Abstract
The present large scale study aimed to assess the prevalence and consequences of malnutrition, based on clinical assessment (body mass index and preoperative weight loss) and severe hypoalbuminemia (<3.1 g/L), in a representative US cohort undergoing IBD surgery. The American College of Surgeons National Quality improvement program (ACS-NSQIP) Public User Files (PUF) between 2005 and 2018 were assessed. A total of 25,431 patients were identified. Of those, 6560 (25.8%) patients had severe hypoalbuminemia, 380 (1.5%) patients met ESPEN 2 criteria (≥10% weight loss over 6 months PLUS BMI < 20 kg/m2 in patients <70 years OR BMI < 22 kg/m2 in patients ≥70 years), and 671 (2.6%) patients met both criteria (severe hypoalbuminemia and ESPEN 2). Patients who presented with malnutrition according to any of the three definitions had higher rates of overall, minor, major, surgical, and medical complications, longer LOS, higher mortality and higher rates of readmission and reoperation. The simple clinical assessment of malnutrition based on BMI and weight loss only, considerably underestimates its true prevalence of up to 50% in surgical IBD patients and calls for dedicated nutritional assessment.
1. Introduction
Infectious complications or medically refractory disease are surgical indications in patients with inflammatory bowel disease (IBD), emphasizing the importance of preoperative optimization strategies, if feasible, to improve surgical outcomes [1,2]. Hence, proper nutritional assessment is mandatory to identify patients at risk and to launch preoperative nutritional support [3,4]. Hypoalbuminemia, as a marker of preoperative systemic inflammation, is associated with intra-abdominal septic complications and may help to identify patients at increased risk [5,6]. Body mass index (BMI) and weight loss are among the screening tools suggested by the European Society for Clinical Nutrition and Metabolism (ESPEN) [7]. However, clinical assessment solely based on these tools may underestimate the true prevalence of malnutrition and hence, entail nutritional undertreatment in IBD patients. This may especially apply to the US population facing an increasing prevalence of obesity in both the general population and IBD patients, with up to half of patients being either overweight or obese [8].
The present large scale study aimed to assess the prevalence and consequences of malnutrition, based on both clinical assessment and severe hypoalbuminemia, in a representative US cohort undergoing IBD surgery.
2. Materials and Methods
2.1. Data
The American College of Surgeons National Quality improvement program (ACS-NSQIP) Public User Files (PUF) between 2005 and 2018 were assessed. The ACS-NSQIP is an externally validated and outcome-based database that was initially created for quality improvement purposes. Each participant center has trained data abstractors who collect surgical data based on standardized definitions. Those data include demographics, anthropometrics, perioperative and post-operative details. The final pooled PUF represent 20% of surgical patients in the US.
2.2. Cohort
All adult patients who underwent surgery for ulcerative colitis (UC) or Crohn’s disease (CD) between 2005 and 2018, and were reported in the ACS-NSQIP PUF, were included in the analysis. Current procedure terminology and international classification of disease codes were used to select patients as specified in Figure 1.
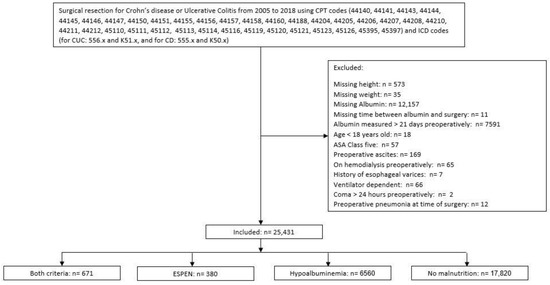
Figure 1.
Study Flow Diagram. ASA: American Society of Anesthesiologists, ESPEN 2: criteria according to the European Society for Nutrition and Metabolism [9], hypoalbuminemia: albumin level < 3.1 g/L.
The patients with missing anthropometric data, albumin level, or who had albumin measured more than 21 days before the index surgery, ASA class 5, age < 18 years old, preoperative ascites, or a history of esophageal varices were excluded from the analysis. Further excluded were patients who were on hemodialysis at the time of surgery, patients who were ventilator-dependent or in coma >24 h preoperatively, or patients who had pneumonia at the time of surgery.
