Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality
Abstract
1. Introduction
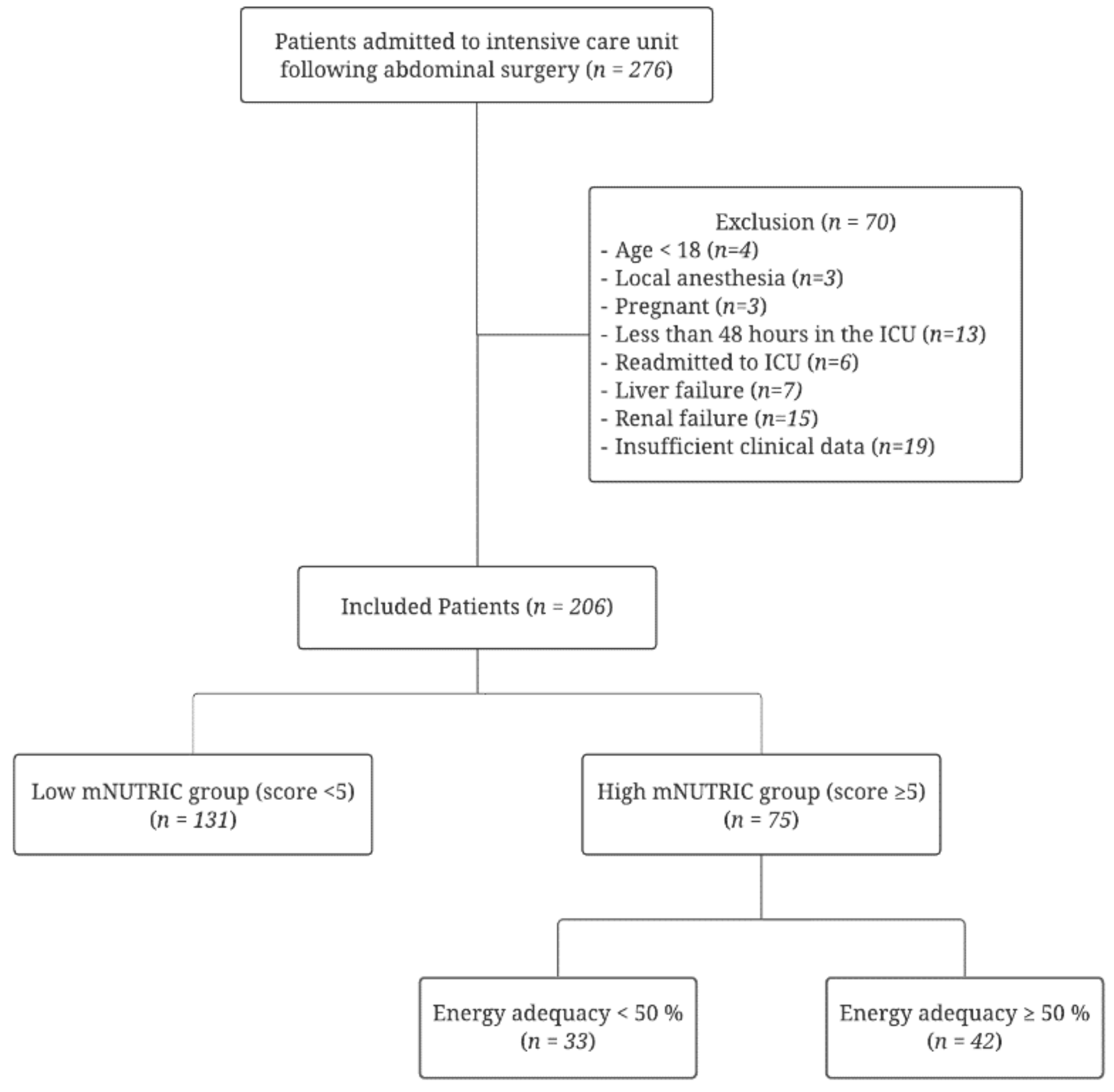
2. Participants and Methods
2.1. Patient Enrollment and Data Collection
2.2. Nutritional Assessment by Modified NUTRIC Scores and Nutritional Supplement Strategies
2.3. Statistical Analysis
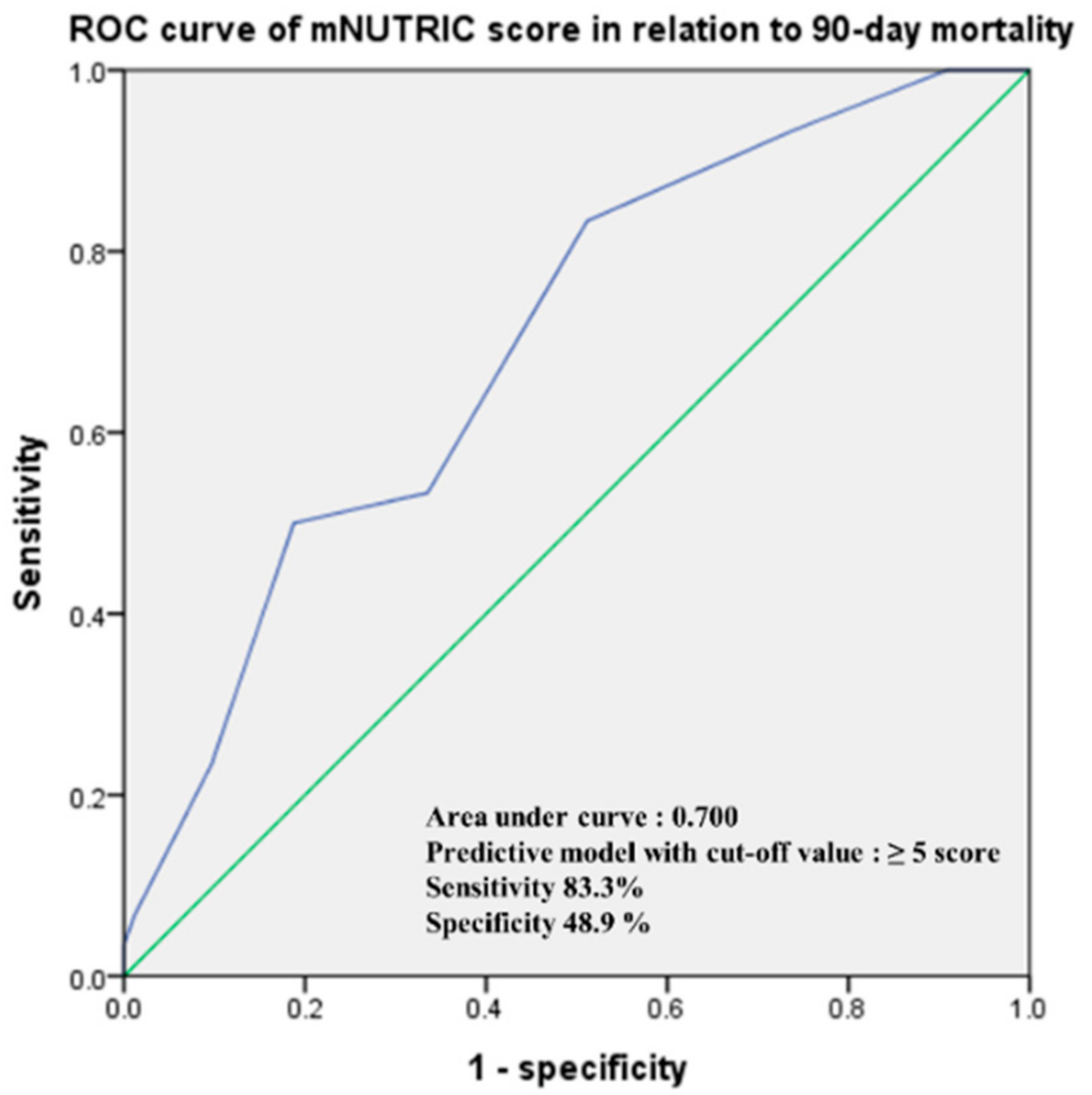
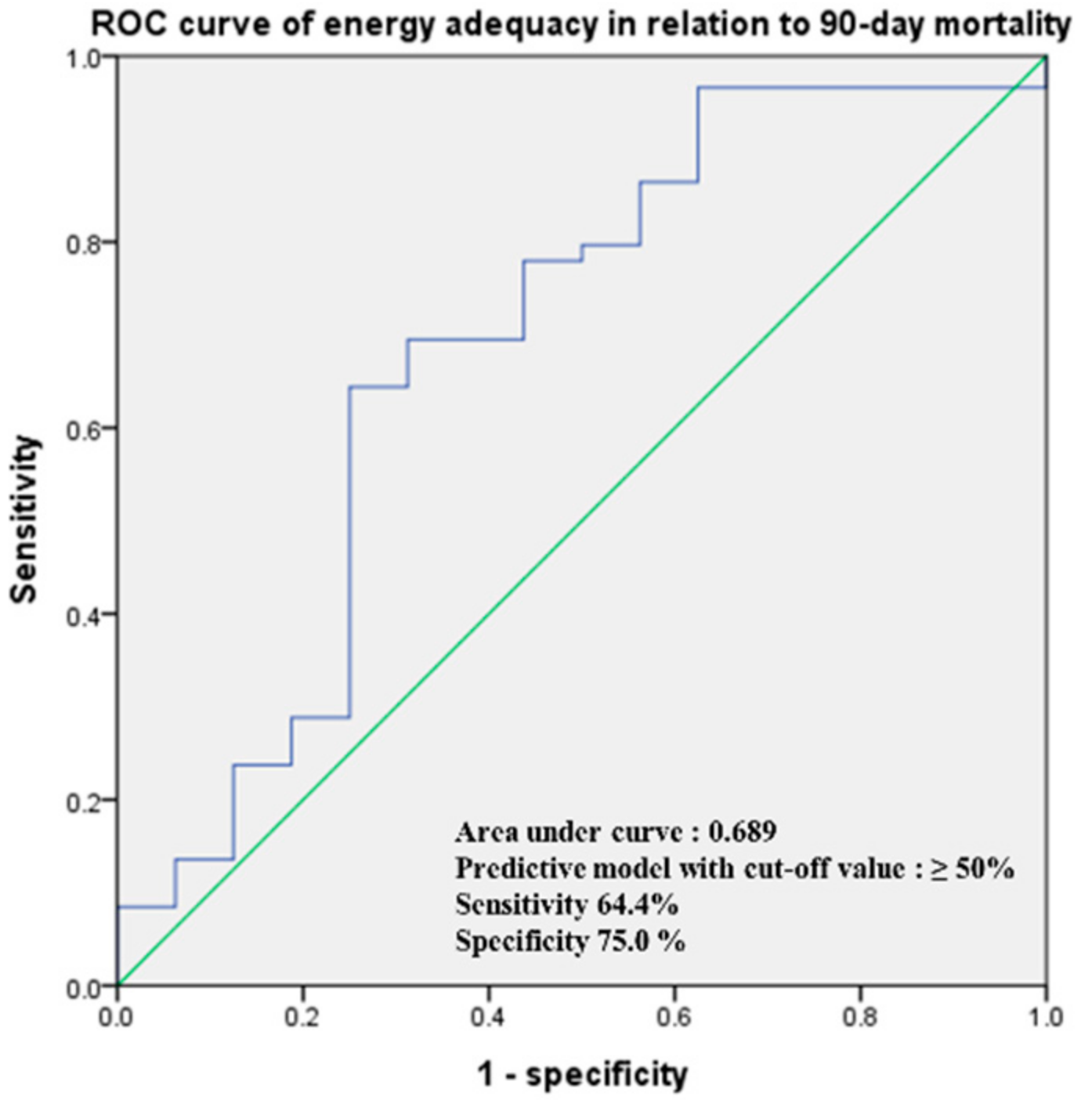
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisinger, K.W.; van Vugt, J.L.; Tegels, J.J.; Snijders, C.; Hulsewé, K.W.; Hoofwijk, A.G.; Stoot, J.H.; Von Meyenfeldt, M.F.; Beets, G.L.; Derikx, J.P.; et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann. Surg. 2015, 261, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Dijkink, S.; Fuentes, E.; Quraishi, S.A.; Cropano, C.; Kaafarani, H.M.; Lee, J.; King, D.R.; DeMoya, M.; Fagenholz, P.; Butler, K.; et al. Nutrition in the Surgical Intensive Care Unit: The Cost of Starting Low and Ramping Up Rates. Nutr. Clin. Pract. 2016, 31, 86–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drover, J.W.; Cahill, N.E.; Kutsogiannis, J.; Pagliarello, G.; Wischmeyer, P.; Wang, M.; Day, A.G.; Heyland, D.K. Nutrition therapy for the critically ill surgical patient: We need to do better! J. Parenter. Enter. Nutr. 2010, 34, 644–652. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- Zheng, C.; Xie, K.; Li, X.K.; Wang, G.M.; Luo, J.; Zhang, C.; Jiang, Z.S.; Wang, Y.L.; Luo, C.; Qiang, Y.; et al. The prognostic value of modified NUTRIC score for patients in cardiothoracic surgery recovery unit: A retrospective cohort study. J. Hum. Nutr. Diet. 2021, 34, 926–934. [Google Scholar] [CrossRef]
