Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation
2.2. Animals
2.3. Experimental Protocol
2.3.1. Maternal Separation
2.3.2. Sub-Threshold Variable Stress
2.3.3. Drug Administration
2.4. Behavioral Tests
2.4.1. Open Field Test (OFT)
2.4.2. Elevated plus Maze (EPM)
2.4.3. Splash Test
2.4.4. Sucrose Preference Test (SPT)
2.4.5. Forced Swimming Test (FST)
2.4.6. Tail Suspension Test (TST)
2.4.7. Nest Building Test
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.7. Western-Blot Analysis
2.8. Immunofluorescence (IF)
2.9. Assays of Serum Biochemical Parameters
2.10. Statistical Analysis
3. Results
3.1. Preemptive Treatment with GLTs Reduces the Susceptibility to Anxiety-like Behaviors in MS Mice
3.2. Preemptive Treatment with GLTs Rescues Depression-like Behaviors in MS Mice
3.3. GLTs Reduces the Peripheral Inflammatory Response in MS Mice
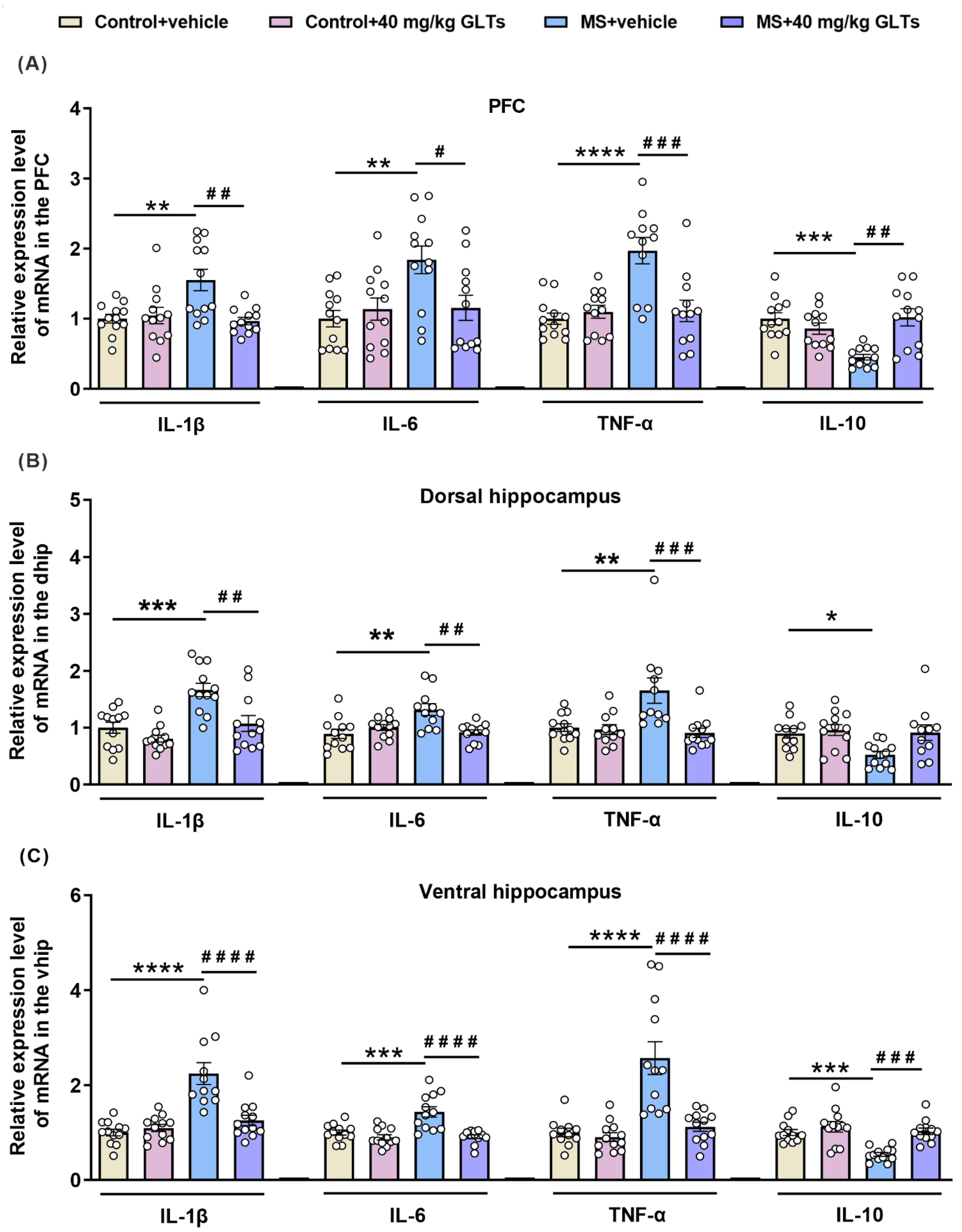
3.4. GLTs Balances the Expression of Pro-Inflammatory and Anti-Inflammatory Factors in the PFC and Hippocampus of MS Mice
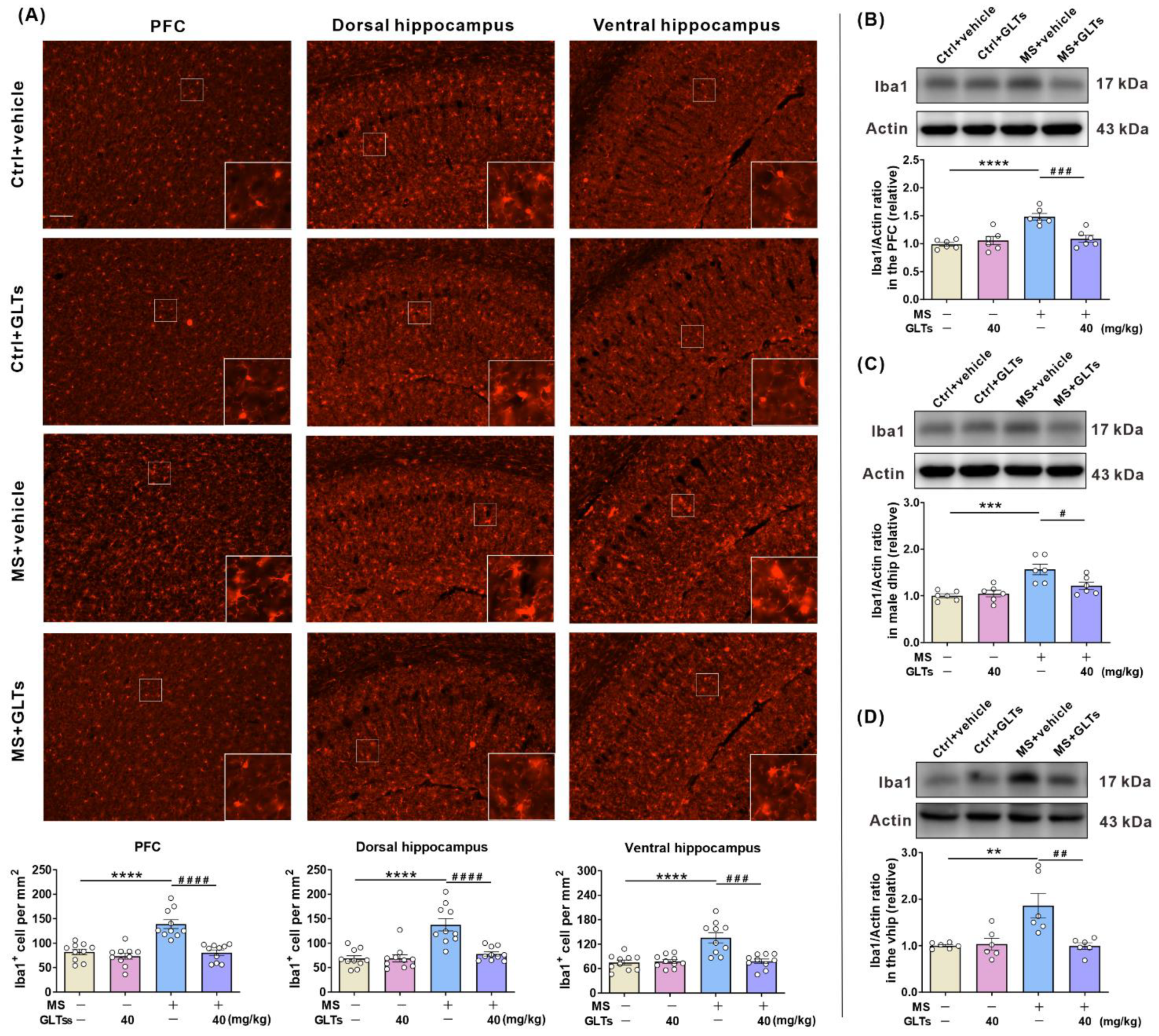
3.5. GLTs Inhibit the Maternal Separation-Induced Activation of the Microglia in the PFC and Hippocampus
3.6. GLTs Have No Adverse Effects on Body Weight and the Functions of Liver and Kidneys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.O.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Darcy, C.; Meng, X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: Systematic review, meta-analysis, and proportional attributable fractions. Psychol. Med. 2015, 46, 717–730. [Google Scholar] [CrossRef] [PubMed]
- LeMoult, J.; Humphreys, K.L.; Tracy, A.; Hoffmeister, J.-A.; Ip, E.; Gotlib, I.H. Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child. Adolesc. Psychiatry 2019, 59, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, L.; Petrelli, C.; Serretti, A. The role of specific early trauma in adult depression: A meta-analysis of published literature. Childhood trauma and adult depression. Eur. Psychiatry 2015, 30, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Infurna, M.R.; Reichl, C.; Parzer, P.; Schimmenti, A.; Bifulco, A.; Kaess, M. Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. J. Affect. Disord. 2016, 190, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010, 21, 848–856. [Google Scholar] [CrossRef]
- Taylor, S.E.; Lehman, B.; Kiefe, C.I.; Seeman, T.E. Relationship of early life stress and psychological functioning to adult c-reactive protein in the coronary artery risk development in young adults study. Biol. Psychiatry 2006, 60, 819–824. [Google Scholar] [CrossRef]
