Suppressive Role of Lactoferrin in Overweight-Related Female Fertility Problems
Abstract
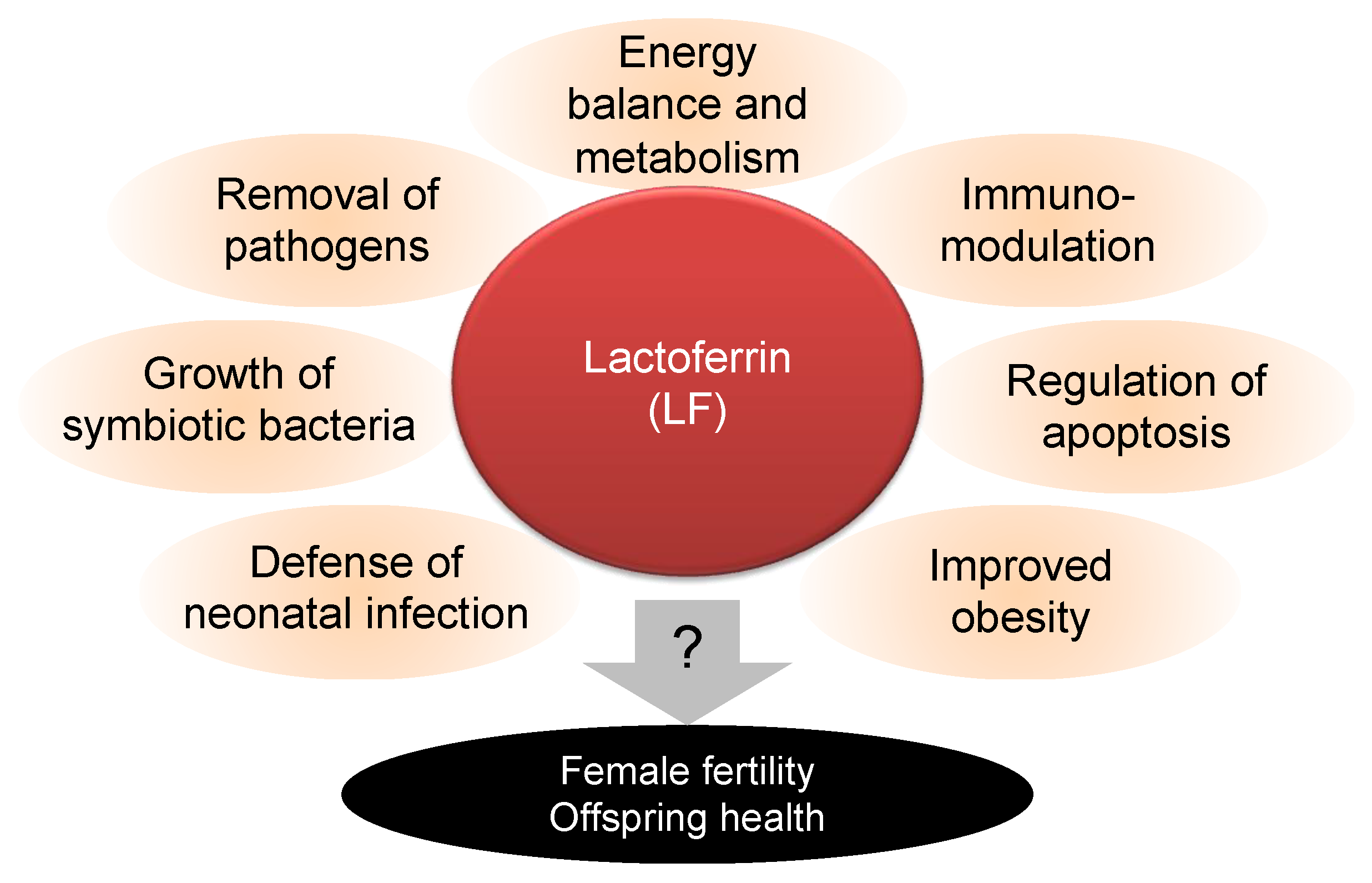
:1. Introduction
2. Materials and Methods
2.1. Diet
2.2. Animals
2.3. Breeding with HF Diets and LF Ingestion
2.4. Measurement of Body Weight
2.5. Estimation of Female Fertility (Litter Size and Implantation)
2.6. Quantitative Estimation of Plasma Key Substances
2.7. Thermal Image by Infrared (IR) Thermography
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
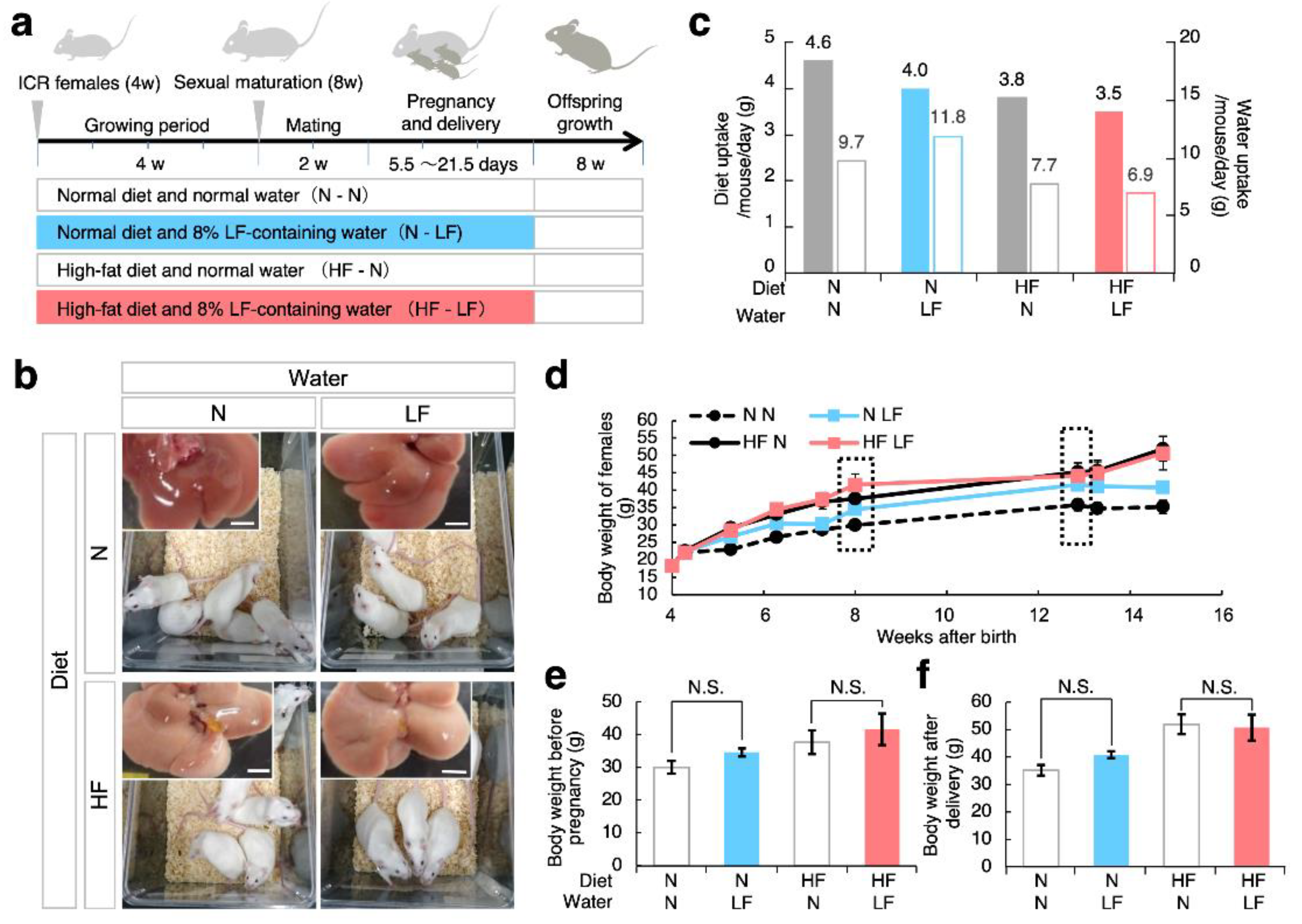
3.1. Effect of LF on Overweight Female Mice before and after Pregnancy
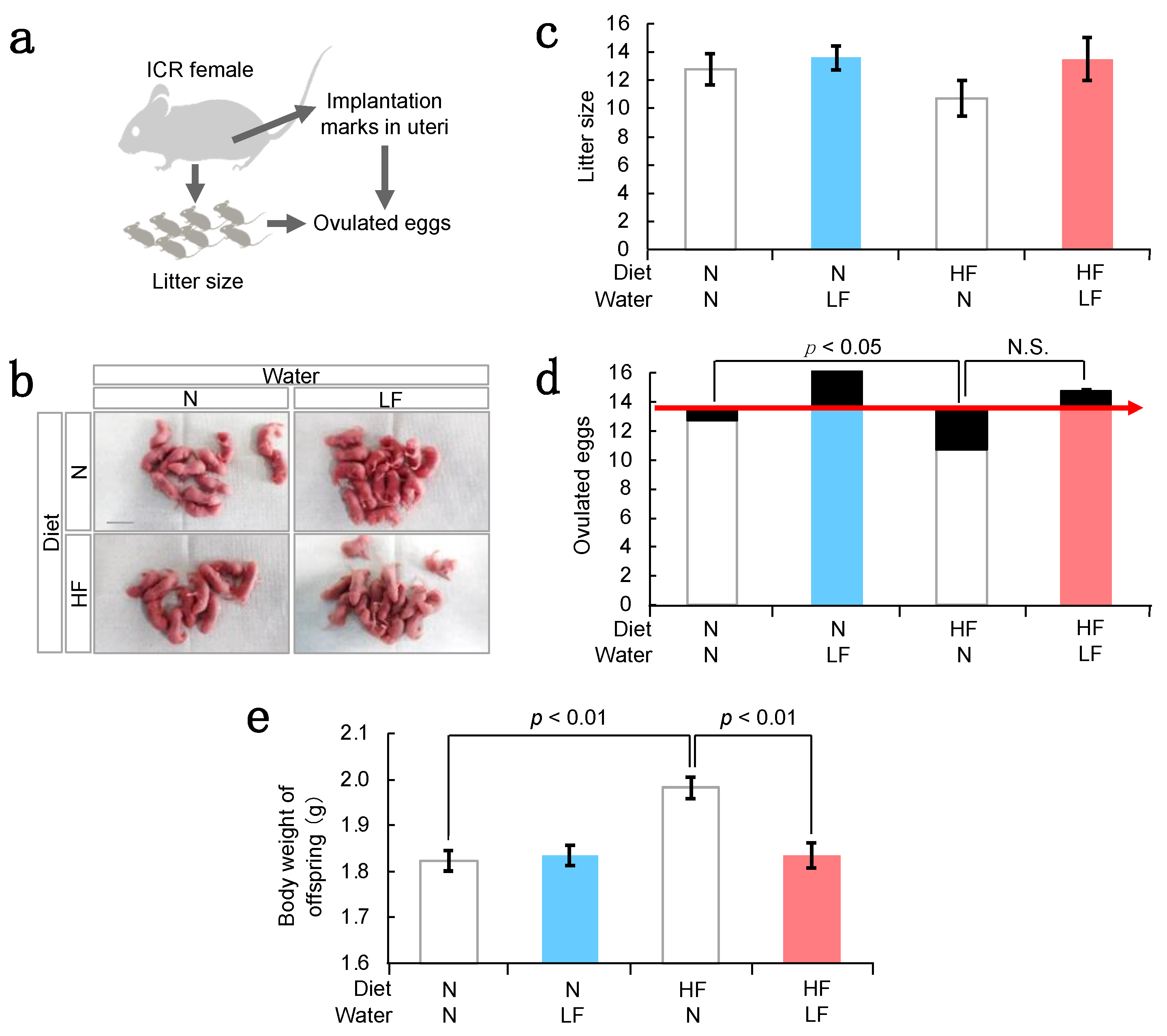
3.2. Effect of LF on Fertility in HF Females and Their Offspring Weight
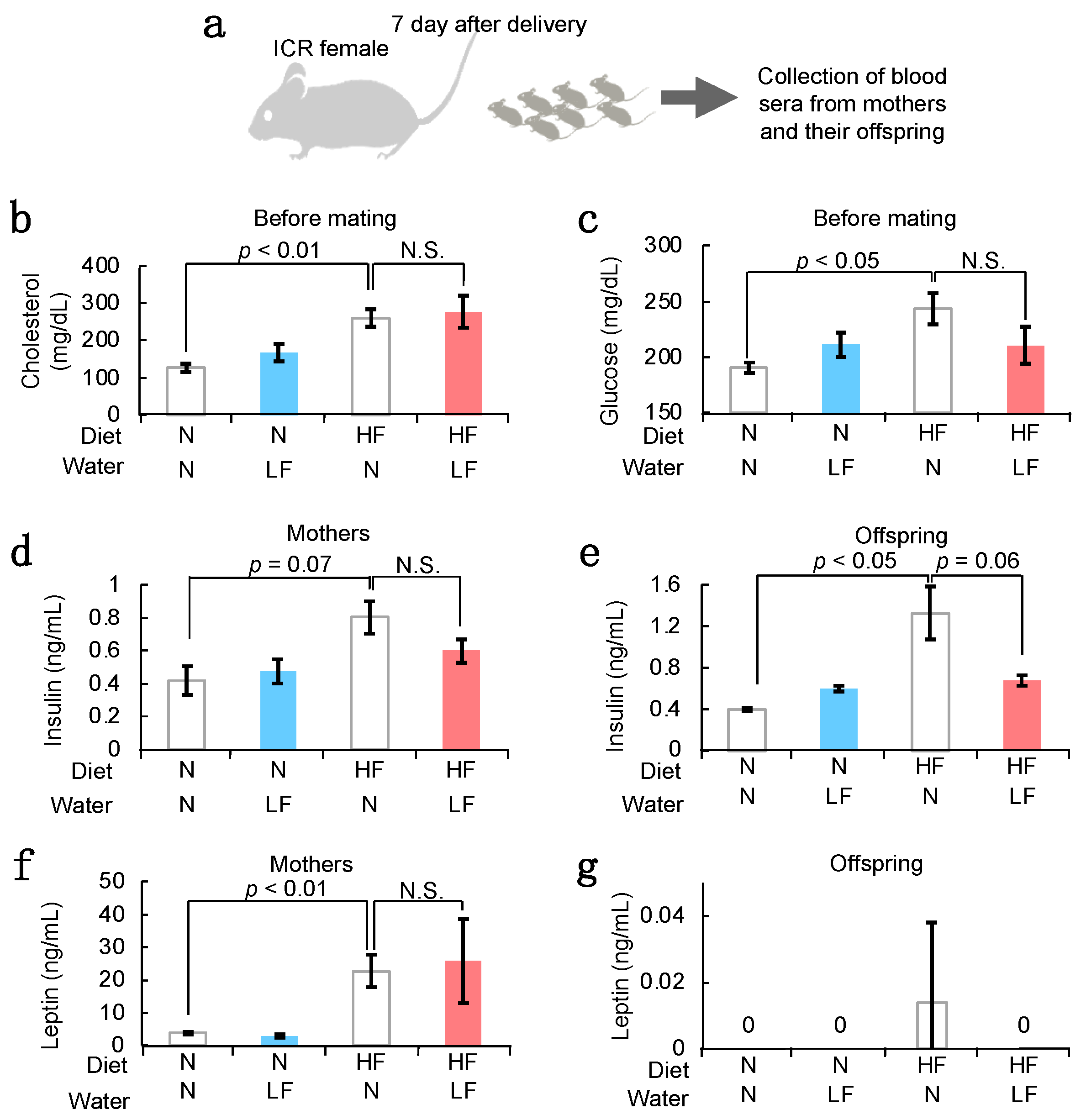
3.3. Serum Components in HF Females and Their Offspring
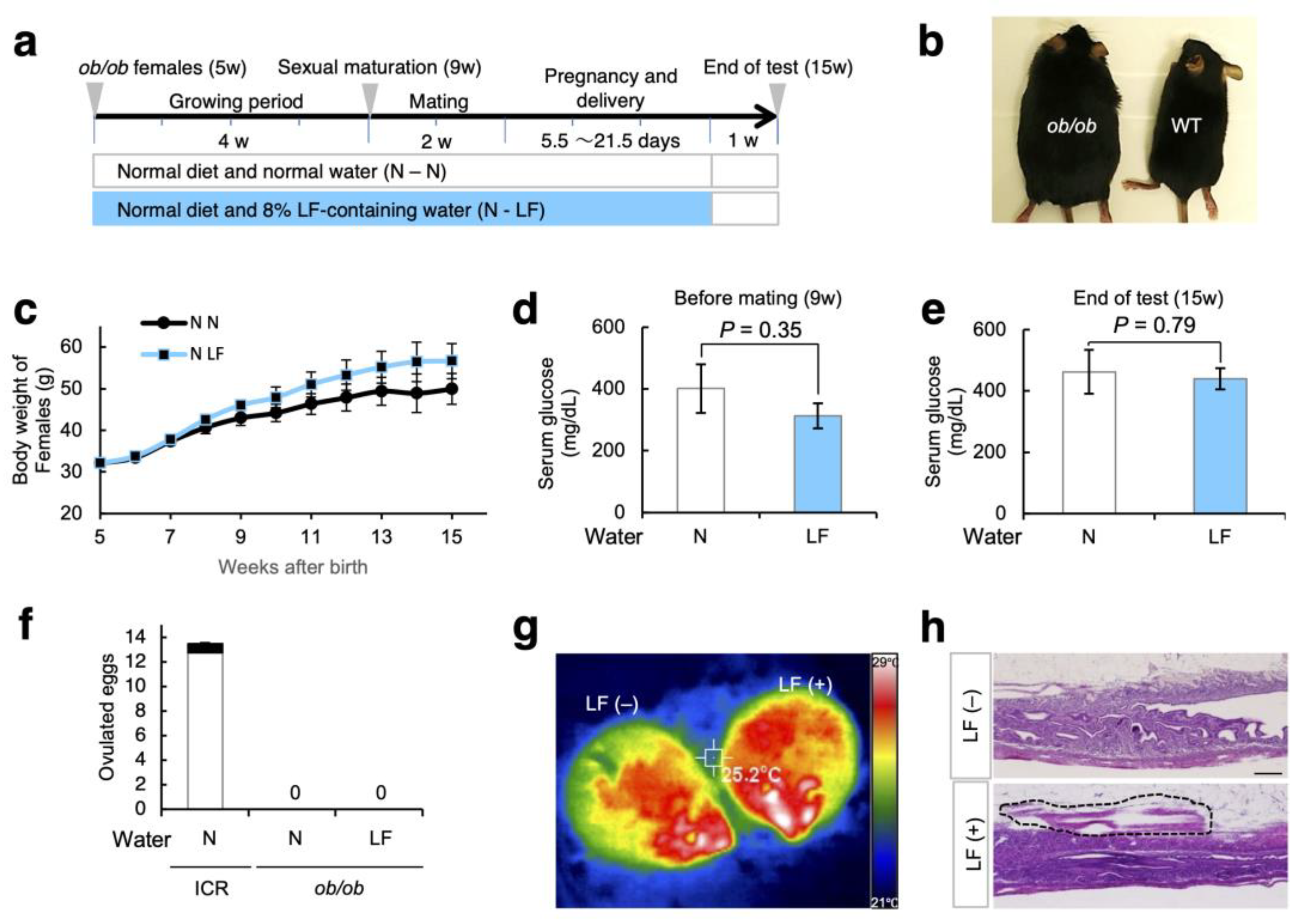
3.4. Effect of LF Ingestion on Fertility Problems in ob/ob Females
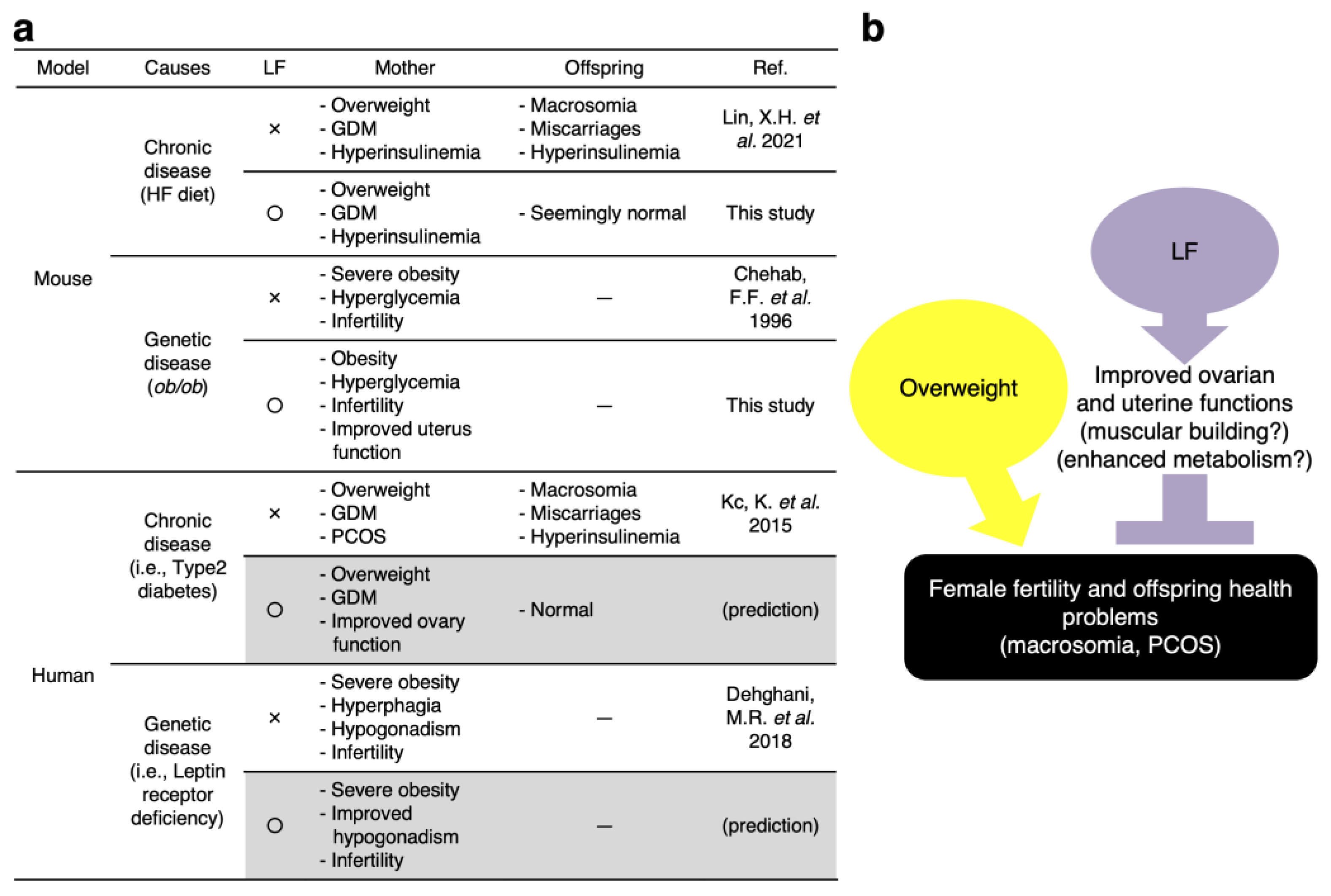
4. Discussion
4.1. Possible Mechanisms of LF for Preventing Fetal Macrosomia
4.2. LF Upregulates the Ovarian Function
