Methodological Aspects of Indirect Calorimetry in Patients with Sepsis—Possibilities and Limitations
Abstract
1. Introduction
2. Materials and Methods
3. Theory
3.1. Energy Expenditure
3.2. Indirect Calorimetry
3.3. Rules for Measuring
4. Results
4.1. Systematic Review
4.2. Aims and Types of Research
4.3. Energy Expenditure and Respiratory Quotient
4.4. Energy Expenditure Measurement Protocol
4.5. Criteria for the Diagnosis of Sepsis and Septic Shock
4.6. Criteria for Participating in the Study
4.7. Limitations of the Analysed Studies
5. Discussion
5.1. Indirect Calorimetry
5.2. Energy Expenditure
5.3. Definition of Sepsis
5.4. Limitations of the Discribed Studies
5.5. Limitations of This Review
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- De Waele, E.; Malbrain, M.L.N.G.; Spapen, H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients 2020, 12, 395. [Google Scholar] [CrossRef]
- Wasyluk, W.; Zwolak, A. Metabolic Alterations in Sepsis. J. Clin. Med. 2021, 10, 2412. [Google Scholar] [CrossRef] [PubMed]
- Wasyluk, W.; Wasyluk, M.; Zwolak, A. Sepsis as a Pan-Endocrine Illness—Endocrine Disorders in Septic Patients. J. Clin. Med. 2021, 10, 2075. [Google Scholar] [CrossRef] [PubMed]
- Mtaweh, H.; Aguero, M.J.S.; Campbell, M.; Allard, J.P.; Pencharz, P.; Pullenayegum, E.; Parshuram, C.S. Systematic review of factors associated with energy expenditure in the critically ill. Clin. Nutr. ESPEN 2019, 33, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211, Correction in JPEN J. Parenter. Enter. Nutr. 2016, 40, 1200. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Singer, P.; Hiesmayr, M.; Biolo, G.; Felbinger, T.W.; Berger, M.M.; Goeters, C.; Kondrup, J.; Wunder, C.; Pichard, C. Pragmatic approach to nutrition in the ICU: Expert opinion regarding which calorie protein target. Clin. Nutr. 2014, 33, 246–251. [Google Scholar] [CrossRef]
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.-P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2016, 36, 651–662. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haugen, H.A.; Chan, L.-N.; Li, F. Indirect Calorimetry: A Practical Guide for Clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef]
- Psota, T.; Chen, K.Y. Measuring energy expenditure in clinical populations: Rewards and challenges. Eur. J. Clin. Nutr. 2013, 67, 436–442. [Google Scholar] [CrossRef]
- Thomas, N.; Das Gupta, R.; Ramachandran, R.; Venkatesan, P.; Anoop, S.; Joseph, M. Indirect calorimetry: From bench to bedside. Indian J. Endocrinol. Metab. 2017, 21, 594–599. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Gomes, F.; Schuetz, P.; Bounoure, L.; Austin, P.; Ballesteros-Pomar, M.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; Poulia, K.-A.; et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2017, 37, 336–353. [Google Scholar] [CrossRef]
- Takala, J.; Keinänen, O.; Väisänen, P.; Kari, A. Measurement of gas exchange in intensive care: Laboratory and clinical validation of a new device. Crit. Care Med. 1989, 17, 1041–1047. [Google Scholar] [CrossRef]
- Weir, J.B.D.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Bursztein, S.; Saphar, P.; Singer, P.; Elwyn, D.H. A mathematical analysis of indirect calorimetry measurements in acutely ill patients. Am. J. Clin. Nutr. 1989, 50, 227–230. [Google Scholar] [CrossRef]
- Wilmore, J.H.; Costill, D.L. Adequacy of the Haldane transformation in the computation of exercise VO2 in man. J. Appl. Physiol. 1973, 35, 85–89. [Google Scholar] [CrossRef]