- Ahmad, F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Wu, G.-S.; Guo, J.-J.; Bao, J.-L.; Li, X.-W.; Chen, X.-P.; Lu, J.-J.; Wang, Y.-T. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—A review. Expert Opin. Investig. Drugs 2013, 22, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.-W.; Gao, Y.-Q.; Xia, B.; Wang, J.-Y.; Liu, X.-N.; Tang, J.-J.; Yin, X.; Gao, J.-M. Ganoderterpene A, a New Triterpenoid from Ganoderma lucidum, Attenuates LPS-induced inflammation and apoptosis via suppressing MAPK and TLR-4/NF-κB pathways in BV-2 cells. J. Agric. Food Chem. 2021, 69, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Han, F.; Luan, S.-S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.-X. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-L.; Lin, Y.-C.; Ni, H.; Mo, F.-E. Ganoderma triterpenoids exert antiatherogenic effects in mice by alleviating disturbed flow-induced oxidative stress and inflammation. Oxidative Med. Cell. Longev. 2018, 2018, 3491703. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.-L.; Guo, J.-B.; Liu, B.-Y.; Lu, J.-Q.; Chen, M.; Liu, B.; Bai, W.-D.; Rao, P.-F.; Ni, L.; Lv, X.-C. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Chang, Q.; Wong, L.K.; Chong, F.S.; Li, R.C. Triterpene antioxidants from ganoderma lucidum. Phytother Res 1999, 13, 529–531. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, J.; Liu, Y.; Guo, W.; Cao, H.; Xiao, J.; Wang, Y.; Liu, B. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2018, 271, 148–156. [Google Scholar] [CrossRef]
- Su, L.; Liu, L.; Jia, Y.; Lei, L.; Liu, J.; Zhu, S.; Zhou, H.; Chen, R.; Lu, H.A.J.; Yang, B. Ganoderma triterpenes retard renal cyst development by downregulating Ras/MAPK signaling and promoting cell differentiation. Kidney Int. 2017, 92, 1404–1418. [Google Scholar] [CrossRef]
- Shao, G.; He, J.; Meng, J.; Ma, A.; Geng, X.; Zhang, S.; Qiu, Z.; Lin, D.; Li, M.; Zhou, H.; et al. Ganoderic Acids Prevent Renal Ischemia Reperfusion Injury by Inhibiting Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 10229. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Huang, Y.; Jiang, Y.; Zou, L.; Liu, X.; Liu, S.; Chen, F.; Luo, J.; Zhu, Y. Ganoderma lucidum Triterpenoids (GLTs) reduce neuronal apoptosis via inhibition of ROCK signal pathway in APP/PS1 transgenic alzheimer’s disease mice. Oxidative Med. Cell. Longev. 2020, 2020, 9894037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yang, X.; Yang, X.; Xue, J.; Yang, Y. Ganoderic Acid A To Alleviate Neuroinflammation of alzheimer’s disease in mice by regulating the imbalance of the Th17/Tregs axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Ran, J.; Ye, Y.; An, Y.; Zhang, Y.; Huang, Z.; Lin, S.; Zhou, H.; Lin, D.; Wang, L.; et al. Ganoderic acid improves 5-fluorouracil-induced cognitive dysfunction in mice. Food Funct. 2021, 12, 12325–12337. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, L.; Wang, S.; Yang, L.; Li, P. Deacetyl ganoderic acid f inhibits LPS-induced neural inflammation via NF-κB pathway both in vitro and in vivo. Nutrients 2019, 12, 85. [Google Scholar] [CrossRef] [Green Version]
