Effects of Creatine Supplementation on Brain Function and Health
Abstract
:1. Introduction
2. Creatine and Guanidinoacetic Acid (GAA) Supplementation on Brain Creatine and Phophorylcreatine (PCr)
2.1. Creatine Monohdyrate Supplementation
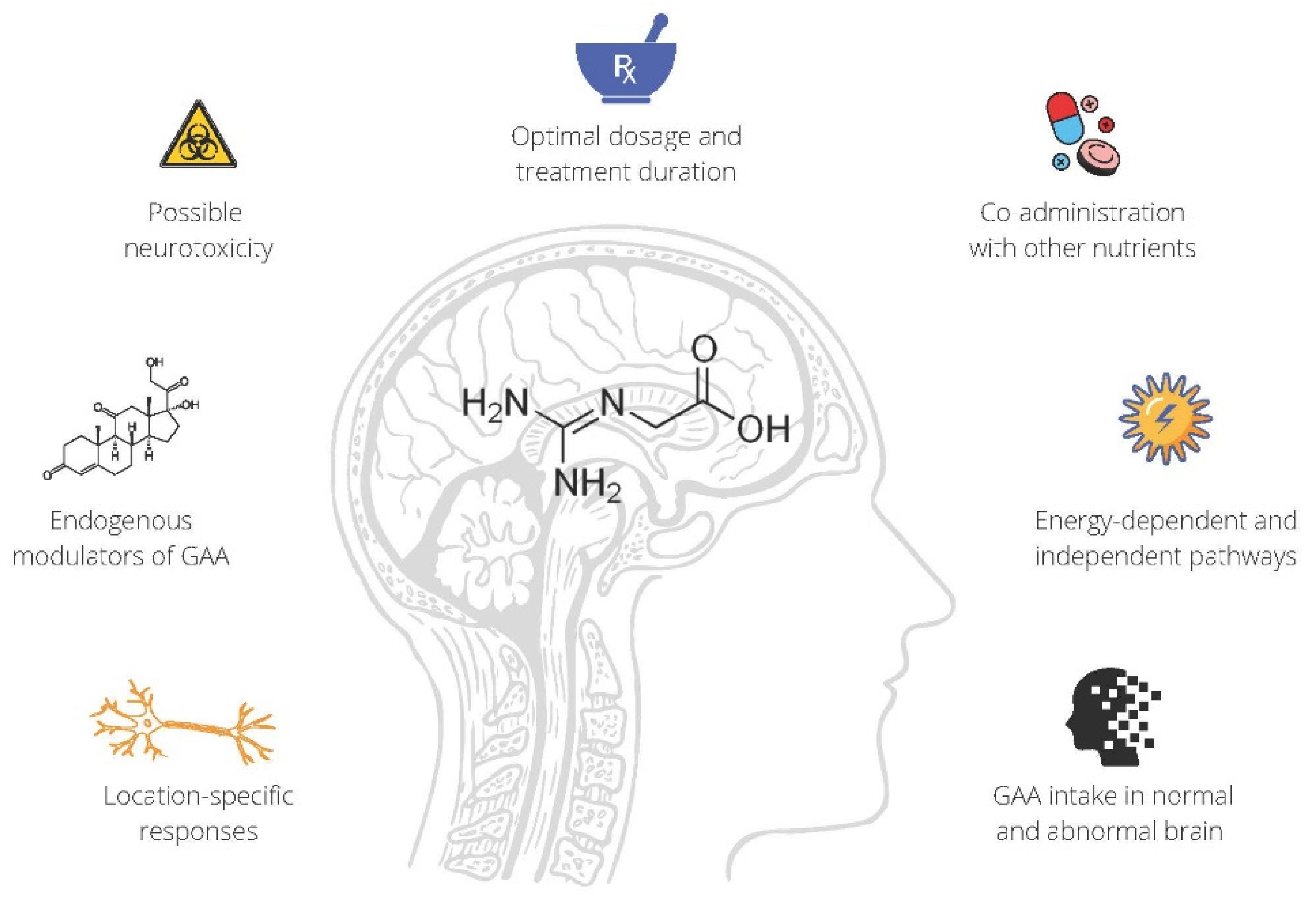
2.2. GAA Supplementation
3. Creatine and Cognitive Function
4. Creatine for Neurodegenerative Diseases
4.1. Amyotrophic Lateral Sclerosis
4.2. Duchenne Muscular Dystrophy
4.3. Huntington’s Disease
4.4. Multiple Sclerosis
4.5. Parkinson’s Disease
5. Creatine and Mental Health
5.1. Depression
5.2. Anxiety and Post-Traumatic Stress Disorder
6. Creatine for Concussion and Traumatic Brain Injury (TBI)
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, P.J. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci. Biobehav. Rev. 2012, 36, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, W.; Cadonic, C.; Cortes-Perez, C.; Roy Chowdhury, S.; Djordjevic, J.; Thomson, E.; Bernstein, M.; Suh, M.; Fernyhough, P.; Albensi, B. Chronic dietary creatine enhances hippocampal-dependent spatial memory, bioenergetics, and levels of plasticity-related proteins associated with NF-κB. Learn. Mem. 2018, 25, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Candow, D.; Ferreira, L.; Souza-Junior, T. Effects of creatine supplementation on properties of muscle, bone, and brain function in older adults: A narrative review. J. Diet. Suppl. 2021, 1–18. [Google Scholar] [CrossRef]
- Ricci, T.; Forbes, S.C.; Candow, D.G. Creatine supplementation: Practical strategies and considerations for mixed martial arts. J. Exerc. Nutr. 2020, 3, s2. [Google Scholar]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Dolder, M.; Schlattner, U.; Eder, M.; Hornemann, T.; Kraft, T.; Stolz, M. Creatine kinase: An enzyme with a central role in cellular energy metabolism. MAGMA 1998, 6, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Pires, G.; Braissant, O. Current and potential new treatment strategies for creatine deficiency syndromes. Mol. Genet. Metab. 2022, 135, 15–26. [Google Scholar] [CrossRef]
- Harris, R.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle Creatine Loading in Men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Kreider, R.; Kalman, D.; Antonio, J.; Ziegenfuss, T.; Wildman, R.; Collins, R.; Candow, D.; Kleiner, S.; Almada, A.; Lopez, H. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; Sewell, D.A.; Hultman, E.; Greenhaff, P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. 1999, 87, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenge, G.R.; Lambourne, J.; Casey, A.; Macdonald, I.A.; Greenhaff, P.L. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E974–E979. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hultman, E.; Macdonald, I.; Sewell, D.; Greenhaff, P. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol. 1996, 271, E821–E826. [Google Scholar] [CrossRef]
- Steenge, G.R.; Simpson, E.J.; Greenhaff, P.L. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J. Appl. Physiol. 2000, 89, 1165–1171. [Google Scholar] [CrossRef]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Tarnopolsky, M.A.; Candow, D.G. Effect of alpha-lipoic acid combined with creatine monohydrate on human skeletal muscle creatine and phosphagen concentration. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 294–302. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 173. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Dechent, P.; Pouwels, P.J.W.; Wilken, B.; Hanefeld, F.; Frahm, J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 46, R698–R704. [Google Scholar] [CrossRef]
- Pan, J.W.; Takahashi, K. Cerebral energetic effects of creatine supplementation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1745. [Google Scholar] [CrossRef] [Green Version]
