig | >3-year-old | [79] |
| Whey protein concentrate | Dairy | 133 | Histidine | 97 | Pig | >3-year-old | [57] |
| Ham (alternatively-cured) | Pork | 133 | Valine | 95 | Pig | >3-year-old | [79] |
| Ribeye (roast, medium) | Beef | 130 | Valine | 95 | Pig | >3-year-old | [76] |
| Bologna | Pork | 128 | Leucine | 97 | Pig | >3-year-old | [76] |
| Ham (conventionally-cured) | Pork | 126 | Valine | 96 | Pig | >3-year-old | [79] |
| Whey protein isolate | Dairy | 125 | Histidine | 100 | Pig | >3-year-old | [57] |
| Ham (non-cured) | Pork | 124 | Valine | 93 | Pig | >3-year-old | [79] |
| Skimmed milk powder | Dairy | 123 | SAA | 99 | Pig | >3-year-old | [57] |
| Egg | Egg | 122 | SAA | 75 | Pig | >3-year-old | [86] |
| Ground beef (raw) | Beef | 121 | Leucine | 99 | Pig | >3-year-old | [76] |
| Beef jerky | Beef | 120 | SAA | 98 | Pig | >3-year-old | [76] |
| Salami | Pork | 120 | Valine | 96 | Pig | >3-year-old | [76] |
| Pork belly (raw) | Pork | 119 | Valine | 97 | Pig | >3-year-old | [79] |
| Milk protein concentrate | Dairy | 118 | SAA | 94 | Rat | 0.5–3-year-old | [72] |
| Pork loin (medium-well done) | Pork | 118 | Valine | 95 | Pig | >3-year-old | [79] |
| Bacon (smoked) | Pork | 117 | Valine | 95 | Pig | >3-year-old | [79] |
| Pork loin (well-done) | Pork | 117 | Valine | 95 | Pig | >3-year-old | [79] |
| Ribeye (roast, medium-rare) | Beef | 111 | Valine | 97 | Pig | >3-year-old | [76] |
| Whey protein isolate | Dairy | 109 | Histidine | 99 | Rat | 0.5–3-year-old | [72] |
| Ribeye (well-done) | Beef | 107 | Valine | 97 | Pig | >3-year-old | [76] |
| Soy flour | Legumes | 105 | SAA | 101 | Pig | >3-year-old | [57] |
| Ground beef (cooked) | Beef | 99 | Leucine | 97 | Pig | >3-year-old | [76] |
| Topside steak (boiled) | Beef | 99 | Valine | 99 | Pig | >3-year-old | [78] |
| Topside steak (pan fried) | Beef | 98 | Valine | 98 | Pig | >3-year-old | [78] |
| Soy protein isolate | Legumes | 98 | SAA | 98 | Pig | >3-year-old | [57] |
| Whey protein concentrate | Dairy | 97 | Histidine | 98 | Rat | 0.5–3-year-old | [72] |
| Topside steak (raw) | Beef | 97 | Valine | 98 | Pig | >3-year-old | [78] |
| Mung beans (cooked) | Legumes | 94 2 | Threonine | 77 | Pig | >3-year-old | [87] |
| Topside steak (roasted) | Beef | 91 | Valine | 98 | Pig | >3-year-old | [78] |
| Soy protein isolate | Legumes | 91 | SAA | 94 | Rat | 0.5–3-year-old | [72] |
| Soy protein isolate | Legumes | 90 | SAA | 92 | Rat | 0.5–3-year-old | [72] |
| Peas (cooked) | Legumes | 88 2 | Valine | 87 | Pig | >3-year-old | [87] |
| Broad beans (cooked) | Legumes | 87 2 | Valine | 91 | Pig | >3-year-old | [87] |
| Pistachio (raw) | Nuts | 86 | Lysine | 87 | Pig | >3-year-old | [74] |
| Pistachio (roasted) | Nuts | 83 | Lysine | 77 | Pig | >3-year-old | [74] |
| Pea protein concentrate | Legumes | 82 | SAA | 95 | Rat | 0.5–3-year-old | [72] |
| Topside steak (grilled) | Beef | 80 | Valine | 97 | Pig | >3-year-old | [78] |
