Feasibility and Effectiveness of a Preventive Care Program during the Compound Humanitarian Crisis and COVID-19 Pandemic in Venezuela
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Inclusion and Exclusion Criteria
2.3. Sampling and Recruitment
2.4. Transculturalization
2.4.1. The Diabetes Prevention Program (DPP)
- Identify the target population: Venezuelan adults.
- Identify the research/clinical question: How can evidence-based preventive care strategies be applied to reduce the T2DM burden?
- Identify, recruit, and involve a team of experts in the source (DPP) and target populations (Venezuelan): a group of Venezuelan experts (four diabetologists, one primary care physician, and two community members) are trained to culturally adapt DPP-GLB content for study in Venezuela.
- Identify, codify, and organize culturally adapted DPP-GLB content using a pragmatic framework: here, DPP-GLB content was interpreted using the ecological validity model (EVM) [18].
2.4.2. Focus on the Low-Energy Liquid Diet
2.5. Intervention
2.5.1. Standard Care (DPP Group)
2.5.2. Hybridized Intervention (LD Group)
- Phase 1: total LD replacement with a low-energy beverage for two months, followed by structured food reintroduction for two weeks, supervised by a nutritionist.
- Phase 2: medical nutritional therapy using a locally adapted tDNA Toolkit, supervised by a nutritionist. Standard care with DPP-GLB content was provided throughout this period, supervised by a coach.
2.6. Impact of the COVID-19 Pandemic
2.7. Data Collection
2.8. Variables Definition
2.9. Data Analysis
2.10. Ethics and Clinical Trial
3. Results
3.1. Feasibility
Overcoming the COVID-19 Pandemic Restrictions
3.2. Dropout Rates
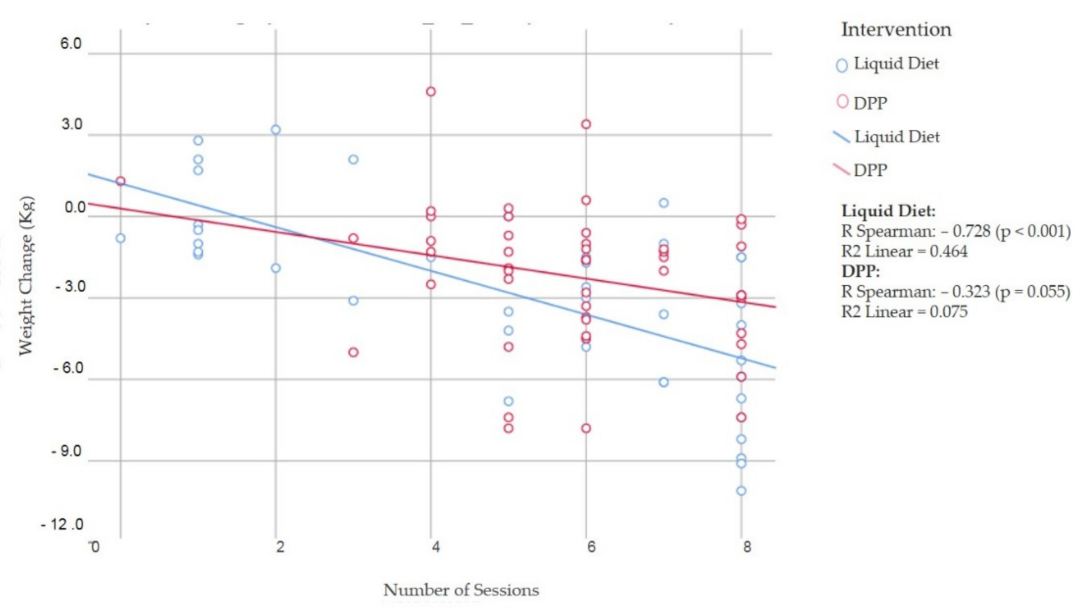
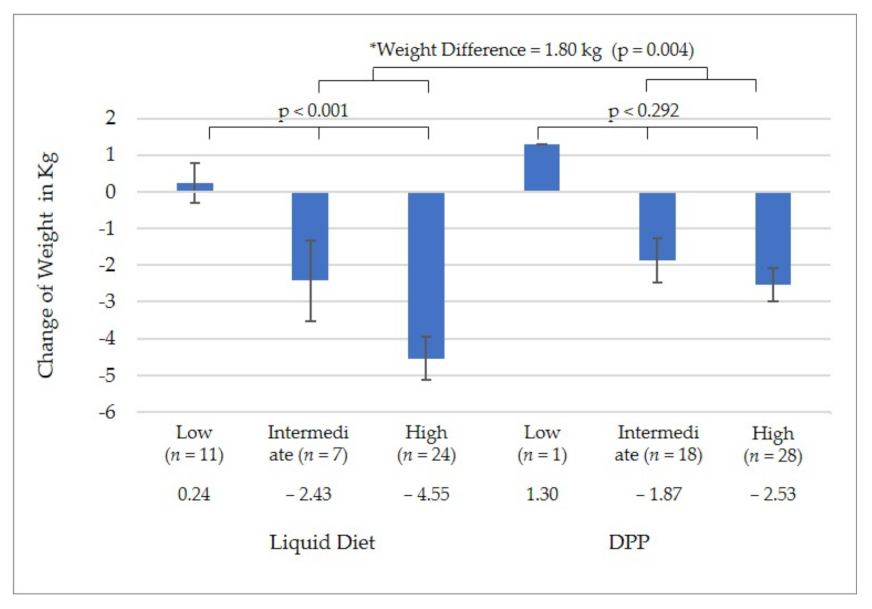
3.3. Fidelity
3.4. Participant Characteristics
3.5. Cardiometabolic Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 DALYs; Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Martínez, R.; Mechanick, J.I.; Brajkovich, I.; Ugel, E.; Risques, A.; Florez, H.; González-Rivas, J.P. Prevalence of diabetes in three regions of Venezuela. The VEMSOLS study results. Prim. Care Diabetes 2018, 12, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Martínez, R.; González-Rivas, J.P.; Ugel, E.; Duran, M.; Dávila, E.; Constantino, R.; García, A.; Mechanick, J.I.; Marulanda, M.I. Cardiometabolic risk factors in Venezuela. The EVESCAM study: A national cross-sectional survey in adults. Prim. Care Diabetes 2021, 15, 106–114. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Ashra, N.; Spong, R.; Carter, P.; Davies, M.; Dunkley, A.; Gillies, C.; Greaves, C.; Khunti, K.; Sutton, S.; Yates, T.; et al. A Systematic Review and Metaanalysis Assessing the Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes Mellitus in Routine Practice; Public Health England: London, UK, 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733053/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf (accessed on 20 January 2019).
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, W.S.; Taylor, R.; Harris, L.; Lean, M.E.J. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: Systematic review and meta-analysis. Int. J. Obes. 2017, 41, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.; Larsen, T.M.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multicentre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef] [Green Version]
- Raben, A.; Vestentoft, P.S.; Brand-Miller, J.; Jalo, E.; Drummen, M.; Simpson, L.; Martinez, J.A.; Handjieva-Darlenska, T.; Stratton, G.; Huttunen-Lenz, M.; et al. The PREVIEW intervention study: Results from a 3-year randomized 2 × 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes, Obes. Metab. 2021, 23, 324–337. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Martínez, R.; González-Rivas, J.P.; Ugel, E.; Marulanda, M.I.; Durán, M.; Mechanick, J.I.; Aschner, P. External validation of the Finnish diabetes risk score in Venezuela using a national sample: The EVESCAM. Prim. Care Diabetes 2019, 13, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Brajkovich, I.; González-Rivas, J.P.; Ugel, E.; Rísquez, A.; Nieto-Martínez, R. Prevalence of Metabolic Syndrome in Three Regions in Venezuela: The VEMSOLS Study. Int. J. Cardiovasc. Sci. 2018, 31, 603–609. [Google Scholar] [CrossRef]
- DPP Group Lifestyle Balance™ Curriculum—Spanish. Available online: https://www.diabetesprevention.pitt.edu/2011-dpp-group-lifestyle-balance-curriculum-spanish/ (accessed on 28 January 2018).