- Ultman, J.S.; Bursztein, S. Analysis of error in the determination of respiratory gas exchange at varying FIO2. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 50, 210–216. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L.; Evidence Analysis Working Group. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Lichtenbelt, W.D.V.M.; Frijns, A.J.H.; Van Ooijen, M.J.; Fiala, D.; Kester, A.M.; Van Steenhoven, A.A. Validation of an individualised model of human thermoregulation for predicting responses to cold air. Int. J. Biometeorol. 2006, 51, 169–179. [Google Scholar] [CrossRef]
- Ueno, S.; Ikeda, K.; Tai, T.; Tai, T. Metabolic Rate Prediction in Young and Old Men by Heart Rate, Ambient Temperature, Weight and Body Fat Percentage. J. Occup. Health 2014, 56, 519–525. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Kiraly, L. The use of indirect calorimetry in the intensive care unit. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 202–208. [Google Scholar] [CrossRef]
- Mtaweh, H.; Tuira, L.; Floh, A.A.; Parshuram, C.S. Indirect Calorimetry: History, Technology, and Application. Front. Pediatr. 2018, 6, 257. [Google Scholar] [CrossRef]
- Macfarlane, D.J. Automated Metabolic Gas Analysis Systems. Sports Med. 2001, 31, 841–861. [Google Scholar] [CrossRef]
- Cunningham, K.F.; Aeberhardt, L.E.; Wiggs, B.R.; Phang, P. Appropriate interpretation of indirect calorimetry for determining energy expenditure of patients in intensive care units. Am. J. Surg. 1994, 167, 547–549. [Google Scholar] [CrossRef]
- McClave, S.; Spain, D.; Skolnick, J.; Lowen, C.; Kleber, M.; Wickerham, P.; Vogt, J.; Looney, S. Achievement of steady state optimizes results when performing indirect calorimetry. J. Parenter. Enter. Nutr. 2003, 27, 16–20. [Google Scholar] [CrossRef]
- El-Orbany, M.; Salem, M.R. Endotracheal Tube Cuff Leaks: Causes, consequences, and management. Anesth. Analg. 2013, 117, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Singer, J. Clinical Guide for the Use of Metabolic Carts: Indirect calorimetry—No longer the orphan of energy estimation. Nutr. Clin. Pract. 2015, 31, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Weissman, C.; Sardar, A.; Kemper, M. In vitro evaluation of a compact metabolic measurement instrument. J. Parenter. Enter. Nutr. 1990, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef]
- Rattanachaiwong, S.; Singer, P. Indirect calorimetry as point of care testing. Clin. Nutr. 2019, 38, 2531–2544. [Google Scholar] [CrossRef]
- De Waele, E.; Van Zwam, K.; Mattens, S.; Staessens, K.; Diltoer, M.; Honoré, P.M.; Czapla, J.; Nijs, J.; La Meir, M.; Huyghens, L.; et al. Measuring resting energy expenditure during extracorporeal membrane oxygenation: Preliminary clinical experience with a proposed theoretical model. Acta Anaesthesiol. Scand. 2015, 59, 1296–1302. [Google Scholar] [CrossRef]
- Wollersheim, T.; Frank, S.; Müller, M.; Skrypnikov, V.; Carbon, N.; Pickerodt, P.; Spies, C.; Mai, K.; Spranger, J.; Weber-Carstens, S. Measuring Energy Expenditure in extracorporeal lung support Patients (MEEP)—Protocol, feasibility and pilot trial. Clin. Nutr. 2017, 37, 301–307. [Google Scholar] [CrossRef]
- Jonckheer, J.; Spapen, H.; Debain, A.; Demol, J.; Diltoer, M.; Costa, O.; Lanckmans, K.; Oshima, T.; Honoré, P.M.; Malbrain, M.; et al. CO2 and O2 removal during continuous veno-venous hemofiltration: A pilot study. BMC Nephrol. 2019, 20, 222. [Google Scholar] [CrossRef]
- Jonckheer, J.; Demol, J.; Lanckmans, K.; Malbrain, M.; Spapen, H.; De Waele, E. MECCIAS trial: Metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin. Nutr. 2020, 39, 3797–3803. [Google Scholar] [CrossRef]
- Matarese, L.E. Indirect Calorimetry: Technical Aspects. J. Am. Diet. Assoc. 1997, 97, S154–S160. [Google Scholar] [CrossRef]