- Kondo, D.G.; Sung, Y.H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellem, T.L.; Sung, Y.-H.; Shi, X.-F.; Pett, M.A.; Latendresse, G.; Morgan, J.; Huber, R.S.; Kuykendall, D.; Lundberg, K.J.; Renshaw, P.F. Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study. J. Dual Diagn. 2015, 11, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.E.; Byblow, W.D.; Gant, N. Creatine Supplementation Enhances Corticomotor Excitability and Cognitive Performance during Oxygen Deprivation. J. Neurosci. 2015, 35, 1773. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Artioli, G.; Otaduy, M.; Leite, C.; Arruda, W.; Veiga, R.; Gualano, B. Effect of age, diet, and tissue type on PCr response to creatine supplementation. J. Appl. Physiol. 2017, 123, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Lyoo, I.K.; Kong, S.W.; Sung, S.M.; Hirashima, F.; Parow, A.; Hennen, J.; Cohen, B.M.; Renshaw, P.F. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003, 123, 87–100. [Google Scholar] [CrossRef]
- Turner, C.E.; Russell, B.R.; Gant, N. Comparative quantification of dietary supplemented neural creatine concentrations with (1)H-MRS peak fitting and basis spectrum methods. Magn. Reson. Imaging 2015, 33, 1163–1167. [Google Scholar] [CrossRef]
- Wilkinson, I.D.; Mitchel, N.; Breivik, S.; Greenwood, P.; Griffiths, P.D.; Winter, E.M.; Van Beek, E.J.R. Effects of creatine supplementation on cerebral white matter in competitive sportsmen. Clin. J. Sport Med. 2006, 16, 63–67. [Google Scholar] [CrossRef]
- Merege-Filho, C.A.A.; Otaduy, M.C.G.; de Sá-Pinto, A.L.; de Oliveira, M.O.; de Souza Gonçalves, L.; Hayashi, A.P.T.; Roschel, H.; Pereira, R.M.R.; Silva, C.A.; Brucki, S.M.D.; et al. Does brain creatine content rely on exogenous creatine in healthy youth? A proof-of-principle study. Appl. Physiol. Nutr. Metab. 2016, 42, 128–134. [Google Scholar] [CrossRef]
- Braissant, O.; Bachmann, C.; Henry, H. Expression and function of AGAT, GAMT and CT1 in the mammalian brain. Subcell. Biochem. 2007, 46, 67–81. [Google Scholar] [CrossRef]
- Andres, R.H.; Ducray, A.D.; Schlattner, U.; Wallimann, T.; Widmer, H.R. Functions and effects of creatine in the central nervous system. Brain Res. Bull. 2008, 76, 329–343. [Google Scholar] [CrossRef]
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomons, G.S.; Van Dooren, S.J.M.; Verhoeven, N.M.; Marsden, D.; Schwartz, C.; Cecil, K.M.; DeGrauw, T.J.; Jakobs, C. X-linked creatine transporter defect: An overview. J. Inherit. Metab. Dis. 2003, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Kerksick, C.M. Creatine Supplementation in Children and Adolescents. Nutrients 2021, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Stöckler, S.; Holzbach, U.; Hanefeld, F.; Marquardt, I.; Helms, G.; Requar, T.M.; Hanicke, W.; Frahm, J. Creatine deficiency in the brain: A new, treatable inborn error of metabolism. Pediatr. Res. 1994, 36, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Borsook, M.; Billig, H.; Golseth, J. Betaine and glycocyamine in the treatment of disability resulting from acute anterior poliomyelitis. Ann. West. Med. Surg. 1952, 6, 423–427. [Google Scholar] [PubMed]
- Fallis, B.D.; Lam, R.L. Betaine and glycocyamine therapy for the chronic residuals of poliomyelitis. J. Am. Med. Assoc. 1952, 150, 851–853. [Google Scholar] [CrossRef]
- Aldes, J. Glycocyamine betaine as an adjunct in the treatment of neuromuscular disease patients. J. Ark. Med. Soc. 1957, 54, 186–194. [Google Scholar]
- Watkins, A.L. Betaine and glycocyamine in the treatment of poliomyelitis. N. Engl. J. Med. 1953, 248, 621–623. [Google Scholar] [CrossRef]
- Liveksedge, L.A. Glycocyamine and betaine in motor-neurone disease. Lancet 1956, 271, 1136–1138. [Google Scholar] [CrossRef]
- McBreairty, L.E.; Robinson, J.L.; Furlong, K.R.; Brunton, J.A.; Bertolo, R.F. Guanidinoacetate is more effective than creatine at enhancing tissue creatine stores while consequently limiting methionine availability in Yucatan miniature pigs. PLoS ONE 2015, 10, e0131563. [Google Scholar] [CrossRef] [Green Version]