- Ata Ur-Rehman, H.M.; Ishtiaq, W.; Yousaf, M.; Bano, S.; Mujahid, A.M.; Akhtar, A. Modified Nutrition Risk in Critically Ill (mNUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study from the Pakistani Population. Cureus 2018, 10, e3786. [Google Scholar] [CrossRef]
- Lin, P.Y.; Yen, Y.T.; Lam, C.T.; Li, K.C.; Lu, M.J.; Hsu, H.S. Use of modified-NUTRIC score to assess nutritional risk in surgical intensive care unit. J. Chin. Med. Assoc. 2021, 84, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.Y.; Hasan, M.S.; Day, A.G.; Ng, C.C.; Ong, S.P.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K. Initial development and validation of a novel nutrition risk, sarcopenia, and frailty assessment tool in mechanically ventilated critically ill patients: The NUTRIC-SF score. J Parenter. Enter. Nutr. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, Z.; Yu, G.; Peng, D.; Feng, Y.; Ling, J.; Wang, Y.; Li, S.; Bian, Y. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin. Nutr. 2021, 40, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Chourdakis, M.; Grammatikopoulou, M.G.; Poulia, K.A.; Passakiotou, M.; Pafili, Z.K.; Bouras, E.; Doundoulakis, I.; Galitsianos, I.; Lappa, T.; Karakatsanis, A.; et al. Translation of the modified NUTRIC score and adaptation to the Greek ICU setting. Clin. Nutr. ESPEN 2019, 29, 72–76. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ziegler, T.R. Parenteral Nutrition in the Critically Ill Patient. N. Engl. J. Med. 2009, 361, 1088–1097. [Google Scholar] [CrossRef]
- Gonzalez-Granda, A.; Schollenberger, A.; Thorsteinsson, R.; Haap, M.; Bischoff, S.C. Impact of an interdisciplinary nutrition support team (NST) on the clinical outcome of critically ill patients. A pre/post NST intervention study. Clin. Nutr. ESPEN 2021, 45, 486–491. [Google Scholar] [CrossRef]