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schurmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Athukorala, C.; Rumbold, A.R.; Willson, K.J.; Crowther, C.A. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Quaresima, P.; Visconti, F.; Interlandi, F.; Puccio, L.; Caroleo, P.; Amendola, G.; Morelli, M.; Venturella, R.; Di Carlo, C. Awareness of gestational diabetes mellitus foetal-maternal risks: An Italian cohort study on pregnant women. BMC Pregnancy Childbirth 2021, 21, 692. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Rascon-Cruz, Q.; Espinoza-Sanchez, E.A.; Siqueiros-Cendon, T.S.; Nakamura-Bencomo, S.I.; Arevalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [Green Version]
- Zumoffen, C.M.; Gil, R.; Caille, A.M.; Morente, C.; Munuce, M.J.; Ghersevich, S.A. A protein isolated from human oviductal tissue in vitro secretion, identified as human lactoferrin, interacts with spermatozoa and oocytes and modulates gamete interaction. Hum. Reprod. 2013, 28, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Nibber, T.; Chelikani, P.K. Whey Protein Components—Lactalbumin and Lactoferrin—Improve Energy Balance and Metabolism. Sci. Rep. 2017, 7, 9917. [Google Scholar] [CrossRef] [Green Version]
- Derraik, J.G.B.; Maessen, S.E.; Gibbins, J.D.; Cutfield, W.S.; Lundgren, M.; Ahlsson, F. Large-for-gestational-age phenotypes and obesity risk in adulthood: A study of 195,936 women. Sci. Rep. 2020, 10, 2157. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Kiilerich, P.; Myrmel, L.S.; Fjaere, E.; Hao, Q.; Hugenholtz, F.; Sonne, S.B.; Derrien, M.; Pedersen, L.M.; Petersen, R.K.; Mortensen, A.; et al. Effect of a long-term high-protein diet on survival, obesity development, and gut microbiota in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E886–E899. [Google Scholar] [CrossRef] [Green Version]
- Kawano, N.; Miyado, K.; Yoshii, N.; Kanai, S.; Saito, H.; Miyado, M.; Inagaki, N.; Odawara, Y.; Hamatani, T.; Umezawa, A. Absence of CD9 reduces endometrial VEGF secretion and impairs uterine repair after parturition. Sci. Rep. 2014, 4, 4701. [Google Scholar] [CrossRef] [Green Version]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef]