- Ostojic, S.M.; Ostojic, J.; Drid, P.; Vranes, M. Guanidinoacetic acid versus creatine for improved brain and muscle creatine levels: A superiority pilot trial in healthy men. Appl. Physiol. Nutr. Metab. 2016, 41, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Ostojic, J.; Drid, P.; Vranes, M.; Jovanov, P. Dietary guanidinoacetic acid increases brain creatine levels in healthy men. Nutrition 2017, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Semeredi, S.; Stajer, V.; Ostojic, J.; Vranes, M.; Ostojic, S.M. Guanidinoacetic acid with creatine compared with creatine alone for tissue creatine content, hyperhomocysteinemia, and exercise performance: A randomized, double-blind superiority trial. Nutrition 2019, 57, 162–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostojic, S.M. Guanidinoacetic acid loading for improved location-specific brain creatine. Clin. Nutr. 2021, 40, 324–326. [Google Scholar] [CrossRef]
- Robinson, J.L.; McBreairty, L.E.; Ryan, R.A.; Randunu, R.; Walsh, C.J.; Martin, G.M.; Brunton, J.A.; Bertolo, R.F. Effects of supplemental creatine and guanidinoacetic acid on spatial memory and the brain of weaned Yucatan miniature pigs. PLoS ONE 2020, 15, e0226806. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef]
- Seper, V.; Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, J.; Nesic, N.; Ostojic, S.M. Guanidinoacetate-Creatine Supplementation Improves Functional Performance and Muscle and Brain Bioenergetics in the Elderly: A Pilot Study. Ann. Nutr. Metab. 2021, 77, 244–247. [Google Scholar] [CrossRef]
- Ostojic, S.M. Tackling guanidinoacetic acid for advanced cellular bioenergetics. Nutrition 2017, 34, 55–57. [Google Scholar] [CrossRef]
- Takahashi, H.; Arai, B.; Koshino, C. Effects of guanidinoacetic acid, gamma-guanidinobutyric acid and gamma-guanidinobutyryl-methylester on the mammalian cerebral cortex. Jpn. J. Physiol. 1961, 11, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Neu, A.; Neuhoff, H.; Trube, G.; Fehr, S.; Ullrich, K.; Roeper, J.; Isbrandt, D. Activation of GABA(A) receptors by guanidinoacetate: A novel pathophysiological mechanism. Neurobiol. Dis. 2002, 11, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Zugno, A.I.; Stefanello, F.M.; Streck, E.L.; Calcagnotto, T.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T.S. Inhibition of Na+, K+-ATPase activity in rat striatum by guanidinoacetate. Int. J. Dev. Neurosci. 2003, 21, 183–189. [Google Scholar] [CrossRef]
- Zugno, A.I.; Scherer, E.B.S.; Schuck, P.F.; Oliveira, D.L.; Wofchuk, S.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T.S. Intrastriatal administration of guanidinoacetate inhibits Na+, K+-ATPase and creatine kinase activities in rat striatum. Metab. Brain Dis. 2006, 21, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zugno, A.I.; Scherer, E.B.S.; Mattos, C.; Ribeiro, C.A.J.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T.S. Evidence that the inhibitory effects of guanidinoacetate on the activities of the respiratory chain, Na+, K+-ATPase and creatine kinase can be differentially prevented by taurine and vitamins E and C administration in rat striatum in vivo. Biochim. Biophys. Acta 2007, 1772, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zugno, A.I.; Oliveira, D.L.; Scherer, E.B.S.; Wajner, M.; Wofchuk, S.; Wyse, A.T.S. Guanidinoacetate inhibits glutamate uptake in rat striatum of rats at different ages. Neurochem. Res. 2007, 32, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Zugno, A.I.; Pereira, L.O.; Mattos, C.; Scherer, E.B.S.; Netto, C.A.; Wyse, A.T.S. Guanidinoacetate administration increases acetylcholinesterase activity in striatum of rats and impairs retention of an inhibitory avoidance task. Metab. Brain Dis. 2008, 23, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zugno, A.I.; Stefanello, F.M.; Scherer, E.B.S.; Mattos, C.; Pederzolli, C.D.; Andrade, V.M.; Wannmacher, C.M.D.; Wajner, M.; Dutra-Filho, C.S.; Wyse, A.T.S. Guanidinoacetate decreases antioxidant defenses and total protein sulfhydryl content in striatum of rats. Neurochem. Res. 2008, 33, 1804–1810. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ostojic, J. Dietary guanidinoacetic acid does not accumulate in the brain of healthy men. Eur. J. Nutr. 2018, 57, 3003–3005. [Google Scholar] [CrossRef]