2.3. Assessment of Malnutrition
The patients were divided into four mutually exclusive, nonoverlapping groups: (1) patients with preoperative albumin levels < 3.1 g/L which we referred to as severe hypoalbuminemia, (2) patients who had clinical parameters for malnutrition according to the ESPEN definition (≥10% weight loss over 6 months PLUS BMI < 20 kg/m2 in patients <70 years OR BMI < 22 kg/m2 in patients ≥70 years) which we referred to as ESPEN 2 criteria [9], (3) patients who fulfilled both of the aforementioned criteria, and (4) patients who had neither of the aforementioned criteria. Of note, body composition and muscle mass are not reported in the ACS-NSQIP and, therefore, the ESPEN 2 group does not meet the full definition of clinical malnutrition as suggested by the ESPEN guidelines.
2.4. Covariates and Outcomes
Baseline demographics, preoperative laboratories (hematocrit, platelets, liver function tests, including serum glutamic oxaloacetic transaminase and international normalized ratio) and surgical details (approach, surgical setting (elective vs. emergency), extent of resection, diversion at time of surgery) were compared between the four cohorts. The primary outcomes were the prevalence of malnutrition, according to the aforementioned definitions, overall and within both disease categories (UC and CD). The secondary outcomes included 30 day complications as defined by the standardized ACS-NSQIP definitions [10].
Surgical complications included any surgical site infection (SSI, superficial, deep or organ space), wound disruption, systemic sepsis (sepsis or septic shock), or the need for blood transfusion. Medical complications were defined as renal complications (progressive renal failure and/or acute kidney injury), respiratory complications (pneumonia, unplanned intubation, and/or on a mechanical ventilator ≥48 h), major adverse cardiovascular events (MACE: stroke, cardiac arrest requiring cardiopulmonary resuscitation, and/or myocardial infarction), and vascular thromboembolism (VTE: pulmonary embolism and/or deep venous thrombosis). Minor complications included UTI and superficial SSI. Major complications included myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, pneumonia, unplanned intubation, the need for a mechanical ventilator for ≥48 h after surgery, deep venous thrombosis, pulmonary embolism, stroke, acute kidney injury, progressive renal insufficiency, deep SSI, organ space infection, blood transfusion, wound disruption, and systemic sepsis. Further assessed were the length of stay (LOS) (index surgery) and prolonged hospitalization defined as LOS > 12 days (3rd quartile for LOS of the whole cohort). In addition, unplanned readmission, unplanned reoperation and 30 day mortality were reported.
2.5. Statistical Analysis
Descriptive statistics were reported as median (interquartile range IQR) for continuous variables and as frequencies and percentages for categorical variables. The differences between the four groups were compared using the chi-squared test for categorical variables and the Independent Samples Kruskal–Wallis test for continuous variables. Outcomes with an alpha level < 0.1 in the univariable analysis were then included in the multivariable binary logistic regression. The odds ratios (OR) with their corresponding 95% confidence intervals (95% CI) are presented. For all analyses, an alpha level < 0.05 was considered statistically significant and all tests were two-sided.
Statistical analysis was conducted using the Statistical Package for the Social Sciences SPSS Advanced Statistics 25 (IBM Software Group, Inc., Armonk, NY, USA).
3. Results
3.1. Prevalence of Malnutrition
A total of 25,431 patients were identified. Of those, 6560 (25.8%) patients had severe hypoalbuminemia, 380 (1.5%) patients met ESPEN 2 criteria only, and 671 (2.6%) patients met both criteria (severe hypoalbuminemia and ESPEN 2) (Figure 1).
A total of 10,702 patients (42.1%) had UC, while 14,729 (57.9%) had CD. Severe hypoalbuminemia was more prevalent in CD patients (p < 0.001) (Figure 2).
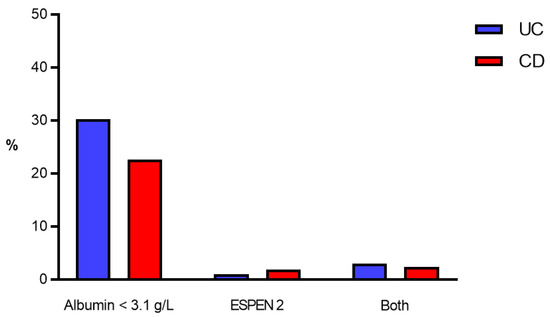
Figure 2.