- Peña, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef] [Green Version]
- Hodes, G.; Pfau, M.L.; Purushothaman, I.; Ahn, H.F.; Golden, S.A.; Christoffel, D.J.; Magida, J.; Brancato, A.; Takahashi, A.; Flanigan, M.E.; et al. Sex differences in nucleus Accumbens transcriptome profiles associated with susceptibility versus resilience to Subchronic variable stress. J. Neurosci. 2015, 35, 16362–16376. [Google Scholar] [CrossRef]
- Peña, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Loh, Y.-H.E.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef] [Green Version]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Hu, W.; Cai, L.; Zeng, G.; Fang, W.; Dai, X.; Ye, Q.; Chen, X.; Zhang, J. Acetate supplementation produces antidepressant-like effect via enhanced histone acetylation. J. Affect. Disord. 2020, 281, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-J.; Li, Y.-D.; Wang, L.; Yang, S.-R.; Yuan, X.-S.; Wang, J.; Cherasse, Y.; Lazarus, M.; Chen, J.-F.; Qu, W.-M.; et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat. Commun. 2018, 9, 1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, D.; Amato, A.; Picone, P.; Terzo, S.; Galizzi, G.; Bonina, F.P.; Mulè, F.; Di Carlo, M. A Natural dietary supplement with a combination of nutrients prevents neurodegeneration induced by a high fat diet in mice. Nutrients 2018, 10, 1130. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, M.P.; Gunnar, M.R. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. NeuroImage 2019, 209, 116493. [Google Scholar] [CrossRef]
- Cao, P.; Chen, C.; Liu, A.; Shan, Q.; Zhu, X.; Jia, C.; Peng, X.; Zhang, M.; Farzinpour, Z.; Zhou, W.; et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 2021, 109, 2573–2589.e9. [Google Scholar] [CrossRef]
- Li, X.; Gao, T.-M. Epigenetic mechanism of depression after early life stress. Neurosci. Bull. 2022, 1. [Google Scholar] [CrossRef]
- Anda, R.F.; Felitti, V.J.; Bremner, J.D.; Walker, J.; Whitfield, C.L.; Perry, B.D.; Dube, S.R.; Giles, W.H. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 256, 174–186. [Google Scholar] [CrossRef]