- Seol, E.M.; Suh, Y.S.; Ju, D.L.; Bae, H.J.; Kim, E.; Lee, H.J. Nutrition Support Team Reconsultation During Nutrition Therapy in Korea. J. Parenter. Enter. Nutr. 2021, 45, 357–365. [Google Scholar] [CrossRef]
- Obata, Y.; Kakutani, N.; Kinugawa, S.; Fukushima, A.; Yokota, T.; Takada, S.; Ono, T.; Sota, T.; Kinugasa, Y.; Takahashi, M.; et al. Impact of Inadequate Calorie Intake on Mortality and Hospitalization in Stable Patients with Chronic Heart Failure. Nutrients 2021, 13, 874. [Google Scholar] [CrossRef]
- Couto, C.F.L.; Dariano, Â.; Texeira, C.; Silva, C.H.D.; Torbes, A.B.; Friedman, G. Adequacy of enteral nutritional support in intensive care units does not affect the short- and long-term prognosis of mechanically ventilated patients: A pilot study. Rev. Bras. Ter. Intensiva 2019, 31, 34–38. [Google Scholar] [CrossRef]
- Chada, R.R.; Chidrawar, S.; Goud, B.A.; Maska, A.; Medanki, R.; Nagalla, B. Association Between Nutrition Delivery, Modified Nutrition Risk In Critically III Score, and 28-Day Mortality. Nutr. Clin. Pract. 2021, 36, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, I.; von Holzen, A.; Gutzwiller, J.P.; Schlumpf, R.; Mühlebach, S.; Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br. J. Surg. 2010, 97, 92–97. [Google Scholar] [CrossRef]