- Terblanche, E.; Remmington, C. Observational study evaluating the nutritional impact of changing from 1% to 2% propofol in a cardiothoracic adult critical care unit. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2020, 34, 413–419. [Google Scholar] [CrossRef]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect calorimetry: The 6 main issues. Clin. Nutr. 2020, 40, 4–14. [Google Scholar] [CrossRef]
- Auxiliadora-Martins, M.; Coletto, F.A.; Campos, A.D.; Basile-Filho, A. Indirect calorimetry can be used to measure cardiac output in septic patients? Acta Cir. Bras. 2008, 23, 118–125. [Google Scholar] [CrossRef]
- Natalini, G.; Schivalocchi, V.; Rosano, A.; Taranto, M.; Pletti, C.; Bernardini, A. Norepinephrine and metaraminol in septic shock: A comparison of the hemodynamic effects. Intensiv. Care Med. 2005, 31, 634–637. [Google Scholar] [CrossRef]
- Marson, F.; Auxiliadora-Martins, M.; Coletto, F.A.; Campos, A.D.; Basile-Filho, A. Correlation between Oxygen Consumption Calculated Using Fick’s Method and Measured with Indirect Calorimetry in Critically Ill Patients. Arq. Bras. Cardiol. 2004, 82, 72–76. [Google Scholar] [CrossRef][Green Version]
- Fernandes, C.J.; Akamine, N.; De Marco, F.V.; De Souza, J.A.; Lagudis, S.; Knobel, E. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit. Care 2001, 5, 362–367. [Google Scholar] [CrossRef]
- Sakka, S.G.; Reinhart, K.; Wegscheider, K.; Meier-Hellmann, A. Variability of splanchnic blood flow in patients with sepsis. Intensiv. Care Med. 2001, 27, 1281–1287. [Google Scholar] [CrossRef]
- Schaffartzik, W.; Sanft, C.; Schaefer, J.H.; Spies, C. Different dosages of dobutamine in septic shock patients: Determining oxygen consumption with a metabolic monitor integrated in a ventilator. Intensiv. Care Med. 2000, 26, 1740–1746. [Google Scholar] [CrossRef]
- Broccard, A.; Hurni, J.-M.; Eckert, P.; Liaudet, L.; Schaller, M.-D.; Lazor, R.; Perret, C.; Feihl, F. Tissue oxygenation and hemodynamic response to no synthase inhibition in septic shock. Shock 2000, 14, 35–40. [Google Scholar] [CrossRef]
- Sakka, S.G.; Reinhard, K.; Wegscheider, K.; Meier-Hellmann, A. Is the placement of a pulmonary artery catheter still justified solely for the measurement of cardiac output? J. Cardiothorac. Vasc. Anesth. 2000, 14, 119–124. [Google Scholar] [CrossRef]
- Opdam, H.; Bellomo, R. Oxygen consumption and lactate release by the lung after cardiopulmonary bypass and during septic shock. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2000, 2, 181–187. [Google Scholar]
- Takemae, A.; Takazawa, T.; Kamiyama, J.; Kanamoto, M.; Tobe, M.; Hinohara, H.; Kunimoto, F.; Saito, S. A novel prediction equation of resting energy expenditure for Japanese septic patients. J. Crit. Care 2020, 56, 236–242. [Google Scholar] [CrossRef]
- Menegueti, M.G.; De Araújo, T.R.; Laus, A.M.; Martins-Filho, O.A.; Basile-Filho, A.; Auxiliadora-Martins, M. Resting Energy Expenditure and Oxygen Consumption in Critically Ill Patients with vs without Sepsis. Am. J. Crit. Care 2019, 28, 136–141. [Google Scholar] [CrossRef]
- Panitchote, A.; Thiangpak, N.; Hongsprabhas, P.; Hurst, C. Energy expenditure in severe sepsis or septic shock in a Thai Medical Intensive Care Unit. Asia Pac. J. Clin. Nutr. 2017, 26, 794–797. [Google Scholar]
- Wu, C.; Wang, X.; Yu, W.; Tian, F.; Liu, S.; Li, P.; Li, J.; Li, N. Hypermetabolism in the Initial Phase of Intensive Care Is Related to a Poor Outcome in Severe Sepsis Patients. Ann. Nutr. Metab. 2015, 66, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Basile-Filho, A.; Auxiliadora-Martins, M.; Marson, F.; Evora, P.R.B. An easy way to estimate energy expenditure from hemodynamic data in septic patients. Acta Cir. Bras. 2008, 23, 112–117. [Google Scholar] [CrossRef]