- Leuner, B.; Glasper, E.R.; Gould, E. Parenting and plasticity. Trends Neurosci. 2010, 33, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Levine, J.L.S.; Avila-Quintero, V.; Bloch, M.; Kaffman, A. Systematic review and meta-analysis: Effects of maternal separation on anxiety-like behavior in rodents. Transl. Psychiatry 2020, 10, 174. [Google Scholar] [CrossRef]
- Enishi, M.; Ehorii-Hayashi, N.; Esasagawa, T. Effects of early life adverse experiences on the brain: Implications from maternal separation models in rodents. Front. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Nishi, M. Effects of early-life stress on the brain and behaviors: Implications of early maternal separation in rodents. Int. J. Mol. Sci. 2020, 21, 7212. [Google Scholar] [CrossRef] [PubMed]
- Seok, B.J.; Jeon, S.; Lee, J.; Cho, S.-J.; Lee, Y.J.; Kim, S.J. Effects of early trauma and recent stressors on depression, anxiety, and anger. Front. Psychiatry 2020, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; da Costa, B.L.S.A.; De Stasi, A.M.; Bacci, A.; Kawasawa, Y.I.; Vaidya, V.A.; Gaspar, P. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatry 2019, 25, 1159–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, R.L.; Portugal, C.; Summavielle, T.; Barbosa, F.; Magalhães, A. Maternal separation effects on mother rodents’ behaviour: A systematic review. Neurosci. Biobehav. Rev. 2019, 117, 98–109. [Google Scholar] [CrossRef]
- Reynolds, K.; Pietrzak, R.H.; El-Gabalawy, R.; Mackenzie, C.S.; Sareen, J. Prevalence of psychiatric disorders in U.S. older adults: Findings from a nationally representative survey. World Psychiatry 2015, 14, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C. Epidemiology of women and depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef]
- Goodwill, H.L.; Nieves, G.M.; Gallo, M.; Lee, H.I.S.; Oyerinde, E.; Serre, T.; Bath, K.G. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 2018, 44, 711–720. [Google Scholar] [CrossRef]
- Bath, K.G. Synthesizing views to understand sex differences in response to early life adversity. Trends Neurosci. 2020, 43, 300–310. [Google Scholar] [CrossRef]
- Gracia-Rubio, I.; Moscoso-Castro, M.; Pozo, O.J.; Marcos, J.; Nadal, R.; Valverde, O. Maternal separation induces neuroinflammation and long-lasting emotional alterations in mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2015, 65, 104–117. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Wang, Q.; Zhang, D.; Zhao, Q.; Zhang, J.; Xie, L.; Liu, G.; You, Z. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bonapersona, V.; Kentrop, J.; Van Lissa, C.; van der Veen, R.; Joëls, M.; Sarabdjitsingh, R. The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neurosci. Biobehav. Rev. 2019, 102, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, T.G.; Willoughby, M.; Moynihan, J.A.; Messing, S.; Sefair, A.V.; Carnahan, J.; Yin, X.; Caserta, M. Early childhood risk exposures and inflammation in early adolescence. Brain, Behav. Immun. 