- Gillessen, T.; Budd, S.L.; Lipton, S.A. Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 2002, 513, 3–40. [Google Scholar] [CrossRef]
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amin. Acids 2016, 48, 1929–1940. [Google Scholar] [CrossRef]
- Mercimek-Andrews, S.; Salomons, G.S. Creatine Deficiency Syndromes. Inborn Metab. Dis. Diagn. Treat. 2015, 239–247. [Google Scholar]
- Kaldis, P.; Hemmer, W.; Zanolla, E.; Holtzman, D.; Wallimann, T. “Hot spots” of creatine kinase localization in brain: Cerebellum, hippocampus and choroid plexus. Dev. Neurosci. 1996, 18, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Borchio, L.; MacHek, S.B.; MacHado, M. Supplemental creatine monohydrate loading improves cognitive function in experienced mountain bikers. J. Sports Med. Phys. Fit. 2020, 60, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMorris, T.; Mielcarz, G.; Harris, R.; Swain, J.; Howard, A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol. Dev. Cogn. B. Aging. Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Hammett, S.T.; Wall, M.B.; Edwards, T.C.; Smith, A.T. Dietary supplementation of creatine monohydrate reduces the human fMRI BOLD signal. Neurosci. Lett. 2010, 479, 201–205. [Google Scholar] [CrossRef]
- Ling, J.; Kritikos, M.; Tiplady, B. Cognitive effects of creatine ethyl ester supplementation. Behav. Pharmacol. 2009, 20, 673–679. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Harris, R.C.; Howard, A.N.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103. [Google Scholar] [CrossRef]
- Smolarek, A.C.; Mcanulty, S.R.; Ferreira, L.H.; Cordeiro, G.R.; Alessi, A.; Rebesco, D.B.; Honorato, I.C.; Laat, E.F.; Mascarenhas, L.P.; Souza-Junior, T.P.; et al. Effect of 16 Weeks of Strength Training and Creatine Supplementation on Strength and Cognition in Older Adults: A Pilot Study. J. Exerc. Physiol. 2020, 23, 88–94. [Google Scholar]
- VAN Cutsem, J.; Roelands, B.; Pluym, B.; Tassignon, B.; Verschueren, J.; DE Pauw, K.; Meeusen, R. Can Creatine Combat the Mental Fatigue-associated Decrease in Visuomotor Skills? Med. Sci. Sports Exerc. 2020, 52, 120–130. [Google Scholar] [CrossRef]
- Watanabe, A.; Kato, N.; Kato, T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci. Res. 2002, 42, 279–285. [Google Scholar] [CrossRef]
- Pires, L.A.M.; Forbes, S.C.; Candow, D.G.; Machado, M. Creatine supplementation on cognitive performance following Creatine supplementation on cognitive performance following exercise in female Muay Thai athletes exercise in female Muay Thai athletes. J. Soc. NeuroSports 2020, 1, 6. [Google Scholar]
- Rawson, E.S.; Lieberman, H.R.; Walsh, T.M.; Zuber, S.M.; Harhart, J.M.; Matthews, T.C. Creatine supplementation does not improve cognitive function in young adults. Physiol. Behav. 2008, 95, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.R.; Filho, C.A.A.M.; Benatti, F.B.; Brucki, S.; Pereira, R.M.R.; de São Pinto, A.L.; Lima, F.R.; Roschel, H.; Gualano, B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: A randomized double-blind study. PLoS ONE 2013, 8, e76301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, C.; Digney, A.L.; McEwan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proc. R. Soc. B Biol. Sci. 2003, 270, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44. [Google Scholar] [CrossRef]
- Klopstock, T.; Elstner, M.; Bender, A. Creatine in mouse models of neurodegeneration and aging. Amino Acids 2011, 40, 1297–1303. [Google Scholar] [CrossRef]
- Xu, C.J.; Klunk, W.E.; Kanfe, J.N.; Xiong, Q.; Miller, G.; Pettegrew, J.W. Phosphocreatine-dependent glutamate uptake by synaptic vesicles. A comparison with atp-dependent glutamate uptake. J. Biol. Chem. 1996, 271, 13435–13440. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F. Neuroprotective effects of creatine. Amino Acids 2011, 40, 1305–1313. [Google Scholar] [CrossRef]