- Maday, K.R. The importance of nutrition in critically ill patients. J. Am. Acad. PAs 2017, 30, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.C.; Silva, S.M.; Leães, D.M.; Novello, C.L.; Silveira, C.R.; Mello, E.D.; Beghetto, M.G. Enteral nutrition: Differences between volume, energy and protein prescribed and administered in adults. Rev. Bras Ter. Intensiva 2010, 22, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Chapple, L.S.; Deane, A.M.; Heyland, D.K.; Lange, K.; Kranz, A.J.; Williams, L.T.; Chapman, M.J. Energy and protein deficits throughout hospitalization in patients admitted with a traumatic brain injury. Clin. Nutr. 2016, 35, 1315–1322. [Google Scholar] [CrossRef]
- Peev, M.P.; Yeh, D.D.; Quraishi, S.A.; Osler, P.; Chang, Y.; Gillis, E.; Albano, C.E.; Darak, S.; Velmahos, G.C. Causes and consequences of interrupted enteral nutrition: A prospective observational study in critically ill surgical patients. J. Parenter. Enter. Nutr. 2015, 39, 21–27. [Google Scholar] [CrossRef]
- Tepaske, R.; Binnekade, J.M.; Goedhart, P.T.; Schultz, M.J.; Vroom, M.B.; Mathus-Vliegen, E.M. Clinically relevant differences in accuracy of enteral nutrition feeding pump systems. J. Parenter. Enter. Nutr. 2006, 30, 339–343. [Google Scholar] [CrossRef]
- Son, D.-H.; Kim, K.-S.; Lee, H.-S.; Lee, J.-W.; Shin, C.-S. Derivation and validation of a new nutritional index for predicting 90 days mortality after ICU admission in a Korean population. J. Formos. Med. Assoc. 2020, 119, 1283–1291. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. 2017, 8, 148–151. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients (n = 206) | Low mNUTRIC Group (mNUTRIC < 5) | High mNUTRIC Group (mNUTRIC ≥ 5) | p-Value |
|---|---|---|---|---|
| n = 206 | n = 131 (63.6%) | n = 75 (36.4%) | ||
| Demographics | ||||
| Age (years) | 62.5 ± 15.4 | 59.4 ± 15.3 | 67.8 ± 14 | <0.001 |