- Otsuki, K.; Yakuwa, K.; Sawada, M.; Hasegawa, A.; Sasaki, Y.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Saito, H.; Okai, T. Recombinant human lactoferrin has preventive effects on lipopolysaccharide-induced preterm delivery and production of inflammatory cytokines in mice. J. Perinat. Med. 2005, 33, 320–323. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 14–20. [Google Scholar] [CrossRef]
- Baumann, M.U.; Deborde, S.; Illsley, N.P. Placental glucose transfer and fetal growth. Endocrine 2002, 19, 13–22. [Google Scholar] [CrossRef]
- Moreli, J.B.; Corrêa-Silva, S.; Damasceno, D.C.; Sinzato, Y.K.; Lorenzon-Ojea, A.R.; Borbely, A.U.; Rudge, M.V.; Bevilacqua, E.; Calderon, I.M. Changes in the TNF-alpha/IL-10 ratio in hyperglycemia-associated pregnancies. Diabetes Res. Clin. Pract. 2015, 107, 362–369. [Google Scholar] [CrossRef]
- Balachandiran, M.; Bobby, Z.; Dorairajan, G.; Gladwin, V.; Vinayagam, V.; Packirisamy, R.M. Decreased maternal serum adiponectin and increased insulin-like growth factor-1 levels along with increased placental glucose transporter-1 expression in gestational diabetes mellitus: Possible role in fetal overgrowth. Placenta 2021, 104, 71–80. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- Chen, X.W.; Li, Y.H.; Zhang, M.J.; Chen, Z.; Ke, D.S.; Xue, Y.; Hou, J.M. Lactoferrin ameliorates aging-suppressed osteogenesis via IGF1 signaling. J. Mol. Endocrinol. 2019, 63, 63–75. [Google Scholar] [CrossRef]
- Wu, L.L.; Dunning, K.R.; Yang, X.; Russell, D.L.; Lane, M.; Norman, R.J.; Robker, R.L. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010, 151, 5438–5445. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Boudoures, A.L.; Chi, M.M.; Wang, Q.; Moley, K.H. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod. Fertil. Dev. 2015, 27, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Moley, K.H. Hyperglycemia and apoptosis: Mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol. Metab. 2001, 12, 78–82. [Google Scholar] [CrossRef]
- Temple, R.; Aldridge, V.; Greenwood, R.; Heyburn, P.; Sampson, M.; Stanley, K. Association between outcome of pregnancy and glycaemic control in early pregnancy in type 1 diabetes: Population based study. BMJ 2002, 325, 1275–1276. [Google Scholar] [CrossRef] [Green Version]
- Dunne, F.; Brydon, P.; Smith, K.; Gee, H. Pregnancy in women with Type 2 diabetes: 12 years outcome data 1990–2002. Diabet. Med. 2003, 20, 734–738. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Murdoch, W.J.; McDonnel, A.C. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction 2002, 123, 743–750. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef] [Green Version]
- Depalo, R.; Nappi, L.; Loverro, G.; Bettocchi, S.; Caruso, M.L.; Valentini, A.M.; Selvaggi, L. Evidence of apoptosis in human primordial and primary follicles. Hum. Reprod. 2003, 18, 2678–2682. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Kuwahara, A.; Taniguchi, Y.; Yamasaki, M.; Tanaka, Y.; Mukai, Y.; Yamashita, M.; Matsuzaki, T.; Yasui, T.; Irahara, M. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries. Reprod. Med. Biol. 2015, 14, 107–115. [Google Scholar] [CrossRef]
- Luque, R.M.; Huang, Z.H.; Shah, B.; Mazzone, T.; Kineman, R.D. Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E891–E899. [Google Scholar] [CrossRef] [Green Version]