- Nieto-Martínez, R.; Hamdy, O.; Marante, D.; Marulanda, M.I.; Marchetti, A.; Hegazi, R.A.; Mechanick, J.I. Transcultural Diabetes Nutrition Algorithm (tDNA): Venezuelan Application. Nutrients 2014, 6, 1333–1363. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Martínez, R.; González-Rivas, J.P.; Florez, H.; Mechanick, J.I. Transcultural Endocrinology: Adapting Type-2 Diabetes Guidelines on a Global Scale. Endocrinol. Metab. Clin. N. Am. 2016, 45, 967–1009. [Google Scholar] [CrossRef]
- Bernal, G.; Bonilla, J.; Bellido, C. Ecological validity and cultural sensitivity for outcome research: Issues for the cultural ad-aptation and development of psychosocial treatments with Hispanics. J. Abnorm. Child Psychol. 1995, 23, 67–82. [Google Scholar] [CrossRef]
- Vera-Cala, L.M.; Orostegui, M.; Valencia-Angel, L.I.; Lopez, N.; Bautista, L.E. Accuracy of the Omron HEM-705 CP for blood pressure measurement in large epidemiologic studies. Arq. Bras. Cardiol. 2011, 96, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm. Ment. Health 2011, 38, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Cundi, J.M. Subjective social status: Construct validity and associations with psychosocial vulnerability and self-rated health. Int. J. Behav. Med. 2013, 20, 148–158. [Google Scholar] [CrossRef]
- Rabin, R.; De Charro, F. EQ-SD: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, K.; Whelton, R.M.C.; Wilbert, S.A.; Donald, E.C., Jr.; Collins, K.J.; Dennison, C.H.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e140–e144. [Google Scholar] [CrossRef]
- ACAP. Humanitarian Overview: An Analysis of Key Crises into 2018. Available online: https://www.acaps.org/special-report/humanitarian-overview-analysis-key-crises-2018 (accessed on 29 January 2019).
- Evaluation of the UNHCR Regional Refugee Response to the Venezuela Situation. Evaluation Report December 2020. Cen-tralized ES/2020/12. Available online: https://www.unhcr.org/research/evalreports/6079a15b4/evaluation-unhcr-regional-refugee-response-venezuela-situation.html?query=venezuela (accessed on 1 June 2021).
- Barengo, N.C.; Acosta, T.; Arrieta, A.; Ricaurte, C.; Smits, D.; Flórez, K.; Tuomilehto, J.O. Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project. Int. J. Environ. Res. Public Health 2019, 16, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillmer, M.; Sandoval, G.A.; Elliott, J.A.; Jain, M.; Barker, T.; Prisniak, A.; Astley, S.; Rosella, L. Diabetes risk reduction in primary care: Evaluation of the Ontario Primary Care Diabetes Prevention Program. Can. J. Public Health 2017, 108, e176–e184. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Masalovich, S.; Ng, B.P.; Soler, R.E.; Jabrah, R.; Ely, E.K.; Smith, B.D. Retention Among Participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012–2017. Diabetes Care 2020, 43, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, K.R.; Sathish, T.; Tapp, R.; Shaw, J.E.; Lotfaliany, M.; Wolfe, R.; Absetz, P.; Mathews, E.; Aziz, Z.; Williams, E.; et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018, 15, e1002575. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.; González-Rivas, J.P.; Jaacks, L.M.; Duran, M.; Marulanda, M.I.; Ugel, E.; Mattei, J.; Chavarro, J.E.; Nieto-Martinez, R. Dietary intake and cardiometabolic risk factors among Venezuelan adults: A nationally representative analysis. BMC Nutr. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]


| n | % | |
|---|---|---|
| Feasibility | ||
| Cases found | 629 | 100 |
| Invited to evaluation with inclusion criteria (% of those screened) | 328 | 52.1 |
| Attended the evaluation (% of those invited) | 148 | 45.1 |
| Selected to participate (% of those evaluated) | 135 | 91.2 |
| Groups | Liquid Diet | Only DPP |
| Started n (%) | 64 | 63 |
| Completed the second-month evaluation n (%) | 46 (71.8%) | 48 (76.1%) |
| Dropout Rates | 18 (28.1%) | 15 (23.8%) |
| Reasons for dropping out | 18 | 15 |
| Migration | 0 (0.0%) | 7 (46.6%) |
| “Didn’t like the intervention” | 8 (44.4%) | 0 (0.0%) |
| COVID-19-related symptoms and so missed the second evaluation | 2 (11.1%) | 0 (0.0%) |
| Pregnancy | 0 (0.0%) | 1 (6.6%) |
| Economic reasons | 1 (5.5%) | 0 (0.0%) |
| Unknown (loss of contact) | 7 (38.8%) | 7 (46.6%) |
| Completed | Dropout Rate | p-Value | |
|---|---|---|---|
| n | 94 | 33 | |
| Women n (%) | 71 (75.5%) | 20 (60.6%) | 0.102 |
| Age (years) | 52.4 ± 12.1 | 51.3 ± 11.9 | 0.675 |
| Height (cm) | 159.4 ± 7.6 | 162.1 ± 9.9 | 0.111 |
| Weight (kg) | 77.2 ± 12.2 | 79.8 ± 16.6 | 0.347 |
| Body mass index (kg/m2) | 30.3 ± 3.6 | 30.1 ± 3.9 | 0.867 |
| Waist circumference (cm) | 103.9 ± 8.1 | 106.3 ± 10.4 | 0.179 |
| Fat percentage (%) | 40.9 ± 7.9 | 38.4 ± 7.2 | 0.108 |
| Systolic blood pressure (mmHg) | 127.9 ± 18.4 | 125.2 ± 18.2 | 0.473 |
| Diastolic blood pressure (mmHg) | 74.3 ± 8.9 | 74.0 ± 12.0 | 0.896 |
| Fasting blood glucose (mg/dL) | 86.7 ± 7.6 | 84.9 ± 8.4 | 0.247 |
| 2-h blood glucose (mg/dL) | 94.1 ± 24.5 | 87.1 ± 23.4 | 0.157 |
| Total cholesterol (mg/dL) | 182.0 ± 45.5 | 175.8 ± 34.8 | 0.477 |
| LDL-c (mg/dL) | 110.6 ± 35.1 | 107.0 ± 25.2 | 0.594 |
| VLDL-c (mg/dL) | 25.5 ± 11.4 | 26.5 ± 9.7 | 0.660 |
| HDL-c (mg/dL) | 41.5 ± 8.2 | 40.0 ± 7.6 | 0.375 |
| Triglycerides (mg/dL) | 137.5 ± 97.9 | 145.3 ± 86.7 | 0.686 |
| EQ-5D | 68.1 ± 15.7 | 75.4 ± 15.5 | 0.022 |
| Social Determinants | |||
| Education Level | |||
| Primary | 5 (5.3%) | 8 (24.2%) | 0.008 |
| Secondary | 22 (23.4%) | 5 (15.2%) | |
| University | 67 (71.3%) | 20 (60.6%) | |
| Self-Perceived SES (Score) | 5.6 ± 1.4 | 5.0 ± 1.5 | 0.036 |
| Employment situation | |||
| Employee | 27 (28.7%) | 6 (18.2%) | 0.368 |
| Unemployed | 8 (8.5%) | 4 (12.1%) | |