2019, 86, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.L.E.; Gawuga, C.; Tyrka, A.R.; Lee, J.K.; Anderson, G.M.; Price, L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010, 35, 2617–2623. [Google Scholar] [CrossRef] [Green Version]
- Réus, G.Z.; Fernandes, G.C.; de Moura, A.B.; Silva, R.H.; Darabas, A.C.; de Souza, T.G.; Abelaira, H.M.; Carneiro, C.; Wendhausen, D.; Michels, M.; et al. Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J. Psychiatr. Res. 2017, 95, 196–207. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia biology: One century of evolving concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [Green Version]
- Catale, C.; Bisicchia, E.; Carola, V.; Viscomi, M.T. Early life stress exposure worsens adult remote microglia activation, neuronal death, and functional recovery after focal brain injury. Brain, Behav. Immun. 2021, 94, 89–103. [Google Scholar] [CrossRef]
- Wang, R.; Wang, W.; Xu, J.; Liu, D.; Wu, H.; Qin, X.; Jiang, H.; Pan, F. Jmjd3 is involved in the susceptibility to depression induced by maternal separation via enhancing the neuroinflammation in the prefrontal cortex and hippocampus of male rats. Exp. Neurol. 2020, 328, 113254. [Google Scholar] [CrossRef]
- Liu, C.; Dunkin, D.; Lai, J.; Song, Y.; Ceballos, C.; Benkov, K.; Li, X.-M. Anti-inflammatory effects of ganoderma lucidum triterpenoid in human Crohnʼs disease associated with downregulation of NF-κB signaling. Inflamm. Bowel Dis. 2015, 21, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic acid a attenuates LPS-induced neuroinflammation in BV2 microglia by activating farnesoid x receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytotherapy Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Lian, C.L.; Hu, T.Y.; Wang, C.F.; Xu, Y.; Xiao, L.; Liu, Z.Q.; Qiu, S.Q.; Cheng, B.H. Two new farnesyl phenolic compounds with anti-inflammatory activities from Ganoderma duripora. Food Chem. 2018, 263, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Liu, J.Q.; Wan, L.S.; Li, X.N.; Yan, Y.X.; Qiu, M.H. Four new polycyclic meroterpenoids from Ganoderma cochlear. Org. Lett. 2014, 16, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Liu, J.Q.; Wang, C.F.; Li, X.Y.; Shu, Y.; Zhou, L.; Qiu, M.H. Hepatoprotective effects of triterpenoids from Ganoderma cochlear. J. Nat. Prod. 2014, 77, 737–743. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 471003" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ganoderic-acid-B (accessed on 24 May 2022).