- Auxiliadora-Martins, M.; Coletto, F.A.; Martins-Filho, O.A.; Marchini, J.S.; Basile-Filho, A. 13CO2 recovery fraction in expired air of septic patients under mechanical ventilation. Braz. J. Med. Biol. Res. 2008, 41, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Cohen, J.; Singer, P. Computerized energy balance and complications in critically ill patients: An observational study. Clin. Nutr. 2006, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zauner, C.; Schuster, B.I.; Schneeweiss, B. Similar metabolic responses to standardized total parenteral nutrition of septic and nonseptic critically ill patients. Am. J. Clin. Nutr. 2001, 74, 265–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uehara, M.; Plank, L.D.; Hill, G.L. Components of energy expenditure in patients with severe sepsis and major trauma. Crit. Care Med. 1999, 27, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Rušavý, Z.; Macdonald, I.A.; Sramek, V.; Lacigova, S.; Tesinsky, P.; Novak, I. Glycemia Influences on Glucose Metabolism in Sepsis during Hyperinsulinemic Clamp. J. Parenter. Enter. Nutr. 2005, 29, 171–175. [Google Scholar] [CrossRef]
- Rusavy, Z.; Sramek, V.; Lacigova, S.; Novak, I.; Tesinsky, P.; Macdonald, I.A. Influence of insulin on glucose metabolism and energy expenditure in septic patients. Crit. Care 2004, 8, R213–R220. [Google Scholar] [CrossRef]
- Saeed, M.; Carlson, G.L.; Little, R.A.; Irving, M.H. Selective impairment of glucose storage in human sepsis. Br. J. Surg. 1999, 86, 813–821. [Google Scholar] [CrossRef]
- Lee, P.S.-P.; Lee, K.L.; Betts, J.A.; Law, K.I. Metabolic Requirement of Septic Shock Patients before and after Liberation from Mechanical Ventilation. J. Parenter. Enter. Nutr. 2016, 41, 993–999. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Yu, W.; Li, P.; Liu, S.; Li, J.; Li, N. Short-term consequences of continuous renal replacement therapy on body composition and metabolic status in sepsis. Asia Pac. J. Clin. Nutr. 2016, 25, 300–307. [Google Scholar]
- Hickmann, C.E.; Roeseler, J.; Castanares-Zapatero, D.; Herrera, E.I.; Mongodin, A.; Laterre, P.-F. Energy expenditure in the critically ill performing early physical therapy. Intensiv. Care Med. 2014, 40, 548–555. [Google Scholar] [CrossRef]
- Gore, D.C.; Wolfe, R.R. Hemodynamic and metabolic effects of selective β1 adrenergic blockade during sepsis. Surgery 2006, 139, 686–694. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensiv. Care Med. 2007, 34, 17–60. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]

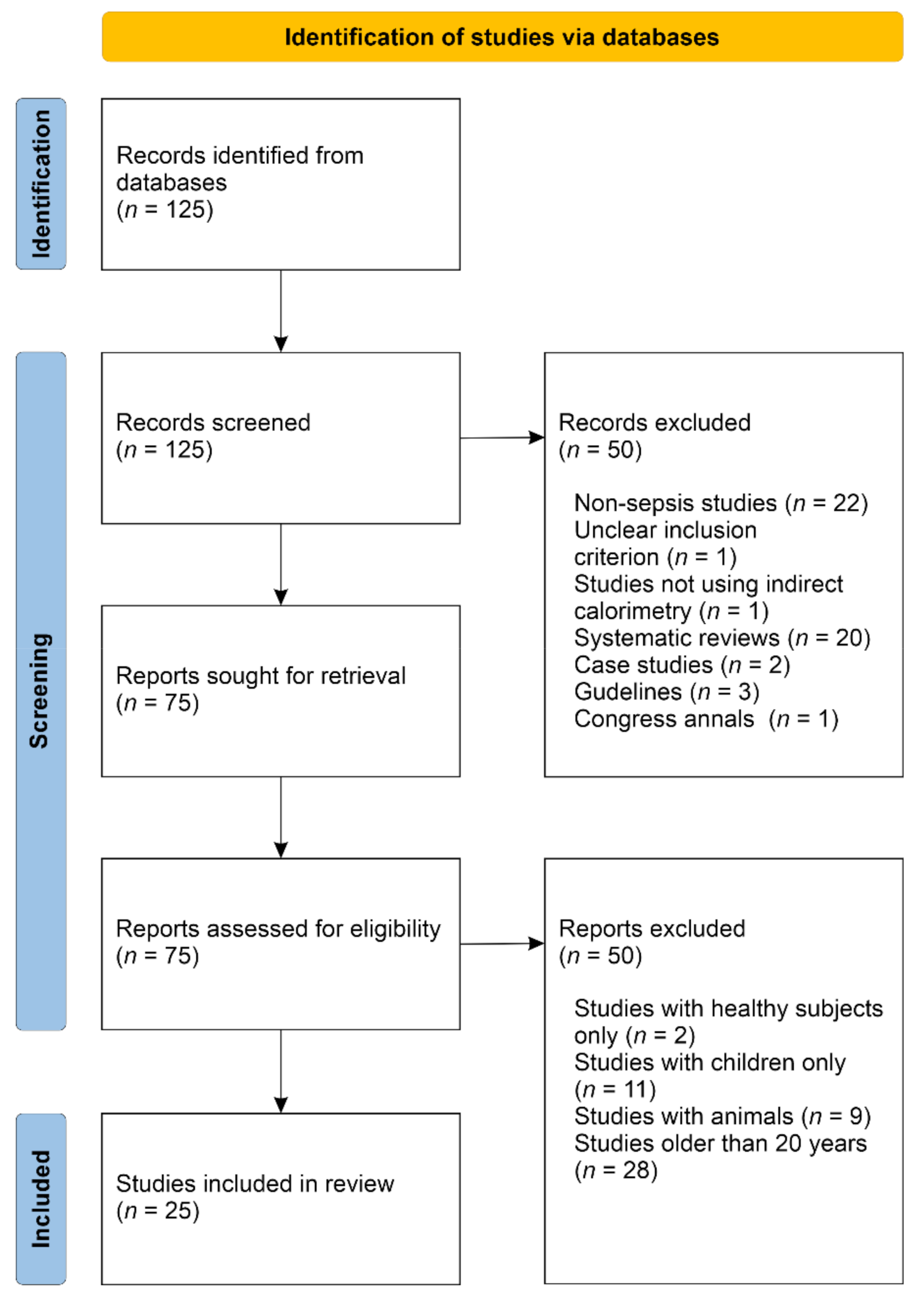

| Reference | Type of Study | Objective of the Study | Only Septic Patients |
|---|---|---|---|
| Takemae et al. (2020) [52] | Retrospective observational study | Development of new equations to estimate the total EE of Japanese patients with sepsis. | Yes |
| Menegueti et al. (2019) [53] | Observational cross-sectional study | Assessment of whether REE, respiratory quotient, oxygen consumption, and carbon dioxide production (measured by IC) differ in critically ill patients with sepsis compared to critically ill patients without sepsis. | No |
| Panitchote et al. (2017) [54] | Prospective observational study | Assessment of the correlation between REE of patients with sepsis/septic shock, measured by IC and estimated using predictive equations. | Yes |
| Lee et al. (2017) [64] | ND | Identification of the difference in EE and substrate utilisation by patients during and upon liberation from mechanical ventilation. | Yes |
