The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence
Abstract
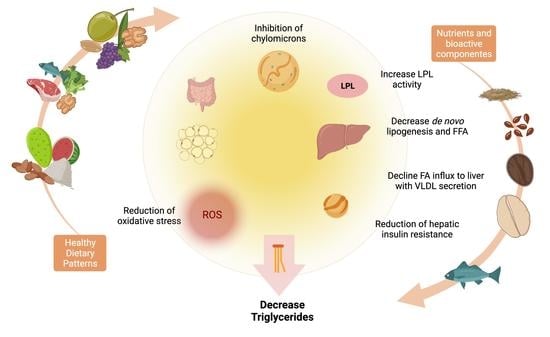
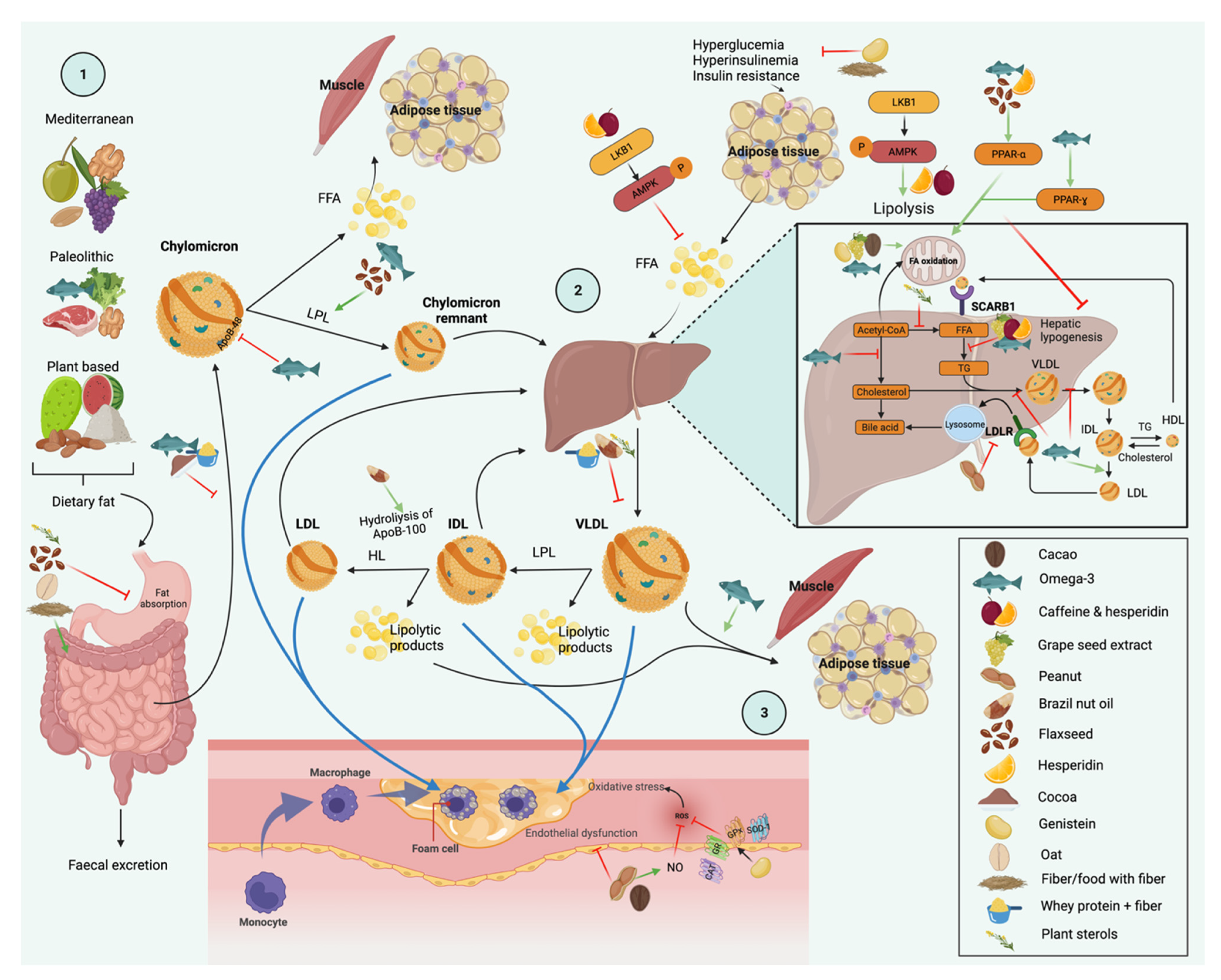
:1. Introduction
2. Materials and Methods
3. Dietary Interventions and Nutrient Compounds on the Improvements of Serum TG
3.1. Diet
3.2. Caloric Restriction
3.3. Fat
3.4. Carbohydrates
3.5. Protein
3.6. Food Combining and Nutritional Quality
3.7. Dietary Patterns
4. Foods
4.1. Nuts and Flaxseed
4.2. Legumes
4.3. Other Foods (Olive Oil, Low-Fat Milk, Spices, Fruits, and Green/Roasted Coffee Blend)
5. Nutrients and Bioactive Compounds
5.1. Omega-3
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main results on Triglyceride Levels |
|---|---|---|---|---|
| Chan, 2016 [99] | Omega-3 + standard care Standard care (control group) | Omega-3 4 g/day (46% EPA and 38% DHA in ethyl ester form) 8 weeks intervention-8 weeks washout-8 weeks intervention | Open-label, randomized, crossover intervention trial 20 subjects with FH (18–70 years) | ↓ TG with omega-3 + standard care 113–75 ± 12.25 mg/dL a 1.30 ± 0.14 mmol/L vs. 91.88 ± 7.88 (1.05 ± 0.09); p = 0.011 (−20%) compared with the control group. |
| Chan, 2016 [100] | Omega-3 + standard care Standard care (control group) | Omega-3 4 g/day (46% EPA and 38% DHA in ethyl ester form) 8 weeks intervention, 8 weeks washout, 8 weeks intervention | Open-label, randomized, crossover intervention trial 20 subjects with FH (18–70 years) | ↓ postprandial TG with omega-3 supplementation total AUCs (−19% b; p < 0.01) and incremental AUCs (−18%; p < 0.05). |
| Koh, 2016 [119] | Fenofibrate + omega-3 Fenofibrate Placebo | Fenofibrate 160 mg + 2 g omega-3 Fenofibrate 160 mg 2 months | Randomized, single-blind, placebo-controlled, parallel study 146 subjects with HTG (54 ± 1 y c) | ↓ TG placebo group (−7% b; p = 0.05), fenofibrate group (−30% b; p = 0.05) and fenofibrate plus omega-3 group (−41% b; p = 0.05). ↓ TG fenofibrate plus omega-3 group compared with placebo and fenofibrate (p = 0.001). |
| Mosca, 2016 [103] | Icosapent ethyl Placebo | 4 g/day 12 weeks | Data of two placebo-controlled, randomized, double-blind trials (MARINE and ANCHOR) 215 womens with elevated and high TG levels (49–73 years) | MARINE ↓ TG with icosapent ethyl 4 g/day −27% (39) d compared to placebo −8.6% (54) (−23%; p = 0.0327) ANCHOR ↓ TAG with icosapent ethyl 4 g/day −20% (25) d compared to placebo 4.5 (40) (−22%; p < 0.0001). |
| Tremblay, 2016 [98] | Omega-3 Corn and soybean oil (placebo group) | 5 g/day of fish oil (3 g/day of EPA (64%) and DHA (36%)) 5 g/day 8 weeks intervention, 12 weeks wash-out period, 8 weeks intervention | Double-blind, randomized, placebo-controlled, crossover study 10 mens with T2DM (54.7 ± 7.6 y c) | ↓ fasting TG omega-3 compared with the placebo (−9.7% b; p = 0.05). |