| Adzuki beans (cooked) | Legumes | 78 2 | SAA | 87 | Pig | >3-year-old | [87] |
| Dehulled oats | Cereals | 77 | Lysine | 85 | Pig | >3-year-old | [80] |
| Kidney beans (cooked) | Legumes | 74 2 | SAA | 68 | Pig | >3-year-old | [87] |
| Pea protein concentrate | Legumes | 73 | SAA | 78 | Pig | >3-year-old | [57] |
| Chickpeas (cooked) | Legumes | 71 2 | Valine | 83 | Pig | >3-year-old | [87] |
| Buckwheat (cooked) | Cereals | 68 | SAA | 86 | Rat | 0.5–3-year-old | [81] |
| Quick oats | Cereals | 67 | Lysine | 83 | Pig | >3-year-old | [77] |
| Oat protein concentrate | Cereals | 67 | Lysine | 86 | Pig | >3-year-old | [88] |
| Polished white rice | Cereals | 64 | Lysine | 92 | Pig | >3-year-old | [80] |
| Rice (cooked) | Cereals | 60 | Lysine | 92 | Rat | 0.5–3-year-old | [72] |
| Kidney beans (cooked) | Legumes | 59 | SAA | 75 | Rat | 0.5–3-year-old | [72] |
| Peas (cooked) | Legumes | 58 | SAA | 89 | Rat | 0.5–3-year-old | [72] |
| Rolled oats (cooked) | Cereals | 54 | Lysine | 84 | Rat | 0.5–3-year-old | [72] |
| Nutridense maize | Cereals | 54 | Lysine | 79 | Pig | >3-year-old | [80] |
| Dehulled barley | Cereals | 51 | Lysine | 74 | Pig | >3-year-old | [80] |
| Yellow dent maize | Cereals | 48 | Lysine | 75 | Pig | >3-year-old | [80] |
| Rey | Cereals | 47 | Lysine | 67 | Pig | >3-year-old | [80] |
| Tartary buckwheat (cooked) | Cereals | 47 | SAA | 72 | Rat | 0.5–3-year-old | [81] |
| Peanuts (roasted) | Legumes | 43 | Lysine | 92 | Rat | 0.5–3-year-old | [72] |
| Wheat | Cereals | 43 | Lysine | 73 | Pig | >3-year-old | [80] |
| Oats (cooked) | Cereals | 43 | Lysine | 83 | Rat | 0.5–3-year-old | [81] |
| Brown rice (cooked) | Cereals | 42 | Lysine | 93 | Rat | 0.5–3-year-old | [81] |
| Wheat bran | Cereals | 41 | Lysine | 73 | Rat | 0.5–3-year-old | [72] |
| Rice protein concentrate | Cereals | 37 | Lysine | 86 | Rat | 0.5–3-year-old | [72] |
| Polished rice cooked | Cereals | 37 | Lysine | 92 | Rat | 0.5–3-year-old | [81] |
| Sorghum | Cereals | 29 | Lysine | 69 | Pig | >3-year-old | [80] |
| Whole wheat (cooked) | Cereals | 20 | Lysine | 96 | Rat | 0.5–3-year-old | [81] |
| Cornflakes | Cereals | 19 | Lysine | 78 | Pig | >3-year-old | [77] |
| Adlay (cooked) | Cereals | 13 | Lysine | 90 | Rat | 0.5–3-year-old | [81] |
| Foxtail millet (cooked) | Cereals | 10 | Lysine | 88 | Rat | 0.5–3-year-old | [81] |
| Proso millet (cooked) | Cereals | 7 | Lysine | 96 | Rat | 0.5–3-year-old | [81] |
| Corn-based breakfast cereal | Cereals | 1 | Lysine | 13 | Rat | 0.5–3-year-old | [72] |
| Food Group | Number of Food items | DIAAS Value (Range) | SID IAAlim (Range) | IAAlim |
|---|---|---|---|---|
| Beef | 11 | 80–130 | 95–99 | Valine (n = 8/5/3), Leucine (n = 2/1/1), SAA * (n = 1/0/1) |
| Cereals | 25 | 1–77 | 13–96 | Lysine (n = 23/23/0), SAA * (n = 2/2/0) |
| Dairy | 8 | 97–144 | 94–101 | SAA (n = 4/0/4), Histidine (n = 4/1/4) |