| Wu et al. (2016) [65] | Prospective observational study | Assessment of the short-term consequence of continuous renal replacement therapy on body composition and pattern of EE. | Yes |
| Wu et al. (2015) [55] | Prospective observational study | Assessment of the incidence of hypermetabolism, defined as high REE, in severe sepsis ICU patients, and evaluate the suitability of excessive RRE as a risk factor of their clinical outcome. | Yes |
| Hickmann et al. (2014) [66] | Prospective observational study | Determining the impact of early exercise on energy requirements to adjust caloric intake accordingly in critically ill patients. | No |
| Auxiliadora-Martins et al. (2008) [43] | Prospective clinical study | Comparison of two different CO monitoring systems based on the thermodilution principle (Thermo-CO) and IC (Fick mixed-CO) in septic patients. | Yes |
| Basile-Filho et al. (2008) [56] | Prospective clinical study | Comparison of REE obtained by IC and the REE calculated by predictive equations (Brandi and Liggett) using the oxygen consumption obtained by Fick‘s method in septic patients. | Yes |
| Auxiliadora-Martins et al. (2008) [57] | Prospective clinical study | Evaluation of the 13CO2 recovery fraction in expired air after continuous intravenous infusion of NaH13CO2, in critically ill patients with sepsis under mechanical ventilation (calculation of substrate oxidation). | Yes |
| Gore et al. (2006) [67] | ND | Investigating the haemodynamic and metabolic effects of cardiac selective beta adrenergic blockade in septic patients. | Yes |
| Dvir et al. (2006) [58] | Prospective observational study | Measuring the daily cumulative energy balance in critically ill patients receiving mechanical ventilation using a bedside computerised information system, and to assess its impact on outcome. | No |
| Rusavy et al. (2005) [61] | ND | Comparing the effects of 2 blood glucose levels (5 and 10 mmol/L) under hyperinsulinemic conditions, and the effect of glycaemia 5 mmol/L with extremely high insulinaemia on glucose metabolism and EE in septic patients. | Yes * |
| Natalini et al. (2005) [44] | Open-label, controlled clinical trial | Comparison of the effects of noradrenaline and metaraminol on haemodynamics in septic shock patients. | Yes |
| Rusavy et al. (2004) [62] | ND | Comparing the effects of two levels of insulinaemia on glucose metabolism and EE in septic patients and volunteers. | Yes * |
| Marson et al. (2004) [45] | Prospective study | Comparison of oxygen consumption index measured by using IC with a portable metabolic cart and calculated according to Fick‘s principle in critically ill patients. | No |
| Fernandes et al. (2001) [46] | Interventional, prospective, randomised, controlled study | Evaluation of the haemodynamic and oxygen utilisation effects of haemoglobin infusion on critically ill septic patients. | Yes |
| Sakka et al. (2001) [47] | Prospective clinical study | Examining the variability of splanchnic blood flow during a 4-h period of unchanged global haemodynamics in patients with sepsis. | Yes |
| Zauner et al. (2001) [59] | Prospective, clinical cohort study | Evaluation of the energy and substrate metabolism in septic and non-septic critically ill patients in the resting state and during the administration of standardised total parenteral nutrition. | No |
| Schaffartzik et al. (2000) [48] | Prospective clinical study | Comparison of oxygen consumption obtained from breathing gases by IC with a metabolic monitor integrated with a respirator and oxygen consumption obtained by the Fick principle in patients with sepsis after an increase in oxygen delivery induced by positive inotropic support. | Yes |