| Gender (male, %) | 143 (69.4) | 95 (72.5) | 48 (64) | 0.212 |
| Body mass index (kg/m−2) | 23.6 ± 4.5 | 23.8 ± 4.5 | 23.3 ± 4.5 | 0.483 |
| Use of vasopressors (%) | 64 (31.1) | 25 (19.1) | 39 (52) | <0.001 |
| SOFA score | 5.8 ± 3.7 | 4.3 ± 2.9 | 8.6 ± 3.5 | <0.001 |
| mNUTRIC score | 4 ± 1.9 | 2.8 ± 1.1 | 6 ± 1 | <0.001 |
| Postoperative complication (%) | 54 (26.2) | 27 (20.6) | 27 (36) | 0.021 |
| 90-day mortality (%) | 30 (14.6) | 14 (10.7) | 16 (21.3) | 0.042 |
| Length of ICU stay (days) | 6.7 ± 6.2 | 5.5 ± 4.5 | 8.9 ± 7.9 | 0.001 |
| Length of hospital stay (days) | 32.3 ± 18.9 | 29.2 ± 17 | 37.6 ± 20.9 | 0.003 |
| Type of nutrition patients received (%) | 0.929 | |||
| PN | 40 (19.4) | 27 (20.6) | 13 (17.3) | 0.715 |
| EN | 9 (4.4) | 6 (4.6) | 3 (4) | 1.000 |
| PN + EN | 20 (9.7) | 12 (9.2) | 8 (10.7) | 0.808 |
| Oral diet | 137 (66.5) | 86 (65.6) | 51 (24.8) | 0.761 |
| Implementation of NST | 77 (37.4) | 39 (29.8) | 38 (50.7) | 0.004 |
| Laboratory test | ||||
| Total protein (g/dL) | 5 ± 0.8 | 5.4 ± 1.1 | 4.8 ± 0.9 | <0.001 |
| Albumin (g/dL) | 3 ± 0.4 | 3.2 ± 0.7 | 2.9 ± 0.7 | 0.001 |
| Prealbumin (mg/dL) | 16.2 ± 7.4 | 15.6 ± 7.8 | 11 ± 6.7 | 0.009 |
| Transferrin (mg/dL) | 130.4 ± 38.4 | 147.1 ± 55.5 | 114.2 ± 38.9 | <0.001 |
| Total cholesterol (mg/dL) | 106.6 ± 41 | 108.6 ± 42.9 | 79.1 ± 30 | <0.001 |
| HDL cholesterol (mg/dL) | 23.7 ± 10.9 | 28.3 ± 12.8 | 19.6 ± 9.5 | <0.001 |
| LDL cholesterol (mg/dL) | 57.7 ± 25.9 | 57.3 ± 26.6 | 40 ± 19.6 | <0.001 |
| Variables | All Patients | Energy Adequacy < 50% | Energy Adequacy ≥ 50% | p-Value |
|---|---|---|---|---|
| n = 75 (100%) | n = 33 (44%) | n = 42 (56%) | ||
| (A) Energy adequacy < 50% | ||||