- Barkan, D.; Hurgin, V.; Dekel, N.; Amsterdam, A.; Rubinstein, M. Leptin induces ovulation in GnRH-deficient mice. FASEB J. 2005, 19, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, T.; Metzger, B.E.; Burns, W.J.; Burns, K. Correlations between antepartum maternal metabolism and intelligence of offspring. N. Engl. J. Med. 1991, 325, 911–916. [Google Scholar] [CrossRef]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.H.; Gao, L.; Tian, S.; Klausen, C.; Guo, M.X.; Gao, Q.; Liu, M.E.; Wang, H.; Wu, D.D.; Zhou, C.L.; et al. Maternal high-fat-diet exposure is associated with elevated blood pressure and sustained increased leptin levels through epigenetic memory in offspring. Sci. Rep. 2021, 11, 316. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Mehrjardi, M.Y.V.; Dilaver, N.; Tajamolian, M.; Enayati, S.; Ebrahimi, P.; Amoli, M.M.; Farooqi, S.; Maroofian, R. Potential role of gender specific effect of leptin receptor deficiency in an extended consanguineous family with severe early-onset obesity. Eur. J. Med. Genet. 2018, 61, 465–467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, B.; Kanai, S.; Sakaguchi, D.; Yajima, K.; Matsumoto, Y.; Morohoshi, K.; Kagaya, S.; Izumo, N.; Ichinose, M.; Kang, W.; et al. Suppressive Role of Lactoferrin in Overweight-Related Female Fertility Problems. Nutrients 2022, 14, 938. https://doi.org/10.3390/nu14050938
Sato B, Kanai S, Sakaguchi D, Yajima K, Matsumoto Y, Morohoshi K, Kagaya S, Izumo N, Ichinose M, Kang W, et al. Suppressive Role of Lactoferrin in Overweight-Related Female Fertility Problems. Nutrients. 2022; 14(5):938. https://doi.org/10.3390/nu14050938
Chicago/Turabian StyleSato, Ban, Seiya Kanai, Daiki Sakaguchi, Kodai Yajima, Yu Matsumoto, Kazunori Morohoshi, Shinji Kagaya, Nobuo Izumo, Minoru Ichinose, Woojin Kang, and et al. 2022. "Suppressive Role of Lactoferrin in Overweight-Related Female Fertility Problems" Nutrients 14, no. 5: 938. https://doi.org/10.3390/nu14050938
APA StyleSato, B., Kanai, S., Sakaguchi, D., Yajima, K., Matsumoto, Y., Morohoshi, K., Kagaya, S., Izumo, N., Ichinose, M., Kang, W., Miyado, M., Miyado, K., & Kawano, N. (2022). Suppressive Role of Lactoferrin in Overweight-Related Female Fertility Problems. Nutrients, 14(5), 938. https://doi.org/10.3390/nu14050938