| Su, 2017 [89] | Omega-3 Omega-3 Olive oil (placebo group) | 2 g/day (920 mg EPA and 760 mg DHA) 4 g/day (1840 mg EPA and 1520 mg DHA) 8 weeks | Multicenter, randomized, double-blind, placebo-controlled, parallel study 251 subjects with HTG (20–79 years) | ↓ TG in the 4 g/day (−32.1%(−38.0–−25.6%)) e and 2 g/day (−29.7% (−35.4–−23.4%)) e groups was larger than in the placebo group (−5.4% (−14.8–5.1%)) e (p < 0.001). |
| Tóth, 2017 [90] | Omega-3 Omega-3 + CoQ10 Unaltered statin therapy (control group) | 2.52 g/day 2.52 g/day omega-3 + 200 mg/day CoQ10 3 months (±1 week) | Randomized double-blind trial with a statin treatment parallel group 105 subjects with dyslipidemia and elevated TG (57.24 ± 11.77 y c) | ↓ TG in omega-3 group to 143.5 ± 70.88 mg/dL (1.64 ± 0.81 mmol/L c; p = 0.004) and omega-3 + CoQ10 to 124.25 ± 56.88 (1.42 ± 0.65 c; p = 0.01) in comparison to the control group 221.37 ± 193.38 (2.53 ± 2.21) c |
| Wang, 2017 [97] | Omega-3 Corn oil (placebo group) | 4 g/day of fish oil (1.34 g EPA and 1.07 g DHA) 6 months | Randomized, double-blind, placebo-controlled trial 100 subjects with T2DM and abdominal obesity (65.4 ± 5.3 y c) | ↓ TG from baseline to 6 months in the omega-3 group 140 ± 80.5 mg/dL c (1.60 ± 0.92 mmol/L) to 151.38 ± 78.75 (1.73 ± 0.90); (p = 0.006). ↓ TG in the omega-3 group compared with the placebo group after 6 months (−21.25% b vs. 2.89% p = 0.007). |
| Zibaeenezhad, 2017 [91] | Omega-3 from fish oil Trout fish twice weekly | 2 g/day (180 mg EPA and 120 mg DHA) Mean of 250 g with 1.4 of omega-3 (280 mg EPA and 160 mg DHA) per 100 g 8 weeks | Open-labeled, randomized trial 93 subjects with hyperlipidemia | ↓ TG significantly in both groups, dietary-fish intake had a more pronounced effect than omega-3 supplementation (−30.75 b vs. −85.04; p = 0.003). |
| Brinton, 2018 [101] | Icosapent ethyl Placebo | 4 g/day 12 weeks | Phase 3, multicenter, placebo-controlled, randomized, double-blind clinical study 146 women with increased CVD risk, TG 200–499 mg/dL and type 2 diabetes (>18 years) | ↓ TG 4 g/day icosapent ethyl −17.4% (33.6) d compared to placebo 5.0 (40.5) (−21.5; p < 0.0001). |
| Kim, 2018 [87] | Rosuvastatin calcium + omega-3 Rosuvastatin + placebo | 4 capsules rosuvastatin calcium 5 mg and 1 g/day omega-3 (380 mg DHA + 460 mg EPA) plus 1 tablet of placebo of rosuvastatin 20 mg/day 20 mg/day of rosuvastatin and 4 capsules of placebo 8 weeks | Multicenter, randomized, double-blind, placebo-controlled study 201 subjects with residual HTG (19 to 80 years) | ↓ TG in the rosuvastatin + omega-3 group compared with the rosuvastatin + placebo group (−26.3% b vs. −11.4%; p < 0.001). |
| Stroes, 2018 [93] | Omega-3 Olive oil (placebo group) | 2 g/day 2 g/day 12 weeks | Randomized, multicenter, double-blind, parallel-group trial 156 subjects with severe HTG (≥18 years) | ↓ TG from baseline to the week 12 endpoint between 2 g omega-3 treatment when compared with the placebo group, −14.2% b (−26.2%, −2.8% d); p = 0.017. |