| Independent | 30 (31.9%) | 11 (33.3%) | |
| Pensioner | 29 (30.9%) | 11 (33.3%) | |
| Self-Perceived Financial Situation | |||
| Fairly good | 7 (7.4%) | 1 (3.0%) | 0.417 |
| Good | 31 (33.0%) | 12 (36.4%) | |
| Regular | 52 (55.3%) | 17 (51.5%) | |
| Very bad | 4. (4.3%) | 2 (6.1%) | |
| Marital Status | |||
| Single | 29 (30.9%) | 10 (30.3%) | 0.238 |
| Married/Partnered | 42 (44.7%) | 16 (48.5%) | |
| Divorced | 19 (20.2%) | 3 (9.1%) | |
| Widower | 4 (4.3%) | 4 (12.1%) |
| Total | Liquid Diet | Only DPP | p-Value | |
|---|---|---|---|---|
| n | 127 | 64 | 63 | |
| Women n (%) | 91 (71.7%) | 49 (76.6%) | 42 (66.7%) | 0.216 |
| Age (years) | 52.1 ± 12.0 | 53.9 ± 12.8 | 50.3 ± 11.0 | 0.092 |
| Height (cm) | 160.1 ± 8.3 | 159.5 ± 8.5 | 160.7 ± 8.1 | 0.402 |
| Weight (kg) | 77.8 ± 13.5 | 76.8 ± 12.6 | 78.9 ± 14.3 | 0.395 |
| Body mass index (kg/m2) | 30.2 ± 3.7 | 30.1 ± 3.6 | 30.3 ± 3.7 | 0.734 |
| Waist circumference (cm) | 104.5 ± 8.8 | 104.6 ± 8.3 | 104.4 ± 9.4 | 0.942 |
| Percentage of fat (%) | 40.3 ± 7.8 | 40.9 ± 7.4 | 39.7 ± 8.2 | 0.380 |
| Systolic blood pressure (mmHg) | 127.2 ± 18.3 | 125.4 ± 16.6 | 129.1 ± 19.9 | 0.259 |
| Diastolic blood pressure (mmHg) | 74.2 ± 9.7 | 72.9 ± 8.5 | 75.6 ± 10.7 | 0.119 |
| Fasting blood glucose (mg/dL) | 86.3 ± 7.9 | 86.2 ± 8.1 | 86.3 ± 7.7 | 0.962 |
| 2-h blood glucose (mg/dL) | 92.3 ± 24.4 | 94.5 ± 27.2 | 90.0 ± 21.1 | 0.296 |
| Total cholesterol (mg/dL) | 180.4 ± 42.9 | 179.8 ± 46.6 | 181.0 ± 39.2 | 0.874 |
| LDL-c (mg/dL) | 109.7 ± 32.8 | 107.3 ± 34.3 | 112.1 ± 31.3 | 0.413 |
| VLDL-c (mg/dL) | 25.8 ± 11.0 | 24.1 ± 9.3 | 27.4 ± 12.3 | 0.096 |
| HDL-c (mg/dL) | 41.1 ± 8.1 | 40.9 ± 8.8 | 41.3 ± 7.3 | 0.783 |
| Triglycerides (mg/dL) | 139.5 ± 94.9 | 141.8 ± 119.3 | 137.2 ± 61.7 | 0.783 |
| EQ-5D | 70.0 ± 15.9 | 70.7 ± 15.7 | 69.3 ± 16.2 | 0.617 |
| Prediabetes (%) | 7 (5.5%) | 4 (6.3%) | 3 (4.8%) | 0.509 |
| Hypertension (%) | 69 (54.3%) | 28 (43.8%) | 41 (65.1%) | 0.013 |
| Anti-hypertensive medication (%) | 25 (19.7%) | 10 (15.6%) | 15 (23.8%) | 0.175 |
| High cholesterol (%) | 39 (30.7%) | 21 (32.8%) | 18 (28.6%) | 0.373 |
| High triglycerides (%) | 35 (27.6%) | 18 (28.1%) | 17 (27.0%) | 0.522 |
| Statin use (%) | 8 (6.3%) | 4 (6.3%) | 4 (6.3%) | 0.632 |
| Social Determinants | ||||
| Education Level | ||||
| Primary | 13 (10.2%) | 8 (12.5%) | 5 (7.9%) | 0.521 |
| Secondary | 27 (21.3%) | 15 (23.4%) | 12 (19.0%) | |
| University | 87 (68.5%) | 41 (64.1%) | 46 (73.0%) | |
| Self-Perceived SES (Score) | 5.5 ± 1.5 | 5.5 ± 1.4 | 5.4 ± 1.5 | 0.577 |
| Employment situation | ||||
| Employee | 33 (26.0%) | 13 (20.3%) | 20 (31.7%) | 0.081 |
| Unemployed | 12 (9.4%) | 5 (7.8%) | 7 (11.1%) | |
| Independent | 41 (32.3%) | 18 (28.1%) | 23 (36.5%) | |
| Pensioner | 40 (31.5%) | 27 (42.2%) | 13 (20.6%) | |
| Self-Perceived Personal Financial Situation | ||||