Prevalence of malnutrition criteria. Prevalence of malnutrition criteria (in %) in UC patients (blue bars) and CD patients (red bars). ESPEN 2—clinical criteria according to the European Society for Nutrition and Metabolism [9]. CD—Crohn’s disease, UC—Ulcerative colitis.
Prevalence of Malnutrition According to Surgical Setting
Overall, 1905 patients (7.5%) underwent emergency surgery. Emergency surgeries were more prevalent in patients with UC compared to patients with CD (910 patients (8.5%) vs. 995 patients (6.8%); p < 0.0001). Malnutrition was more prevalent in patients who underwent emergency surgery compared to patients who had elective surgery, regardless of the underlying disease and the definition used for malnutrition (Figure 3).
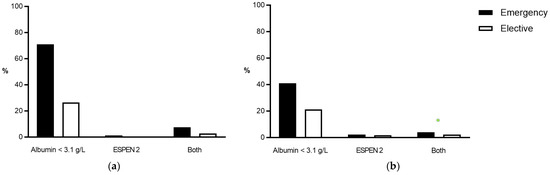
Figure 3.
Prevalence of malnutrition criteria according to surgical setting. Comparison of the prevalence of malnutrition (in %) before emergency (black bars) and elective operations (white bars) in (a) UC and (b) CD patients. ESPEN 2—clinical criteria according to the European Society for Nutrition and Metabolism. CD—Crohn’s disease, UC—Ulcerative colitis.
3.2. Baseline Characteristics
Baseline and surgical characteristics of the four comparative groups are displayed in Table 1.

Table 1.
Baseline characteristics.
3.3. Outcomes
Patients who presented with malnutrition according to any of the three definitions had higher rates of overall, minor, major, surgical, and medical complications, longer LOS, higher mortality, and higher rates of readmission and reoperation, as specified in Table 2.

Table 2.
Unadjusted complications rates.
After adjusting for baseline characteristics, the patients who had clinical malnutrition had higher adjusted odds of overall, major, surgical and medical complications compared to the patients without severe malnutrition (reference group). The patients who had severe hypoalbuminemia had higher adjusted odds of overall complications and prolonged LOS compared to patients who had clinical malnutrition alone (ESPEN 2). There was no statistically significant difference in the adjusted odds regarding major, surgical and medical complications, or in the overall mortality between patients who had severe hypoalbuminemia compared to patients who had clinical malnutrition alone (ESPEN 2) (Figure 4).
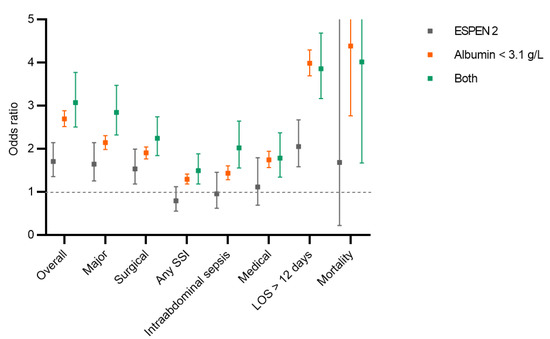
Figure 4.
Postoperative complications. Comparison of different postoperative complications, prolonged length of hospital stay and mortality between patients with hypoalbuminemia, ESPEN 2 criteria [9] and both criteria. All complications were adjusted for demographic and surgical risk factors. Displayed are odds ratios (squares) with 95% confidence interval. None of the three criteria met was used as a reference. SSI: surgical site infection, LOS: length of stay.
4. Discussion
This large scale study confirms both the high prevalence and predictive value of severe preoperative hypoalbuminemia (<3.1 g/L) as a marker of surgical risk in IBD patients. In contrast, simple clinical assessment of malnutrition based on the ESPEN 2 recommendations, combining BMI and weight loss, considerably underestimates the true prevalence of malnutrition, while being strongly associated with postoperative adverse outcomes.