| Postoperative complication (%) | 27 (36) | 13 (39.4) | 14 (33.3) | 0.634 |
| 90-day mortality (%) | 16 (21.3) | 12 (36.4) | 4 (9.5) | 0.009 |
| Length of ICU stay (days) | 8.9 ± 7.9 | 9.2 ± 7.7 | 8.6 ± 8.2 | 0.763 |
| Length of hospital stay (days) | 37.6 ± 20.9 | 36.8 ± 17.2 | 38.3 ± 23.5 | 0.756 |
| (B) Energy adequacy < 60% | ||||
| Postoperative complication (%) | 27 (36) | 19 (36.5) | 8 (34.8) | 1.000 |
| 90-day mortality (%) | 16 (21.3) | 12 (23.1) | 4 (17.4) | 0.762 |
| Length of ICU stay (days) | 8.9 ± 7.9 | 8.6 ± 7.4 | 9.5 ± 9.1 | 0.660 |
| Length of hospital stay (days) | 37.6 ± 20.9 | 36.4 ± 19.9 | 40.4 ± 23.2 | 0.459 |
| (C) Energy adequacy < 70% | ||||
| Postoperative complication (%) | 27 (36) | 22 (34.4) | 5 (45.5) | 0.511 |
| 90-day mortality (%) | 16 (21.3) | 14 (21.9) | 2 (18.2) | 1.000 |
| Length of ICU stay (days) | 8.9 ± 7.9 | 8.7 ± 7.4 | 9.9 ± 10.8 | 0.640 |
| Length of hospital stay (days) | 37.6 ± 20.9 | 36.6 ± 2.5 | 44 ± 24.5 | 0.277 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| (A) Total participants | ||||
| Age | 1.034 (1.006–1.064) | 0.017 | 1.021 (0.990–1.053) | 0.180 |
| Use of vasopressor | 1.590 (0.715–3.534) | 0.255 | ||
| Mechanical ventilation | 3.757 (1.684–8.380) | 0.001 | 2.327 (0.939–5.765) | 0.068 |
| SOFA score | 1.099 (0.991–1.219) | 0.074 | ||
| mNUTRIC score | 1.499 (1.198–1.875) | <0.001 | 1.215 (0.793–1.859) | 0.371 |
| Energy adequacy < 50% | 4.333 (1.928–9.737) | <0.001 | 1.389 (0.299–6.449) | 0.675 |
| (B) High mNUTRIC group (mNUTRIC score ≥ 5) | ||||
| Age | 1.022 (0.980–1.066) | 0.308 | ||
| Use of vasopressor | 0.903 (0.299–2.728) | 0.857 | ||
| Mechanical ventilation | 2.431 (0.779–7.582) | 0.126 | ||