| Broccard et al. (2000) [49] | ND | Evaluation of the tissue oxygenation and haemodynamic effects of NOS inhibition in clinical severe septic shock. | Yes |
| Sakka et al. (2000) [50] | Prospective clinical study | Comparison of four clinical techniques of measuring cardiac output in critically ill patients: pulmonary artery thermodilution, transpulmonary aortic thermodilution, Fick principle-derived, and continuous pulmonary artery measurements. | Yes |
| Opdam et al. (2000) [51] | Prospective observational study | Determining whether there is a correlation between lung lactate release and lung oxygen consumption by studying adult intensive care patients, either after cardiopulmonary bypass or with septic shock. | No |
| Uehara et al. (1999) [60] | Prospective study | Obtaining accurate values for the components of EE in critically ill patients with sepsis or trauma during the first 2 weeks after admission to the ICU. | No |
| Saeed et al. (1999) [63] | ND | Assessment of the effect of sepsis on total glucose utilisation, oxidation and storage, and the energetic costs of these metabolic processes. | Yes * |
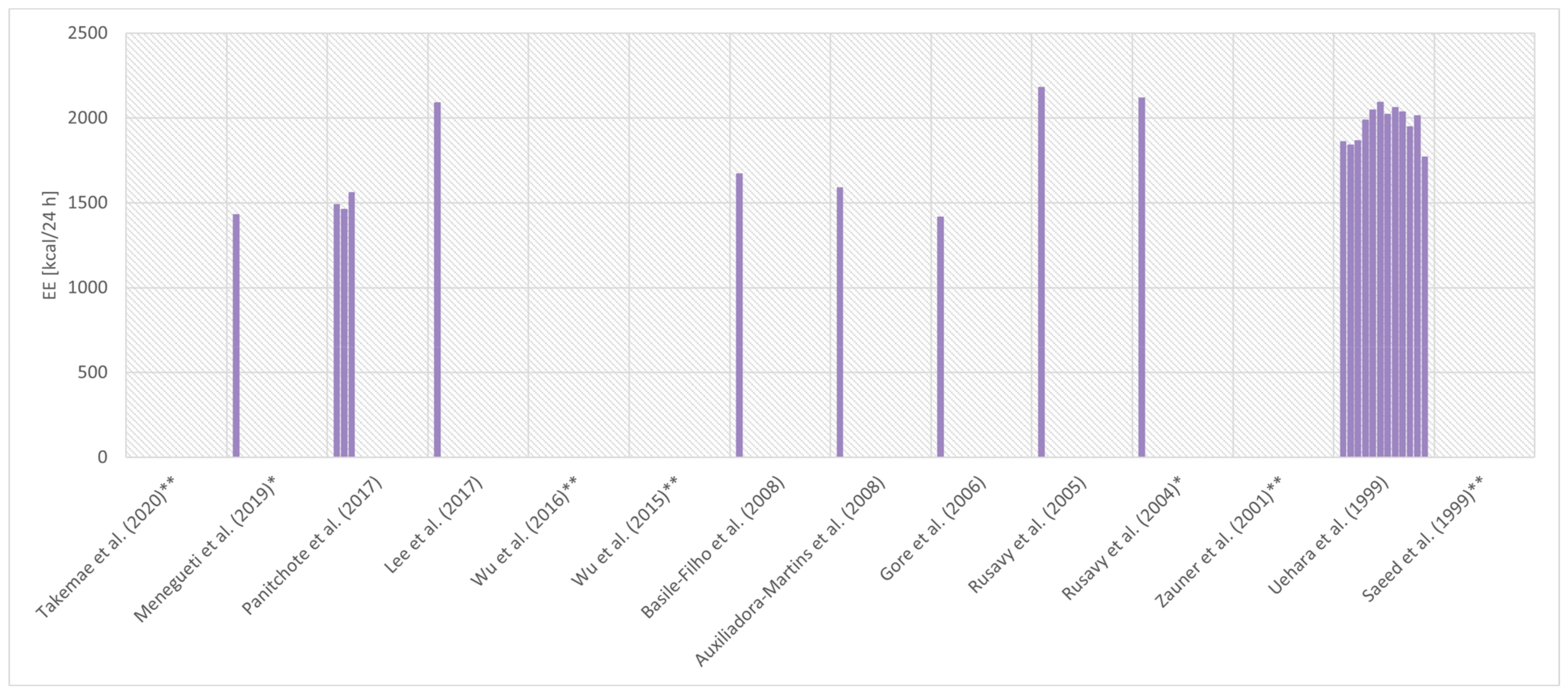
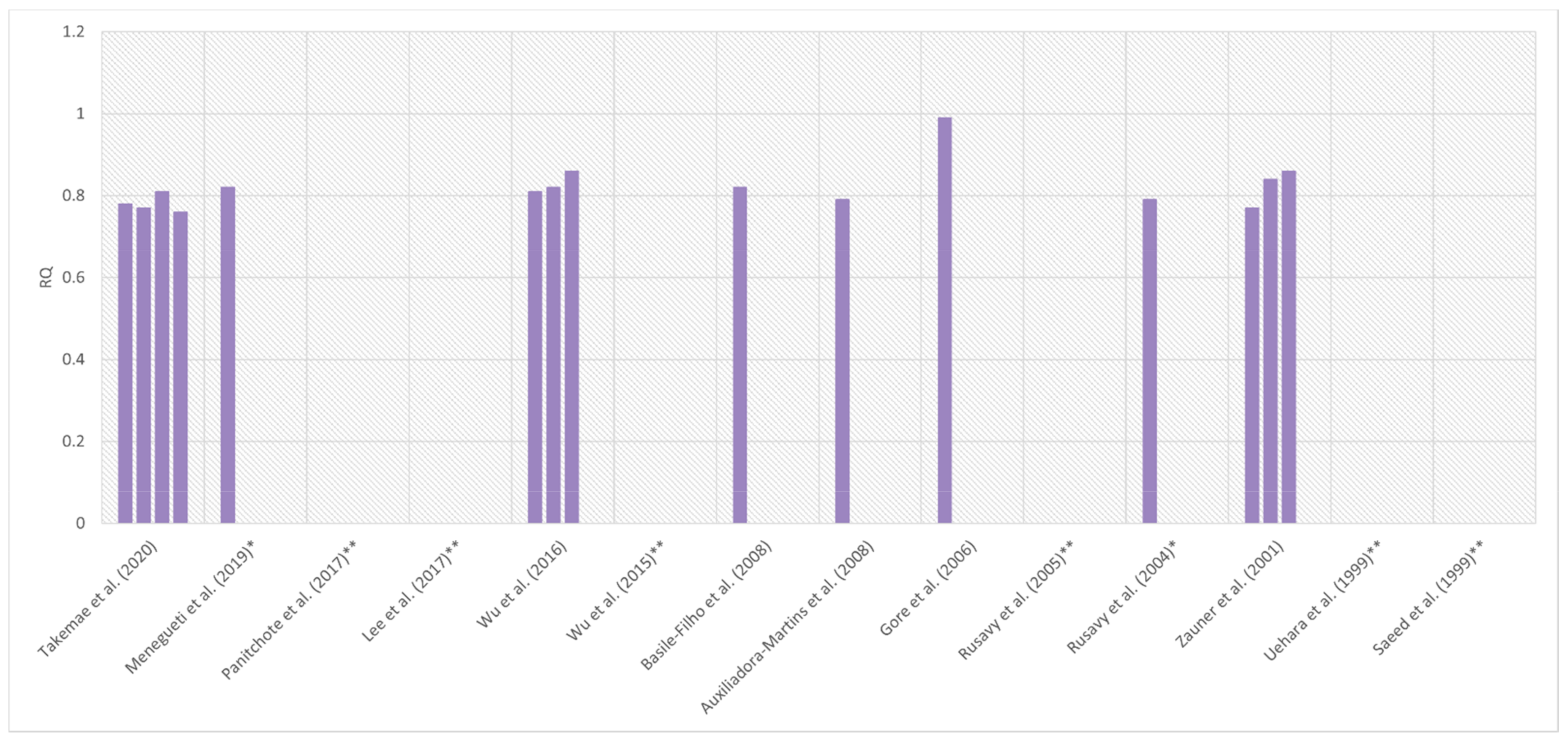
| Reference | Diagnosis | Criteria for Sepsis AND Septic Shock | Sample Size | % of Women | Age (years) | Body Mass (kg) | BMI (kg/m2) | APACHE II (points) | Mechanical Ventilation (%) | Device | Nutrition during IC | Day of Measurement | EE (kcal/24 h) | EE (kcal/kg/24 h) | RQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takemae et al. (2020) [52] | Severe sepsis | SEPSIS-2 [68] SSC 2012 [69] | 42 | 0% | 68 ± 14 | 60 ± 14 | 22.2 ± 4.7 | 24.2 ± 5.8 | 100% | M-COVX® (Datex-Ohmeda, Helsinki, Finland) | ≥4 h between changes in the feeding method and IC | 1st day of the intubation period | ND | ND | 0.78 ± 0.09 |
| 24 | 100% | 60 ± 16 | 48 ± 16 | 20.4 ± 5.3 | 27.6 ± 6.0 | 0.77 ± 0.9 | |||||||||
| 19 | 0% | 66 ± 13 | 62 ± 10 | 23.0 ± 2.9 | 26.9 ± 5.7 | 0.81 ± 0.11 | |||||||||
| 10 | 100% | 56 ± 15 | 60 ± 17 | 25.1 ± 7.1 | 34.8 ± 8.0 | 0.76 ± 0.12 | |||||||||
| Menegueti et al. (2019) [53] | Sepsis/septic shock | SSC 2008 [70] | 91 | 42% | 58 (19–89) m(r) | ND | 26 (17–45) m(r) | 25 (9–47) m(r) | 100% | Deltatrac II® (Datex-Ohmeda) | IC before the beginning of nutrition | First 48 h of admission | 1430 (540–2420) m(r) | ND | 0.82 (0.6–1.24) m(r) |
| Panitchote et al. (2017) [54] | Severe sepsis/septic shock | ND | 16 | 44% | 71.6 ± 5.5 | ND | 22.0 ± 2.9 | 26.9 ± 4.0 | 100% | Engström Carestation® (GE Healthcare, Chicago, IL, USA) | ND | 24 h | 1488 ± 261 | 26.7 ± 5.3 | ND |
| 48 h | 1459 ± 270 | ||||||||||||||
| 72 h | 1560 ± 363 | ||||||||||||||
| Lee et al. (2017) [64] | Septic shock | ND | 37 | 43% | 69 ± 10 | 59.01 ± 7.63 | ND | 22 m | 100% | CCM Express® (Medical Graphics Corporation, St Paul, MN, USA) | Suspended 4 h before IC | ND | 2090 ± 489 | ND | ND |
| Wu et al. (2016) [65] | Sepsis and CRRT requirement | SSC 2012 [69] | 27 | 41% | 48.2 ± 22.0 | 62.8 ± 14.7 | 22.0 ± 1.4 | ND | 48.1% | Metabolic cart (Cosmed, Roma, Italy) | Suspended ≥1.5 h before IC | At admission | ND | 27.9 ± 5.9 | 0.81 ± 0.06 |
| Before CRRT a | 29.9 ± 5.6 | 0.82 ± 0.06 | |||||||||||||
| 6 h after CRRT a | 26.6 ± 4.3 | 0.86 ± 0.05 | |||||||||||||
| Wu et al. (2015) [55] | Severe sepsis/septic shock | SSC 2012 [69] | 62 | 35% | 57.1 ± 19.5 | 79.1 ± 10.3 | 21.6 ± 3.1 | 20.2 ± 4.1 | 37.5% | Metabolic cart (Med Graphics) | Suspended ≥1.5 h before IC | 1st, 2nd, 3rd, 4th, 5th day | ND | ND | ND |