- Mazzini, L.; Balzarini, C.; Colombo, R.; Mora, G.; Pastore, I.; De Ambrogio, R.; Caligari, M. Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: Preliminary results. J. Neurol. Sci. 2001, 191, 139–144. [Google Scholar] [CrossRef]
- Groeneveld, G.J.; Veldink, J.H.; Van der Tweel, I.; Kalmijn, S.; Beijer, C.; De Visser, M.; Wokke, J.H.J.; Franssen, H.; Van den Berg, L.H. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann. Neurol. 2003, 53, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.; King, R.M.; Jackson, C.E.; Bedlack, R.S.; Barohn, R.J.; Dick, A.; Phillips, L.H.; Chapin, J.; Gelinas, D.F.; Lou, J.S. Creatine monohydrate in ALS: Effects on strength, fatigue, respiratory status and ALSFRS. Amyotroph. Lateral Scler. 2008, 9, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefner, J.M.; Cudkowicz, M.E.; Schoenfeld, D.; Conrad, T.; Taft, J.; Chilton, M.; Urbinelli, L.; Qureshi, M.; Zhang, H.; Pestronk, A.; et al. A clinical trial of creatine in ALS. Neurology 2004, 63, 1656–1661. [Google Scholar] [CrossRef]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Cecio, M.R.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in DuchenneMuscular Dystrophy: Retrospective analysisof 835 patients. Acta Myol. 2012, 31, 125. [Google Scholar]
- Louis, M.; Lebacq, J.; Poortmans, J.R.; Belpaire-Dethiou, M.-C.; Devogelaer, J.-P.; Van Hecke, P.; Goubel, F.; Francaux, M. Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve 2003, 27, 604–610. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Mahoney, D.J.; Vajsar, J.; Rodriguez, C.; Doherty, T.J.; Roy, B.D.; Biggar, D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 2004, 62, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- Escolar, D.; Buyse, G.; Henricson, E.; Leshner, R.; Florence, J.; Mayhew, J.; Tesi-Rocha, C.; Gorni, K.; Pasquali, L.; Patel, K.; et al. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann. Neurol. 2005, 58, 151–155. [Google Scholar] [CrossRef]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Blamire, A.M.; Manners, D.N.; Rajagopalan, B.; Styles, P.; Schapira, A.H.V.; Warner, T.T. High-dose creatine therapy for Huntington disease: A 2-year clinical and MRS study. Neurology 2005, 64, 1655–1656. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Blamire, A.M.; Manners, D.N.; Rajagopalan, B.; Styles, P.; Schapira, A.H.V.; Warner, T.T. Creatine therapy for Huntington’s disease: Clinical and MRS findings in a 1-year pilot study. Neurology 2003, 61, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Verbessem, P.; Lemiere, J.; Eijnde, B.O.; Swinnen, S.; Vanhees, L.; Van Leemputte, M.; Hespel, P.; Dom, R. Creatine supplementation in Huntington’s disease: A placebo-controlled pilot trial. Neurology 2003, 61, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Auer, D.P.; Merl, T.; Reilmann, R.; Saemann, P.; Yassouridis, A.; Bender, J.; Weindl, A.; Dose, M.; Gasser, T.; et al. Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J. Neurol. 2005, 252, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Schifitto, G.; Oakes, D.; Bredlau, A.L.; Meyers, C.M.; Nahin, R.; Rosas, H.D. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology 2017, 89, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, C.P.; Archer, R.L.; Carrithers, J.A.; Fink, W.J.; Evans, W.J.; Trappe, T.A. Influence of creatine monohydrate ingestion on muscle metabolites and intense exercise capacity in individuals with multiple sclerosis. Arch. Phys. Med. Rehabil. 2003, 84, 1206–1210. [Google Scholar] [CrossRef]
- Malin, S.K.; Cotugna, N.; Fang, C.S. Effect of creatine supplementation on muscle capacity in individuals with multiple sclerosis. J. Diet. Suppl. 2008, 5, 20–32. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Calingasan, N.Y.; Wille, E.J.; Cormier, K.; Smith, K.; Ferrante, R.J.; Flint Beal, M. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J. Neurochem. 2009, 109, 1427–1439. [Google Scholar] [CrossRef] [Green Version]