| SOFA score | 1.067 (0.903–1.261) | 0.049 | 0.935 (0.752–1.164) | 0.547 |
| mNUTRIC score | 2.108 (1.143–3.885) | 0.017 | 2.548 (1.177–5.514) | 0.018 |
| Energy adequacy < 50% | 5.429 (1.554–18.963) | 0.008 | 6.427 (1.674–24.674) | 0.007 |
| Variables | All Patients | NST Implementation (+) | NST Implementation (−) | p-Value |
|---|---|---|---|---|
| n = 131 (100%) | n = 39 (29.8%) | n = 92 (70.2%) | ||
| (A) Low mNUTRIC group (mNUTRIC score < 5) | ||||
| Average energy delivered (kcal/kg/day) | 11 ± 5.1 | 12.4 ± 6.7 | 10.4 ± 4.2 | 0.09 |
| Average protein delivered (g/kg/day) | 0.51 ± 0.24 | 0.55 ± 0.3 | 0.49 ± 0.2 | 0.224 |
| Energy adequacy (%) | 44 ± 20.5 | 49.6 ± 26.9 | 41.6 ± 16.7 | 0.09 |
| (B) High mNUTRIC group (mNUTRIC score ≥ 5) | ||||
| Average energy delivered (kcal/kg/day) | 12.7 ± 6 | 14.7 ± 6 | 10.7 ± 5.4 | 0.003 |
| Average protein delivered (g/kg/day) | 0.58 ± 0.28 | 0.66 ± 0.26 | 0.49 ± 0.27 | 0.009 |
| Energy adequacy (%) | 50.9 ± 24 | 58.8 ± 23.9 | 42.8 ± 21.6 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, K.M.; Kim, E.Y. Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients 2022, 14, 946. https://doi.org/10.3390/nu14050946
Im KM, Kim EY. Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients. 2022; 14(5):946. https://doi.org/10.3390/nu14050946
Chicago/Turabian StyleIm, Kyoung Moo, and Eun Young Kim. 2022. "Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality" Nutrients 14, no. 5: 946. https://doi.org/10.3390/nu14050946
APA StyleIm, K. M., & Kim, E. Y. (2022). Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients, 14(5), 946. https://doi.org/10.3390/nu14050946