| Basile-Filho et al. (2008) [56] | Septic shock | SEPSIS-1 [71] | 15 | 27% | 41.3 ± 18.9 | 68.5 ± 9.2 | ND | 22.6 ± 7.2 | 100% | Deltatrac II® (Datex–Ohmeda) | ND | 3rd–5th day | 1669 ± 271 | ND | 0.82 ± 0.11 |
| Auxiliadora-Martins et al. (2008) [57] | Sepsis/septic shock | SEPSIS-1 [71] | 10 | 60% | 55.1 ± 19 | ND | ND | 25.9 ± 7.4 | 100% | Deltatrac II® (Datex-Ohmeda) | ND | 2nd–5th day | 1587 ± 430 b | ND | 0.79 ± 0.10 |
| Gore et al. (2006) [67] | Sepsis | ND | 6 | ND | 41 ± 7 | 81 ± 18 | ND | 17 ± 2 | 100% | Delta Trac® (Sensormedics, Yorba Linda, CA, USA) | EN 40 cal/h during IC | ND | 1414 ± 134 | ND | 0.99 ± 0.06 |
| Rusavy et al. (2005) [61] | Sepsis | ND | 10 | ND | ND | ND | ND | 18.4 ± 2.12 | 100% | Deltatrac II® (Datex, Instrumentarium, Helsinki, Finland) | ND | ND | 2179± 354 | ND | ND |
| Rusavy et al. (2004) [62] | Sepsis | ND | 20 | ND | 65 (52–68) m(IQR) | ND | 26 (24.6–27.8) m(IQR) | 20.2 (18.3–22.4) m(IQR) | 100% | Deltatrac II® (Datex-Ohmeda) | Suspended 9 h before IC | 3rd–7th day | 2116 (1880–2455) m(IQR) | ND | 0.79 (0.77–0.85) m(IQR) |
| Zauner et al. (2001) [59] | Severe sepsis/septic shock | SEPSIS-1 [71] | 14 | 43% | 57.5 ± 12.92 | 71.4 ± 12.7 | 24.1 ± 4.2 | ND c | ND | MMC 2900® (SensorMedics) | TPN was started after the first IC | At admission | ND d | ND | 0.77 ± 0.05 |
| 2nd day | 0.84 ± 0.05 | ||||||||||||||
| 7th day | 0.86 ± 0.05 | ||||||||||||||
| Uehara et al. (1999) [60] | Severe sepsis | SEPSIS-1 [71] | 12 | 33% | 67 (25–84) m(r) | Day 0: 78.4 ± 3.8 Day 5: 74.2 ± 3.3 Day 10: 70.2 ± 3.4 mean±SEM | ND | 23 (15–34) m(r) | 100% | Deltatrac MBM-100® (Datex/Instrumentarium) | ND | 2nd 3rd 4th 5t 6th 7th 8th 9th 10th 11th 12th 23rd day | 1859 ± 140 1840 ± 119 1864 ± 139 1988 ± 121 2047 ± 141 2091 ± 140 2022 ± 150 2061 ± 138 2036 ± 147 1947 ± 126 2013 ± 140 1770 ± 116 mean±SEM | ND | ND |
| Saeed et al. (1999) [63] | Sepsis | SEPSIS-1 [71] | 24 | 42% | 52.2 ± 15.6 | 77.2 ± 11.7 | ND | ND | ND | Deltratrac® (Datex) | PN overnight fast before IC | ND | ND e | ND | ND |
| Reference | Takemae et al. (2020) [52] | Menegueti et al. (2019) [53] | Panitchote et al. (2017) [54] | Lee et al. (2017) [64] | Wu et al. (2016) b [65] | Wu et al. (2015) [55] | Basile-Filho et al. (2008) [56] | Auxiliadora-Martins et al. (2008) [57] | Gore et al. (2006) c [67] | Rusavy et al. (2005) d [61] | Rusavy et al. (2004) d [62] | Zauner et al. (2001) [59] | Uehara et al. (1999) e [60] | Saeed et al. (1999) d [63] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Exclusion Criterion a | |||||||||||||||
| Age (years) | <18 | <18 | <18 | <18 | <18 | <15 >80 | <15 >85 | ||||||||
| Chest tube/drain | + | + | + | ||||||||||||
| Bronchopleural fistula | + | ||||||||||||||
| PEEP (cm H2O) | >12 | >14 | >12 | >12 | |||||||||||
| FiO2 | ≥0.6 | >0.6 | >0.6 | >0.6 | > 0.6 | >0.6 | >0.6 | >0.7 | >0.55 | ||||||
| MAP (mm Hg) | <50 | <50 | <70 | <75 | |||||||||||
| Diuresis (ml/h) | <50 | <50 | |||||||||||||
| Cardiac index | <3 | <3 | |||||||||||||
| Respiratory rate (breath/min) | >35 | ||||||||||||||
| Lactate (mmol/L) | ↑ trend | ↑ trend | >5 | ||||||||||||
| Changes in buffer base in 12 h | >10% | >10% | |||||||||||||
| Haemodialysis | + | + | + | + | |||||||||||
| CRRT | + | ||||||||||||||
| ECMO | + | ||||||||||||||
| Brain death | + | + | |||||||||||||
| Pregnancy | + | + | |||||||||||||
| Endocrine/metabolic disorders | + | + | + | ||||||||||||
| Triacylglycerol (mmol/L) | >5.1 | ||||||||||||||
| Oliguric renal insufficiency | + | ||||||||||||||
| Haemodynamic shock | + | ||||||||||||||
| Major pulmonary complications | + | ||||||||||||||
| Malignant disease | + | ||||||||||||||