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Müller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264. [Google Scholar] [CrossRef]
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural Repair 2007, 21, 107–115. [Google Scholar] [CrossRef]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Wai, T.C.; Demler, O.; Walters, E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palay, J.; Taillieu, T.L.; Afifi, T.O.; Turner, S.; Bolton, J.M.; Enns, M.W.; Smith, M.; Lesage, A.; Bakal, J.A.; Rush, B.; et al. Prevalence of Mental Disorders and Suicidality in Canadian Provinces. Can. J. Psychiatry 2019, 64, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Masand, P.S. Tolerability and adherence issues in antidepressant therapy. Clin. Ther. 2003, 25, 2289–2304. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Usala, T.; Clavenna, A.; Zuddas, A.; Bonati, M. Randomised controlled trials of selective serotonin reuptake inhibitors in treating depression in children and adolescents: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2008, 18, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Doran, C.M.; Kinchin, I. A review of the economic impact of mental illness. Aust. Heal. Rev. 2019, 43, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Fons, C.; Campistol, J. Creatine Defects and Central Nervous System. Semin. Pediatr. Neurol. 2016, 23, 285–289. [Google Scholar] [CrossRef]
- Faulkner, P.; Paioni, S.L.; Kozhuharova, P.; Orlov, N.; Lythgoe, D.J.; Daniju, Y.; Morgenroth, E.; Barker, H.; Allen, P. Relationship between depression, prefrontal creatine and grey matter volume. J. Psychopharmacol. 2021, 35, 1464–1472. [Google Scholar] [CrossRef]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol. Biochem. Behav. 2012, 101, 588–601. [Google Scholar] [CrossRef] [Green Version]
- Bakian, A.V.; Huber, R.S.; Scholl, L.; Renshaw, P.F.; Kondo, D. Dietary creatine intake and depression risk among U.S. adults. Transl. Psychiatry 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology 2010, 35, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, P.J.; Debold, J.F.; Rios, M.; Kanarek, R.B. Chronic high-dose creatine has opposing effects on depression-related gene expression and behavior in intact and sex hormone-treated gonadectomized male and female rats. Pharmacol. Biochem. Behav. 2015, 130, 22–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanekar, S.; Ettaro, R.; Hoffman, M.D.; Ombach, H.J.; Brown, J.; Lynch, C.; Sheth, C.S.; Renshaw, P.F. Sex-Based Impact of Creatine Supplementation on Depressive Symptoms, Brain Serotonin and SSRI Efficacy in an Animal Model of Treatment-Resistant Depression. Int. J. Mol. Sci. 2021, 22, 8195. [Google Scholar] [CrossRef]
- Okwuofu, E.O.; Ogundepo, G.E.; Akhigbemen, A.M.; Abiola, A.L.; Ozolua, R.I.; Igbe, I.; Chinazamoku, O. Creatine attenuates seizure severity, anxiety and depressive-like behaviors in pentylenetetrazole kindled mice. Metab. Brain Dis. 2021, 36, 571–579. [Google Scholar] [CrossRef]
- Rosa, J.M.; Pazini, F.L.; Cunha, M.P.; Colla, A.R.S.; Manosso, L.M.; Mancini, G.; Souza, A.C.G.; de Bem, A.F.; Prediger, R.D.; Rodrigues, A.L.S. Antidepressant effects of creatine on amyloid β 1-40-treated mice: The role of GSK-3β/Nrf 2 pathway. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 270–278. [Google Scholar] [CrossRef]
- Warrington, T.P.; Bostwick, J.M. Psychiatric adverse effects of corticosteroids. Mayo Clin. Proc. 2006, 81, 1361–1367. [Google Scholar] [CrossRef] [Green Version]
- Pazini, F.L.; Cunha, M.P.; Azevedo, D.; Rosa, J.M.; Colla, A.; de Oliveira, J.; Ramos-Hryb, A.B.; Brocardo, P.S.; Gil-Mohapel, J.; Rodrigues, A.L.S. Creatine Prevents Corticosterone-Induced Reduction in Hippocampal Proliferation and Differentiation: Possible Implication for Its Antidepressant Effect. Mol. Neurobiol. 2017, 54, 6245–6260. [Google Scholar] [CrossRef]
- Pazini, F.L.; Rosa, J.M.; Camargo, A.; Fraga, D.B.; Moretti, M.; Siteneski, A.; Rodrigues, A.L.S. mTORC1-dependent signaling pathway underlies the rapid effect of creatine and ketamine in the novelty-suppressed feeding test. Chem. Biol. Interact. 2020, 332, 109281. [Google Scholar] [CrossRef]