| Blom, 2019 [106] | Low-fat spread with plant sterols and (EPA) + (DHA) from fish oil Low-fat spread with sunflower oil and no added plant sterols (placebo group) | 2.0 g/day plant sterols and 1.0 g/day EPA + DHA 4 weeks | Randomized, double-blind, placebo-controlled, parallel group 259 subjects with elevated LDL cholesterol and borderline-high to high TG concentrations (18–75 years) | ↓ TG in the treatment group compared with the placebo group (− 16.0% b to − 4.9); p < 0.001. |
| Miller, 2019 [88] | Icosapent ethyl Icosapent ethyl Placebo | 2 g/day 4 g/day 12 weeks | Phase 3, multicenter, placebo-controlled, randomized, double-blind clinical study 246 statin-treated subjects at increased cardiovascular risk with TG 200–499 mg/dL (>18 years) | ↓ TG 4 g/day icosapent ethyl group –16.6% b (32.3) d compared to placebo group 5 (44.4) (−19.9%; p < 0.0001). |
| Sezai, 2019 [105] | Icosapent ethyl EPA + DHA | 1.8 g/day 0.93 g/day EPA + 0.75 g/day DHA 3 years | Single-blind study 87 cardiac surgery patients with HTG (≥20 to <90 years) | ↓ TG in both groups from baseline to the 6 months, 1, 2 and 3 years; p < 0.05 ↓ TG after 2 (p = 0.032) and 3 (p =0.005) years in the EPA + DHA group compared with the EPA group. |
| Vijayaraghavan, 2019 [102] | Icosapent ethyl Icosapent ethyl Placebo | 2 g/day 4 g/day 12 weeks | Phase 3, multicenter, placebo-controlled, randomized, double-blind, clinical study 83 subjects with TG 200–499 mg/dL and chronic kidney disease in stage 3 (>18 years) | ↓ TG 4 g/day icosapent ethyl group –17.1% b (43.2) d compared to placebo group 5.9 (36.0) d (−16.9%; p = 0.0074). |
| Zhou, 2019 [92] | EPA + DHA from fish oil EPA + DHA from fish oil ALA from flaxseed oil ALA from flaxseed oil Corn oil (placebo group) | 1.8 g/day 3.6 g/day 4.2 g/day 7.2 g/day 12 weeks | RCT, double-blind trial 123 subjects with moderate to high hypercholesterolemia (40–65 years) | ↓ TG in 1.8 g EPA + DHA group (−11.99% b; p = 0.008) and 3.6 g EPA + DHA (−15.78; p = 0.024). The changes in those two groups differed significantly compared with the placebo group; p < 0.05. |
| Jun, 2020 [118] | Atorvastatin calcium + omega-3 Atorvastatin + placebo (placebo group) | 4 capsules with atorvastatin calcium 5 mg and 1 g/day omega-3 plus 1 tablet of placebo of atorvastatin 20 mg/day 20 mg/day of atorvastatin and 4 capsules of placebo (olive oil 1000 mg) 8 weeks | Randomized, double-blind, placebo-controlled, parallel-group, and phase III multicenter study 200 subjects with mixed dyslipidemia (20–79 years) | ↓ TG in the omega-3 plus atorvastatin group after intervention (−29.8 ± 3.2 a; p < 0.001). ↓ TG in the omega-3 plus atorvastatin group than in the placebo group (−29.8 ± 3.2% a vs. 3.6 ± 3.7); p < 0.001. |
| Nicholls, 2020 [95] | Omega-3 Corn oil (placebo group) | 4 g/day (EPA + DHA) 4 g/day Median of 38.2 months | Double-blind, randomized, multicenter trial 13,078 subjects with high cardiovascular risk, HTG, and low levels of HDL cholesterol (62.5 years c) | ↓ TG in the omega-3 treatment group compared with the placebo group (−19.0% b vs. −0.9; p < 0 .001). |