- Yang, M.; Wang, X.; Guan, S.; Xia, J.; Sun, J.; Guo, H.; Guo, D.A. Analysis of triterpenoids in ganoderma lucidum using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 21632955, methyl ganoderate B" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/methyl-ganoderate-B (accessed on 24 May 2022).
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 20055990, CID 20055990" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/20055990 (accessed on 24 May 2022).
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 23247892, Lucidenic acid E" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lucidenic-acid-E. (accessed on 24 May 2022).
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 20055991, Ganoderic Acid J" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ganoderic-Acid-J. (accessed on 24 May 2022).
- Chen, L.X.; Chen, X.Q.; Wang, S.F.; Bian, Y.; Zhao, J.; Li, S.P. Analysis of triterpenoids in Ganoderma resinaceum using liquid chromatography coupled with electrospray ionization quadrupole - time - of - flight mass spectrometry. Int. J. Mass. Spectrom. 2019, 436, 42–51. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 14193982" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14193982 (accessed on 24 May 2022).
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 102004760, CID 102004760" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/102004760. (accessed on 24 May 2022).
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 72728372, CID 72728372" PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72728372. (accessed on 24 May 2022).




| Gene | Primer Sequences | |
|---|---|---|
| Actin | Forward | TGTCCACCTTCCAGCAGATGT |
| Reverse | AGCTCAGTAACAGTCCGCCTAG | |
| IL-1β | Forward | TCGCAGCAGCACATCAACAAGAG |
| Reverse | AGGTCCACGGGAAAGACACAGG | |
| IL-6 | Forward | CTCCCAACAGACCTGTCTATAC |
| Reverse | CCATTGCACAACTCTTTTCTCA | |
| TNF-α | Forward | ACTGGCAGAAGAGGCACTCC |
| Reverse | GCCACAAGCAGGAATGAGAA | |
| IL-10 | Forward | GCTCTTACTGACTGGCATGAG |
| Reverse | CGCAGCTCTAGGAGCATGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, X.; Zeng, G.-R.; Liu, J.-Q.; Luo, Z.-S.; Zhang, L.; Dai, X.-M.; Fang, W.-T.; Zhang, J.; Chen, X.-C. Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain. Nutrients 2022, 14, 2268. https://doi.org/10.3390/nu14112268
Mi X, Zeng G-R, Liu J-Q, Luo Z-S, Zhang L, Dai X-M, Fang W-T, Zhang J, Chen X-C. Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain. Nutrients. 2022; 14(11):2268. https://doi.org/10.3390/nu14112268
Chicago/Turabian StyleMi, Xue, Gui-Rong Zeng, Jie-Qing Liu, Zhou-Song Luo, Ling Zhang, Xiao-Man Dai, Wen-Ting Fang, Jing Zhang, and Xiao-Chun Chen. 2022. "Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain" Nutrients 14, no. 11: 2268. https://doi.org/10.3390/nu14112268
APA StyleMi, X., Zeng, G.-R., Liu, J.-Q., Luo, Z.-S., Zhang, L., Dai, X.-M., Fang, W.-T., Zhang, J., & Chen, X.-C. (2022). Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain. Nutrients, 14(11), 2268. https://doi.org/10.3390/nu14112268