| Legumes | 15 | 43–105 | 75–101 | SAA * (n = 10/9/1) Valine (n = 3/3/0) Lysine (n = 1/1/0) Threonine (n = 1/1/0) |
| Pork | 11 | 117–142 | 93–97 | Valine (n = 10/0/10) Leucine (n = 1/0/1) |
| Egg | 1 | 122 | 75 | SAA * (n = 1/0/1) |
| Nuts | 2 | 83–86 | 77–87 | Lysine (n = 2/2/0) |
| All | 73 | 1–144 | 13–101 | Lysine (n = 26/26) Valine (n = 8/21) SAA * (n = 11/18) Histidine (n = 1/4) Leucine (n = 1/3) Threonine (n = 1/1) |
| Food | Processing | Processing Conditions | Protein Digestibility (%) | Digestibility Method | References | |
|---|---|---|---|---|---|---|
| Raw | Processed | |||||
| Eggs | Microwave | - | 51 | 91 | TD | [107] |
| Ground beef | Cooking | Fully cooked, 72 °C | 104 | 105 | SID (pig) | [76] |
| Topside steak | Boiling | boiled at 80 °C completely submersed in water | 97 | 98 | SID (pig) | [78] |
| Grilling | 225 °C, internal temperature 35.5 °C | 97 | 96 | SID (pig) | ||
| Frying | 186 °C, internal temperature 35.5 °C | 97 | 98 | SID (pig) | ||
| Roasting | oven roasting at 160 °C | 97 | 98 | SID (pig) | ||
| Canadian cowpea | Soaking | room temp 1:5 (w/v) seed to water, 18 h | 83 | 87 | IVPD | [111] |
| Boiling | 35 min | 83 | 98 | IVPD | ||
| Roasting | 180 °C for 15 min | 83 | 78 | IVPD | ||
| Autoclaving | 15 lb pressure and 121 °C for 20 min | 83 | 90 | IVPD | ||
| Microwave | 1200 Watt for 15 min | 83 | 93 | IVPD | ||
| Micronization | 90 °C with 115V infrared for 2.5 min | 83 | 80 | IVPD | ||
| Egyptian cowpea | Soaking | room temp 1:5 (w/v) seed to water, 22 h | 82 | 87 | IVPD | [111] |
| Boiling | 35 min | 82 | 97 | IVPD | ||
| Roasting | 180 °C for 15 min | 82 | 77 | IVPD | ||
| Autoclaving | 15lb pressure and 121 °C for 20 min | 82 | 90 | IVPD | ||
| Microwave | 1200 Watt for 15 min | 82 | 92 | IVPD | ||
| Micronization | 90 °C with 115V infrared for 2.5 min | 82 | 79 | IVPD | ||
| Canadian kidney bean | Soaking | room temp 1:5 (w/v) seed to water, 18 h | 71 | 76 | IVPD | [111] |
| Boiling | 45 min | 71 | 87 | IVPD | ||
| Roasting | 180 °C for 20 min | 71 | 65 | IVPD | ||
| Autoclaving | 15 lb pressure and 121 °C for 20 min | 71 | 79 | IVPD | ||
| Microwave | 1200 Watt for 20 min | 71 | 82 | IVPD | ||
| Micronization | 90 °C with 115 V infrared for 3 min | 71 | 68 | IVPD | ||
| Egyptian kidney bean | Soaking | room temp 1:5 (w/v) seed to water, 20 h | 78 | 83 | IVPD | [111] |
| Boiling | boiled for 45 min | 78 | 94 | IVPD | ||
| Roasting | 180 °C for 20 min | 78 | 73 | IVPD | ||
| Autoclaving | 15 lb pressure and 121 °C for 20 min | 78 | 86 | IVPD | ||
| Microwave | 1200 Watt for 20 min | 78 | 89 | IVPD | ||
| Micronization | 90 °C with 115 V infrared for 3 min | 78 | 75 | IVPD | ||
| Canadian pea | Soaking | room temp 1:5 (w/v) seed to water, 18 h | 78 | 84 | IVPD | [111] |
| Boiling | boiled for 35min, 1:5 (w/v) | 78 | 94 | IVPD | ||
| Roasting | 180 °C for 15 min | 78 | 73 | IVPD | ||