| Saraswathi, 2020 [104] | Omega-3 Naproxen + Omeprazole Omega-3 + Naproxen + Omeprazole Standard nutrition counseling (control group) | 4 g/day (300 mg EPA and 200 mg of DHA per gram) 440 mg/day Naproxen + omeprazole 20 mg/day 4 g/day omega-3 (300 mg EPA and 200 mg of DHA per gram) + 440 mg/day naproxen + omeprazole 20 mg/day 12 weeks | Randomized prospective open-label study 34 obese dyslipidemic (39–58 years) | ↔ absolute fold change TG in omega-3 (−57 ± 24) a and omega-3 + naproxen-treated subjects (−63 ± 23) a compared with control group (−6 ± 26) a |
| Teramoto, 2020 [94] | Omega-3 Omega-3 | 2 g/day 4 g/day 8 weeks | Multicenter, open-label, randomized study 37 subjects with HTG (≥ 20 years) | ↓ fasting TG over the 8-week study period from baseline 194.4 ± 48.6 mg/dL c (2.2 ± 0.55 mmol/L) to 144.9 ± 43.1 (1.65 ± 0.49); p < 0.001 and mean 4 h postprandial TG 278.2 ± 70.5 mg/dL (3.17 ± 0.8 mmol/L) to 202.3 ± 78.3 (2.31 ± 0.89) only in the 4 g group (p < 0.001). |
| Lee, 2021 [96] | HMR Regular dietary pattern (control group) | Higher protein and fat content, lower carbohydrate content, and a lower omega-6/omega-3 ratio (<4) 4 weeks intervention, 2 weeks wash out period, 4 weeks intervention | Monocentric, controlled, randomized crossover trial 64 obese and cardiometabolically unhealthy subjects (≥ 40 years) | TG were reduced in the HMR group 134.1 ± 64.6 mg/dL c (1.53 ± 0.74 mmol/L) to 115.6 ± 48.4 (1.32 ± 0.55); p < 0.01. |
5.2. Flavonoids
5.3. Dietary Fiber
6. Molecular Nutrition
6.1. Genetic Variants
6.2. Gene Expression
7. Conclusions and Future Perspectives
- The execution of systematic reviews and meta-analyses for specific nutritional strategies with enough evidence to obtain precise recommendations for each one.
- To replicate the results of dietary intervention efficient to lower TG in several phenotypes.
- To design and perform RCT with the main objective of measuring the effectiveness in reducing TG with dietary interventions; many have measured it secondarily.
- Future RCT should be with a larger number of participants and long term (at least 6 months).
- New molecular nutrition studies should clarify the diet mechanism of action on HTG through gene level.
- Increase knowledge about food science focused on maintaining healthy TG.
- To encourage the evaluation of the effect of modifying eating behaviors (healthy and unhealthy) through several techniques to reduce TG.
- To conduct more studies where joint strategies with prior evidence are assessed to decrease TG, examples: diet + one or more supplements of bioactive compounds or functional food.
- The use of technology in new dietary interventions on HTG could improve adherence to treatment.
- A new review could describe in detail the impact of the consumption of ultra-processed products on TG. The guidelines recommend reducing them in general, but sometimes this is not feasible for the population. For this reason, a more precise recommendation is needed.