| Fairly good | 8 (6.3%) | 6 (9.4%) | 2 (3.2%) | 0.081 |
| Good | 43 (33.9%) | 15 (23.4%) | 28 (44.4%) | |
| Regular | 69 (54.3%) | 38 (59.4%) | 31 (49.2%) | |
| Very bad | 6 (4.7%) | 4 (6.3%) | 2 (3.2%) | |
| Marital Status | ||||
| Single | 39 (30.7%) | 21 (32.8%) | 18 (28.6%) | 0.106 |
| Married/Partnered | 58 (45.7%) | 23 (35.9%) | 35 (55.6%) | |
| Divorced | 22 (17.3%) | 15 (23.4%) | 7 (11.1%) | |
| Widower | 8 (6.3%) | 5 (7.8%) | 3 (4.8%) |
| Variables | Intervention | Baseline (n = 94) | 2 Months | Change | p-Value | ≠ | p-Value |
|---|---|---|---|---|---|---|---|
| Weight | LD | 74.2 ± 10.6 | 71.3 ± 9.4 | −2.9 | <0.001 | 0.7 | 0.170 |
| DPP | 79.1 ± 13.5 | 76.9 ± 13.8 | −2.2 | <0.001 | |||
| Body mass index (kg/m2) | LD | 29.9 ± 3.5 | 28.8 ± 3.4 | −1.2 | <0.001 | 0.4 | 0.112 |
| DPP | 30.6 ± 3.7 | 29.8 ± 3.8 | −0.8 | 0.124 | |||
| Waist circumference (cm) | LD | 103.0 ± 7.0 | 99.8 ± 6.3 | −3.2 | <0.001 | 1.4 | 0.113 |
| DPP | 104.4 ± 9.1 | 102.6 ± 9.0 | −1.8 | <0.001 | |||
| Fat percentage (%) | LD | 42.1 ± 7.5 | 40.7 ± 7.4 | −1.4 | <0.001 | −0.5 | 0.323 |
| DPP | 40.5 ± 8.4 | 38.6 ± 8.2 | −1.9 | <0.001 | |||
| Systolic blood pressure (mm/Hg) | LD | 125.5 ± 16.5 | 115.0 ± 15.0 | −10.5 | <0.001 | 2.1 | 0.521 |
| DPP | 130.4 ± 19.9 | 122.0 ± 16.2 | −8.4 | 0.002 | |||
| Diastolic blood pressure (mm/Hg) | LD | 72.7 ± 8.1 | 70.6 ± 10.1 | −2.1 | 0.112 | 0.3 | 0.441 |
| DPP | 75.7 ± 9.4 | 73.9 ± 8.8 | −1.8 | <0.001 | |||
| Fasting blood glucose | LD | 87.0 ± 7.4 | 86.6 ± 8.5 | −0.4 | 0.812 | −2.0 | 0.320 |
| DPP | 86.5 ± 8.0 | 84.1 ± 9.3 | −2.4 | 0.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rivas, J.P.; Infante-García, M.M.; Nieto-Martinez, R.; Mechanick, J.I.; Danaei, G. Feasibility and Effectiveness of a Preventive Care Program during the Compound Humanitarian Crisis and COVID-19 Pandemic in Venezuela. Nutrients 2022, 14, 939. https://doi.org/10.3390/nu14050939
González-Rivas JP, Infante-García MM, Nieto-Martinez R, Mechanick JI, Danaei G. Feasibility and Effectiveness of a Preventive Care Program during the Compound Humanitarian Crisis and COVID-19 Pandemic in Venezuela. Nutrients. 2022; 14(5):939. https://doi.org/10.3390/nu14050939
Chicago/Turabian StyleGonzález-Rivas, Juan P., María M. Infante-García, Ramfis Nieto-Martinez, Jeffrey I. Mechanick, and Goodarz Danaei. 2022. "Feasibility and Effectiveness of a Preventive Care Program during the Compound Humanitarian Crisis and COVID-19 Pandemic in Venezuela" Nutrients 14, no. 5: 939. https://doi.org/10.3390/nu14050939
APA StyleGonzález-Rivas, J. P., Infante-García, M. M., Nieto-Martinez, R., Mechanick, J. I., & Danaei, G. (2022). Feasibility and Effectiveness of a Preventive Care Program during the Compound Humanitarian Crisis and COVID-19 Pandemic in Venezuela. Nutrients, 14(5), 939. https://doi.org/10.3390/nu14050939