Malnutrition in IBD patients is common with a prevalence of 16%, according to a recent Spanish multicenter study using the Subjective Global Assessment tool and bioelectrical impedance [11]. In surgical IBD patients facing disease complications leading to both malabsorption and nutrient loss, malnutrition affects up to 50% of patients, according to the Global Leadership Initiative on Malnutrition (GLIM) tool [12]. Hence, its prevalence is highly dependent on the used screening modality and a gold standard method is still lacking [13]. Official ESPEN guidelines suggest both BMI and preoperative weight loss as screening tools for preliminary clinical assessment [9]. However, BMI may be an inaccurate measure of body fat composition since it does not take into account muscle mass and bone density among other factors [14]. Assessing body fat composition in the surgical patient may be of importance given the increasing evidence demonstrating a direct relationship between postoperative hospital stay and surgical outcomes [15,16]. Furthermore, BMI scores may remain within normal ranges despite significant preoperative cachexia, especially in the US population facing an increasing prevalence of obesity [17]. The present study confirms these findings, given the very low prevalence of about 4% when using this composite clinical screening tool, which may lead to the underdetection of patients at risk. The assessment of body composition through anthropometric measures may be more accurate and indispensable in this setting [18,19]. Furthermore, validated official nutritional screening tools such as the Nutritional Risk Score (NRS-2002), the Malnutrition Universal Screening Tool (MUST) or (GLIM) need to be considered, together with dedicated assessment by nutritional specialists [12,20,21].
Hypoalbuminemia has been repeatedly described as a risk factor for postoperative adverse outcomes and, in particular, intraabdominal septic complications in surgical IBD patients [5,22]. Preoperative albumin levels correlate with systemic inflammation and thus, reflect disease severity. Hence, albumin represents a surrogate of disease activity rather than a marker of malnutrition. This is further supported by the recently published position paper of the American Society for Parenteral and Enteral Nutrition (ASPEN) [23]. According to these guidelines, serum albumin must be recognized as an inflammatory marker associated with “nutrition risk” in the context of nutrition assessment, rather than with malnutrition per se. Furthermore, serum albumin does not serve as a valid proxy measure of total body protein or total muscle mass. Serum albumin, as an acute phase reactant in proinflammatory states, decreases as a result of increased vessel permeability, increased clearance, hepatic repriorization, and alterations in liver synthesis [23,24]. Importantly, the present study tried to adjust for unrelated causes of hypoalbuminemia by excluding patients with underlying hepatopathy. The high prevalence of severe hypoalbuminemia (< 3.1 g/L) of over 25% in this cohort of all-comers underlines the fragility of surgical IBD patients and calls for preoperative optimization strategies in this vulnerable patient population, including the correction of anemia, weaning of steroids, the treatment of malnutrition, and intraabdominal infection control among others [25]. The present study further suggests a cumulative deleterious effect of both hypoalbuminemia and clinical malnutrition, which may be even more pronounced in the emergency setting. As a consequence, patients should benefit from a global conditioning concept.
The preconditioning of surgical IBD patients is mandatory to achieve better surgical outcomes [3]. Specific recommendations to face preoperative anemia have been described by the European Crohn’s and Colitis Organization (ECCO) [26]. Medical optimization (i.e., steroid weaning) and the treatment of malnutrition represent further strategies to facilitate and improve surgical management [27,28]. Preoperative nutritional supplementation strategies in patients at risk have been recommended by the ESPEN in dedicated guidelines and need to be tailored to the individual patient and risk profile. As a common denominator, enteral support strategies should be preferred over parenteral nutrition whenever possible [3,13]. The present study emphasizes the importance of these optimization strategies in the light of increased postoperative surgical, medical and infectious complications associated with severe hypoalbuminemia and malnutrition.
This study has limitations associated but not exclusively linked to the ACS-NSQIP, with its unselected 20% sample of the surgical US population. First, the comparative baseline group not fulfilling any of the aforementioned criteria may have met other criteria for malnutrition not accounted for. Second, the chosen definition, ESPEN 2, relies on a previously described definition [9]. However, both the ESPEN and the ASPEN endorse further screening tools (i.e., muscle mass, lean body mass) which were not available in this dataset [13,23]. Third, the large scale of this study impedes in-depth analysis of individual institutional practices regarding nutritional screening and therapy, which has to be considered when interpreting the results. Finally, albumin as an unspecific marker of disease activity should not replace dedicated nutritional screening but may help to assess surgical risk in a busy clinical practice.
5. Conclusions
In conclusion, this analysis revealed a low prevalence of malnutrition when defined as a low BMI in conjunction with significant weight. In order to tailor preoperative support strategies and to identify and treat patients at nutrition risk, nutritional screening through validated scores and referral to dedicated specialists to implement preoperative support should be strongly advocated. Albumin as an unspecific marker of disease activity may help to define whether preoperative support strategies are needed.