- We invite those interested in implementing new clinical trials to include postprandial TG measurements, in addition to fasting. Fasting TG have long been associated with CVD and other cardiometabolic conditions. Evidence suggests that non-fasting TG (i.e., measured within 8 h of eating) better predict CVD than fasting TG, which has led several organizations to recommend non-fasting lipid panels as the new clinical standard [159].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ALA | α-linolenic acid |
| CG | Control group |
| CoQ10 | Coenzyme Q10 |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FDA | Food and Drug Administration |
| FH | Familial hypercholesterolaemia |
| G/day | Grams per day |
| HTG | Hypertriglyceridemia |
| IG | Intervention group |
| kcal/day | Kcal per day |
| LF | Low fat |
| LPL | Lipoprotein lipase |
| MCFA | Medium chain fatty acids |
| mg/day | Milligrams per day |
| mmol/L | Millimoles per litter |
| MS | Metabolic syndrome |
| MUFA | Monounsaturated fatty acids |
| PPAR | Peroxisome proliferator-activated receptors |
| PPAR-α | Peroxisome proliferator-activated receptors alpha |
| PUFA | Polyunsaturated fatty acids |
| RCT | Randomized clinical trial |
| SFA | Saturated fatty acids |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| UFA | Unsaturated fatty acids |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| VLDL | Very low-density lipoprotein |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
References
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–22. ISBN 978-0-470-65071-4. [Google Scholar]
- Goldberg, I.J.; Eckel, R.H.; McPherson, R. Triglycerides and Heart Disease: Still a Hypothesis? ATVB 2011, 31, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Afonso, C.B.; Spickett, C.M. Lipoproteins as Targets and Markers of Lipoxidation. Redox Biol. 2019, 23, 101066. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Triacylglycerol-Rich Lipoproteins and the Generation of Small, Dense Low-Density Lipoprotein. Biochem. Soc. Trans. 2003, 31, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Rahmany, S.; Jialal, I. Biochemistry, Chylomicron. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical Review on Triglycerides. Eur. Heart J. 2020, 41, 99–109c. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simha, V. Management of Hypertriglyceridemia. BMJ 2020, 371, m3109. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G.; Laufs, U. The Diagnosis and Treatment of Hypertriglyceridemia. Dtsch. Arztebl. Int. 2019, 116, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braxas, H.; Rafraf, M.; Karimi Hasanabad, S.; Asghari Jafarabadi, M. Effectiveness of Genistein Supplementation on Metabolic Factors and Antioxidant Status in Postmenopausal Women With Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Huang, L.-S. The Insulin Resistance Syndrome: Impact on Lipoprotein Metabolism and Atherothrombosis. Eur. J. Cardiovasc. Risk 2000, 7, 325–331. [Google Scholar] [CrossRef]
- Dron, J.S.; Hegele, R.A. Genetics of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 455. [Google Scholar] [CrossRef]