- Amital, D.; Vishne, T.; Rubinow, A.; Levine, J. Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am. J. Psychiatry 2006, 163, 1840–1841. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Yoon, S.; Kim, T.-S.; Hwang, J.; Kim, J.E.; Won, W.; Bae, S.; Renshaw, P.F. A Randomized, Double-Blind Placebo-Controlled Trial of Oral Creatine Monohydrate Augmentation for Enhanced Response to a Selective Serotonin Reuptake Inhibitor in Women with Major Depressive Disorder. Am. J. Psychiatry 2012, 169, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roitman, S.; Green, T.; Osher, Y.; Karni, N.; Levine, J. Creatine monohydrate in resistant depression: A preliminary study. Bipolar Disord. 2007, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, R.; Fernandes, F.; Silva, M.; Dias, R.; Lafer, B. Cognitive effects of creatine monohydrate adjunctive therapy in patients with bipolar depression: Results from a randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 2017, 224, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, R.A.; Silva, M.; de Brito Ferreira Fernandes, F.; de Mello Siqueira Amaral, J.A.; da Silva Dias, R.; Lafer, B. A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression. J. Neural Transm. 2018, 125, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Creatine for the Treatment of Depression. Biomolecules 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemets, B.; Levine, J. A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression. Int. Clin. Psychopharmacol. 2013, 28, 127–133. [Google Scholar] [CrossRef]
- Watson, P. PTSD as a Public Mental Health Priority. Curr. Psychiatry Rep. 2019, 21, 61. [Google Scholar] [CrossRef]
- Coplan, J.D.; Mathew, S.J.; Mao, X.; Smith, E.L.P.; Hof, P.R.; Coplan, P.M.; Rosenblum, L.A.; Gorman, J.M.; Shungu, D.C. Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: Relationship to IQ and early trauma. Psychiatry Res. 2006, 147, 27–39. [Google Scholar] [CrossRef]
- Schuff, N.; Neylan, T.C.; Fox-Bosetti, S.; Lenoci, M.; Samuelson, K.W.; Studholme, C.; Kornak, J.; Marmar, C.R.; Weiner, M.W. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008, 162, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, G.; Petropoulos, H.; Hamilton, D.A.; Rowland, L.M.; Horan, W.P.; Griego, J.A.; Moreshead, M.; Hart, B.L.; Brooks, W.M. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: Preliminary results. Can. J. Psychiatry 2002, 47, 666–670. [Google Scholar] [CrossRef]
- Amital, D.; Vishne, T.; Roitman, S.; Kotler, M.; Levine, J. Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J. Clin. Psychiatry 2006, 67, 836–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashbaugh, A.; McGrew, C. The Role of Nutritional Supplements in Sports Concussion Treatment. Curr. Sports Med. Rep. 2016, 15, 16–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsley Dean, P.J.; Arikan, G.; Opitz, B.; Sterr, A. Potential for use of creatine supplementation following mild traumatic brain injury. Concussion 2017, 2, CNC34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassol, G.; Godinho, D.B.; de Zorzi, V.N.; Farinha, J.B.; Della-Pace, I.D.; de Carvalho Gonçalves, M.; Oliveira, M.S.; Furian, A.F.; Fighera, M.R.; Royes, L.F.F. Potential therapeutic implications of ergogenic compounds on pathophysiology induced by traumatic brain injury: A narrative review. Life Sci. 2019, 233, 116684. [Google Scholar] [CrossRef] [PubMed]
- Pender, S.C.; Smith, A.M.; Finnoff, J.T.; Huston, J.; Stuart, M.J. Concussions in Ice Hockey—Moving Toward Objective Diagnoses and Point-of-care Treatment: A Review. Curr. Sports Med. Rep. 2020, 19, 380–386. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef] [Green Version]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury—An Update. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 373–393. [Google Scholar] [CrossRef]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef]