Author Contributions
Conceptualization, F.G. and M.A.A.-E.-A.; methodology, F.G., M.A.A.-E.-A. and D.W.L.; formal analysis, M.A.A.-E.-A.; interpretation of results, M.A.A.-E.-A., M.H., N.D., D.W.L. and F.G.; writing—original draft preparation, M.A.A.-E.-A. and F.G.; writing—review and editing, M.H. and N.D.; supervision, D.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No ethical approval was needed as this study utilized a national database with no attempt to identify the patients.
Informed Consent Statement
Patient consent was waived in the setting of this de-identified national database for quality improvement purposes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Grass, F.; Pache, B.; Martin, D.; Hahnloser, D.; Demartines, N.; Hübner, M. Preoperative Nutritional Conditioning of Crohn’s Patients-Systematic Review of Current Evidence and Practice. Nutrients 2017, 9, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.S.; Bachour, S.; Jia, X.; Holubar, S.D.; Hull, T.L.; Achkar, J.P.; Philpott, J.; Qazi, T.; Rieder, F.; Cohen, B.L.; et al. Hypoalbuminaemia, Not Biologic Exposure, Is Associated with Postoperative Complications in Crohn’s Disease Patients Undergoing Ileocolic Resection. J. Crohns Colitis 2021, 15, 1142–1151. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2017, 9, 829. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Johnson, A.M.; Harmsen, W.S.; Aniwan, S.; Tremaine, W.J.; Abu Dayyeh, B.K.; Loftus Jr, E.V. Prevalence and Impact of Obesity on Disease-specific Outcomes in a Population-based Cohort of Patients with Ulcerative Colitis. J. Crohns Colitis 2021, 15, 1816–1823. [Google Scholar] [CrossRef]
- McKenna, N.P.; Bews, K.A.; Al-Refaie, W.B.; Colibaseanu, D.T.; Pemberton, J.H.; Cima, R.R.; Habermann, E.B. Assessing Malnutrition Before Major Oncologic Surgery: One Size Does Not Fit All. J. Am. Coll. Surg. 2020, 230, 451–460. [Google Scholar] [CrossRef]
- Raval, M.V.; Pawlik, T.M. Practical Guide to Surgical Data Sets: National Surgical Quality Improvement Program (NSQIP) and Pediatric NSQIP. JAMA Surg. 2018, 153, 764–765. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Fiorindi, C.; Luceri, C.; Dragoni, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020, 12, 2222. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahima, R.S.; Lazar, M.A. Physiology. The health risk of obesity--better metrics imperative. Science 2013, 341, 856–858. [Google Scholar] [CrossRef]
- Tsaousi, G.; Kokkota, S.; Papakostas, P.; Stavrou, G.; Doumaki, E.; Kotzampassi, K. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur. J. Cancer Care 2017, 26, e12491. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Malnutrition at Hospital Admission-Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. J. Parenter. Enter. Nutr. 2016, 40, 487–497. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.P.; Kedia, S.; Madhusudhan, K.S.; Bopanna, S.; Goyal, S.; Jain, S.; Vikram, N.K.; Sharma, R.; Makharia, G.K.; Ahuja, V. Body Composition in Crohn’s Disease and Ulcerative Colitis: Correlation with Disease Severity and Duration. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1215035. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Hübner, M.; Becce, F.; Koerfer, J.; Collinot, J.A.; Demartines, N.; Hahnloser, D.; Grass, F.; Martin, D. Sarcopenia and major complications in patients undergoing oncologic colon surgery. J. Cachexia Sarcopenia Muscle 2021, 12, 1757–1763. [Google Scholar] [CrossRef]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef]
- Wagner, I.J.; Rombeau, J.L. Nutritional support of surgical patients with inflammatory bowel disease. Surg. Clin. N. Am. 2011, 91, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Du, L.; Chong, R.Y.; Jackson, T.D. Hypoalbuminaemia and Postoperative Outcomes in Inflammatory Bowel Disease: The NSQIP Surgical Cohort. J. Crohns Colitis. 2019, 13, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr Clin Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangenberg, M.S.; Horesh, N.; Kopylov, U.; El-Hussuna, A. Preoperative optimization of patients with inflammatory bowel disease undergoing gastrointestinal surgery: A systematic review. Int. J. Colorectal. Dis. 2017, 32, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro-Sanchez, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohns Colitis 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohns Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).