- Pivonello, R.; Auriemma, R.S.; Grasso, L.F.S.; Pivonello, C.; Simeoli, C.; Patalano, R.; Galdiero, M.; Colao, A. Complications of Acromegaly: Cardiovascular, Respiratory and Metabolic Comorbidities. Pituitary 2017, 20, 46–62. [Google Scholar] [CrossRef]
- Rygiel, K. Hypertriglyceridemia—Common Causes, Prevention and Treatment Strategies. CCR 2018, 14, 67–76. [Google Scholar] [CrossRef]
- Santos-Baez, L.S.; Ginsberg, H.N. Hypertriglyceridemia—Causes, Significance, and Approaches to Therapy. Front. Endocrinol. 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Brunzell, J.D.; Goldberg, A.C.; Goldberg, I.J.; Sacks, F.; Murad, M.H.; Stalenhoef, A.F.H. Evaluation and Treatment of Hypertriglyceridemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2969–2989. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Adams-Huet, B.; Franklin, B.; Phillips, M.; Mitchell, J. The Effects of High-Protein and High-Monounsaturated Fat Meals on Postprandial Lipids, Lipoprotein Particle Numbers, Cytokines, and Leptin Responses in Overweight/Obese Subjects. Metab. Syndr. Relat. Disord. 2018, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Brindal, E.; James-Martin, G.; Noakes, M. Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes. Nutrients 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroofi, M.; Nasrollahzadeh, J. Effect of Intermittent versus Continuous Calorie Restriction on Body Weight and Cardiometabolic Risk Markers in Subjects with Overweight or Obesity and Mild-to-Moderate Hypertriglyceridemia: A Randomized Trial. Lipids Health Dis. 2020, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of Low-Carbohydrate Diets Versus Low-Fat Diets on Metabolic Risk Factors: A Meta-Analysis of Randomized Controlled Clinical Trials. Am. J. Epidemiol. 2012, 176, S44–S54. [Google Scholar] [CrossRef] [Green Version]
- Johansen, R.; Søndergaard, E.; Sørensen, L.; Jurik, A.; Nielsen, S. Measuring VLDL1-Triglyceride and VLDL2-Triglyceride Kinetics in Men: Effects of Dietary Control on Day-to-Day Variability. Horm. Metab. Res. 2017, 49, 604–611. [Google Scholar] [CrossRef]
- Blackburn, G. Effect of Degree of Weight Loss on Health Benefits. Obes. Res. 1995, 3, 211s–216s. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Moszak, M.; Klupczynska, A.; Kanikowska, A.; Kokot, Z.; Zawada, A.; Grzymisławska, M.; Grzymisławski, M. The Influence of a 3-Week Body Mass Reduction Program on the Metabolic Parameters and Free Amino Acid Profiles in Adult Polish People with Obesity. Adv. Clin. Exp. Med. 2018, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lin, X. Effects of Fasting-Mimicking Diet and Specific Meal Replacement Foods on Blood Glucose Control in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2020, 2020, 6615295. [Google Scholar] [CrossRef] [PubMed]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Benjak Horvat, I.; Šuša, L.; Rahelić, D.; Klobučar Majanović, S. Metabolic and Hepatic Effects of Energy-Reduced Anti-Inflammatory Diet in Younger Adults with Obesity. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6649142. [Google Scholar] [CrossRef]