| Autoclaving | 15 lb pressure and 121 °C for 20 min | 78 | 87 | IVPD | ||
| Microwave | 1200 Watt for 15 min | 78 | 89 | IVPD | ||
| Micronization | 90 °C with 115V infrared for 2.5 min | 78 | 76 | IVPD | ||
| Egyptian pea | Soaking | room temp 1:5 (w/v) seed to water, 20 h | 80 | 85 | IVPD | [111] |
| Boiling | pre-soaked (4 h) seeds boiled for 35min, 1:5 (w/v) | 80 | 96 | IVPD | ||
| Roasting | sandbathe at 180 °C for 15 min | 80 | 75 | IVPD | ||
| Autoclaving | 15 lb pressure and 121 °C for 20 min | 80 | 88 | IVPD | ||
| Microwave | with 1:5 (w/v) water at 1200 Watt for 15 min | 80 | 91 | IVPD | ||
| Micronization | tempered overnight moisture 24/100, heated at 90 °C with 115V infrared for 2.5 min | 80 | 78 | IVPD | ||
| Chickpea | Boiling | 90 min | 84 | 89 | IVPD | [112] |
| Autoclaving | 35 min at 15 lb pressure and 121 °C | 84 | 90 | IVPD | ||
| Microwave | 15 min at 2450 MHz and dried at 50 °C for 20 h | 84 | 89 | IVPD | ||
| Bambara groundnut | Soaking | overnight at room temp | 79 | 76 | IVPD | [109] |
| Boiling | soaked and boiled for 120 min | 79 | 49 | IVPD | ||
| Boiling | unsoaked and boiled for 120 min | 79 | 52 | IVPD | ||
| Boiling | unsoaked and boiled in 2% NaCl for 120 min | 79 | 51 | IVPD | ||
| Roasting | roasted at 230 °C until colour change | 79 | 42 | IVPD | ||
| Sorghum grain flour | Boiling | flour 1:10 (w/v) in water cooked in water bath for 20 min | 53 | 30 | IVPD | [110] |
| Dry heating | 90 min | 53 | 50 | IVPD | ||
| Popping | popped in hot-air oven and ground to powder | 53 | 54 | IVPD | ||
| Pistachio | Roasting | 115 °C for 30 min | 94 | 85.19 | SID (pig) | [74] |
| Soybean (ground) | Wet heating | 80 °C for 1 min | 46 | 52 | SID (pig) | [108] |
| Wet heating | 100 °C for 6 min | 46 | 73 | SID (pig) | ||
| Wet heating | 100 °C for 16 min | 46 | 80 | SID (pig) | ||
| Autoclaving | 110 °C for 15 min | 46 | 82 | SID (pig) | ||
| Autoclaving | 110 °C for 30 min | 46 | 83 | SID (pig) | ||
| Autoclaving | 110 °C for 45 min | 46 | 84 | SID (pig) | ||
| Autoclaving | 110 °C for 60 min | 46 | 82 | SID (pig) | ||
| Soybean dried (whole) | Roasting | 110–115 °C | 53 | 72 | SID (pig) | [113] |
| Red sorghum | Extrusion | Extruded at 182 °C and 14% moisture | 53 | 70 | IVPD | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. https://doi.org/10.3390/nu14050947
Adhikari S, Schop M, de Boer IJM, Huppertz T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients. 2022; 14(5):947. https://doi.org/10.3390/nu14050947
Chicago/Turabian StyleAdhikari, Shiksha, Marijke Schop, Imke J. M. de Boer, and Thom Huppertz. 2022. "Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications" Nutrients 14, no. 5: 947. https://doi.org/10.3390/nu14050947
APA StyleAdhikari, S., Schop, M., de Boer, I. J. M., & Huppertz, T. (2022). Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients, 14(5), 947. https://doi.org/10.3390/nu14050947