- Wang, Y.; Bartels, H.M.; Nelson, L.D. A Systematic Review of ASL Perfusion MRI in Mild TBI. Neuropsychol. Rev. 2020, 1–32. [Google Scholar] [CrossRef]
- Signoretti, S.; Di Pietro, V.; Vagnozzi, R.; Lazzarino, G.; Amorini, A.M.; Belli, A.; D’Urso, S.; Tavazzi, B. Transient alterations of creatine, creatine phosphate, N-acetylaspartate and high-energy phosphates after mild traumatic brain injury in the rat. Mol. Cell. Biochem. 2010, 333, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, R.; Signoretti, S.; Floris, R.; Marziali, S.; Manara, M.; Amorini, A.M.; Belli, A.; Di Pietro, V.; D’Urso, S.; Pastore, F.S.; et al. Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. J. Head Trauma Rehabil. 2013, 28, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakellaris, G.; Kotsiou, M.; Tamiolaki, M.; Kalostos, G.; Tsapaki, E.; Spanaki, M.; Spilioti, M.; Charissis, G.; Evangeliou, A. Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: An open label randomized pilot study. J. Trauma 2006, 61, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Sakellaris, G.; Nasis, G.; Kotsiou, M.; Tamiolaki, M.; Charissis, G.; Evangeliou, A. Prevention of traumatic headache, dizziness and fatigue with creatine administration. A pilot study. Acta Paediatr. 2008, 97, 34. [Google Scholar] [CrossRef] [PubMed]
- Sakellaris, G.; Partalis, N.; Nasis, G.; Kotsiou, M.; Tamiolaki, M.; Bouloukaki, E.; Evangeliou, A. Outcome of Traumatic Dysarthria and Lingual Problems of Understanding with Creatine Administration. An Open Label Randomized Pilot Study. J. Trauma Treat. 2012, 1, 120. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.; Geiger, J.; Mattson, M.; Scheff, S. Dietary supplement creatine protects against traumatic brain injury. Ann. Neurol. 2000, 48, 723–729. [Google Scholar] [CrossRef]
- Scheff, S.W.; Dhillon, H.S. Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem. Res. 2004, 29, 469–479. [Google Scholar] [CrossRef]
- Masse, I.; Moquin, L.; Bouchard, C.; Gratton, A.; De Beaumont, L. Efficacy of prophylactic versus therapeutic administration of the NMDA receptor antagonist MK-801 on the acute neurochemical response to a concussion in a rat model combining force and rotation. J. Neurosurg. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Genius, J.; Geiger, J.; Bender, A.; Möller, H.J.; Klopstock, T.; Rujescu, D. Creatine protects against excitoxicity in an in vitro model of neurodegeneration. PLoS ONE 2012, 7, e30554. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, A.L.L.; Ferreira, A.P.O.; Silva, L.F.A.; Hoffmann, M.S.; Dutra, F.D.; Furian, A.F.; Oliveira, M.S.; Fighera, M.R.; Royes, L.F.F. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res. Bull. 2012, 87, 180–186. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forbes, S.C.; Cordingley, D.M.; Cornish, S.M.; Gualano, B.; Roschel, H.; Ostojic, S.M.; Rawson, E.S.; Roy, B.D.; Prokopidis, K.; Giannos, P.; et al. Effects of Creatine Supplementation on Brain Function and Health. Nutrients 2022, 14, 921. https://doi.org/10.3390/nu14050921
Forbes SC, Cordingley DM, Cornish SM, Gualano B, Roschel H, Ostojic SM, Rawson ES, Roy BD, Prokopidis K, Giannos P, et al. Effects of Creatine Supplementation on Brain Function and Health. Nutrients. 2022; 14(5):921. https://doi.org/10.3390/nu14050921
Chicago/Turabian StyleForbes, Scott C., Dean M. Cordingley, Stephen M. Cornish, Bruno Gualano, Hamilton Roschel, Sergej M. Ostojic, Eric S. Rawson, Brian D. Roy, Konstantinos Prokopidis, Panagiotis Giannos, and et al. 2022. "Effects of Creatine Supplementation on Brain Function and Health" Nutrients 14, no. 5: 921. https://doi.org/10.3390/nu14050921
APA StyleForbes, S. C., Cordingley, D. M., Cornish, S. M., Gualano, B., Roschel, H., Ostojic, S. M., Rawson, E. S., Roy, B. D., Prokopidis, K., Giannos, P., & Candow, D. G. (2022). Effects of Creatine Supplementation on Brain Function and Health. Nutrients, 14(5), 921. https://doi.org/10.3390/nu14050921