| Significant postoperative bleeding | + | ||||||||||||||
| Isolation protocol | + | ||||||||||||||
| Comfort care directives | + | ||||||||||||||
| Expected ICU stay (days) | <5 | ||||||||||||||
| Corticosteroid treatment | + | + | |||||||||||||
| Catecholamine treatment | + | ||||||||||||||
| β-adrenoceptor antagonist treatment | + | ||||||||||||||
| Thyroid hormones treatment | + | ||||||||||||||
| Clinical conditions resulting in false data of body composition parameters | + | ||||||||||||||
| Refusal to participate | + | ||||||||||||||
| Reference | Limitations |
|---|---|
| Takemae et al. (2020) [52] | No specific protocol to control nutrition during patient intubation; A small number of REE data were acquired per day. |
| Menegueti et al. (2019) [53] | The REE was measured only at admission to the ICU; The study was conducted in a single centre |
| Panitchote et al. (2017) [54] | Difficulties in obtaining steady state; The small sample size; The IC was measured only 6 h per day and did not occur randomly during the day; Activities were not recorded during the measurements. |
| Lee et al. (2017) [64] | Heterogeneous nature of the cohort; Patients whose disease progression warrants admission to the ICU can be in their late and more severe stages; Variability in sedation management and body mass. |
| Wu et al. (2016) [65] | A short-term self-control study in surgical ICU–mortality outcomes of enrolled patients were not followed; A small-size study at a single department; Plasma cytokine concentration and ultrafiltration were not tested due to operational difficulties. |
| Wu et al. (2015) [55] | The effect of medical procedures on the REE determination has not been evaluated in each individual patient included; The IC measurement was performed around noon every day; A single centre, small sample study; Some patients entered the ICU directly without prior hospitalisation, while others were admitted from the ward or postoperatively–the included patients were at various stages in the course of their disease. |
| Basile-Filho et al. (2008) [56] | ND |
| Auxiliadora-Martins et al. (2008) [57] | ND |
| Gore et al. (2006) [67] | ND |
| Rusavy et al. (2005) [61] | ND |
| Rusavy et al. (2004) [62] | The volunteers were younger, and had lower fasting glycaemia and EE–increased age decreases insulin sensitivity; Calculation of carbohydrate and fat utilisation on the basis of nonprotein RQ can lead to errors if the rates of gluconeogenesis and ketogenesis are changing. |
| Zauner et al. (2001) [59] | ND |
| Uehara et al. (1999) [60] | ND |
| Saeed et al. (1999) [63] | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasyluk, W.; Zwolak, A.; Jonckheer, J.; De Waele, E.; Dąbrowski, W. Methodological Aspects of Indirect Calorimetry in Patients with Sepsis—Possibilities and Limitations. Nutrients 2022, 14, 930. https://doi.org/10.3390/nu14050930
Wasyluk W, Zwolak A, Jonckheer J, De Waele E, Dąbrowski W. Methodological Aspects of Indirect Calorimetry in Patients with Sepsis—Possibilities and Limitations. Nutrients. 2022; 14(5):930. https://doi.org/10.3390/nu14050930
Chicago/Turabian StyleWasyluk, Weronika, Agnieszka Zwolak, Joop Jonckheer, Elisabeth De Waele, and Wojciech Dąbrowski. 2022. "Methodological Aspects of Indirect Calorimetry in Patients with Sepsis—Possibilities and Limitations" Nutrients 14, no. 5: 930. https://doi.org/10.3390/nu14050930
APA StyleWasyluk, W., Zwolak, A., Jonckheer, J., De Waele, E., & Dąbrowski, W. (2022). Methodological Aspects of Indirect Calorimetry in Patients with Sepsis—Possibilities and Limitations. Nutrients, 14(5), 930. https://doi.org/10.3390/nu14050930