- Petrisko, M.; Kloss, R.; Bradley, P.; Birrenkott, E.; Spindler, A.; Clayton, Z.S.; Kern, M. Biochemical, Anthropometric, and Physiological Responses to Carbohydrate-Restricted Diets Versus a Low-Fat Diet in Obese Adults: A Randomized Crossover Trial. J. Med. Food 2020, 23, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Veum, V.L.; Laupsa-Borge, J.; Eng, Ø.; Rostrup, E.; Larsen, T.H.; Nordrehaug, J.E.; Nygård, O.K.; Sagen, J.V.; Gudbrandsen, O.A.; Dankel, S.N.; et al. Visceral Adiposity and Metabolic Syndrome after Very High–Fat and Low-Fat Isocaloric Diets: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Le, T.; Flatt, S.W.; Natarajan, L.; Pakiz, B.; Quintana, E.L.; Heath, D.D.; Rana, B.K.; Rock, C.L. Effects of Diet Composition and Insulin Resistance Status on Plasma Lipid Levels in a Weight Loss Intervention in Women. JAHA 2016, 5, e002771. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Keogh, J.; Clifton, P. Differential Effects of Red Meat/Refined Grain Diet and Dairy/Chicken/Nuts/Whole Grain Diet on Glucose, Insulin and Triglyceride in a Randomized Crossover Study. Nutrients 2016, 8, 687. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Consumption of Red and Processed Meat and Refined Grains for 4 Weeks Decreases Insulin Sensitivity in Insulin-Resistant Adults: A Randomized Crossover Study. Metabolism 2017, 68, 173–183. [Google Scholar] [CrossRef]
- Maki, K.C.; Palacios, O.M.; Lindner, E.; Nieman, K.M.; Bell, M.; Sorce, J. Replacement of Refined Starches and Added Sugars with Egg Protein and Unsaturated Fats Increases Insulin Sensitivity and Lowers Triglycerides in Overweight or Obese Adults with Elevated Triglycerides. J. Nutr. 2017, 147, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Mateo-Gallego, R.; Marco-Benedí, V.; Perez-Calahorra, S.; Bea, A.M.; Baila-Rueda, L.; Lamiquiz-Moneo, I.; de Castro-Orós, I.; Cenarro, A.; Civeira, F. Energy-Restricted, High-Protein Diets More Effectively Impact Cardiometabolic Profile in Overweight and Obese Women than Lower-Protein Diets. Clin. Nutr. 2017, 36, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals With Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD Study: A Randomised Controlled Trial Using a Whole Food Plant-Based Diet in the Community for Obesity, Ischaemic Heart Disease or Diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef]
- Øyri, L.K.L.; Hansson, P.; Bogsrud, M.P.; Narverud, I.; Florholmen, G.; Leder, L.; Byfuglien, M.G.; Veierød, M.B.; Ulven, S.M.; Holven, K.B. Delayed Postprandial TAG Peak after Intake of SFA Compared with PUFA in Subjects with and without Familial Hypercholesterolaemia: A Randomised Controlled Trial. Br. J. Nutr. 2018, 119, 1142–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Murphy, K.J. A Mediterranean Diet Supplemented with Dairy Foods Improves Markers of Cardiovascular Risk: Results from the MedDairy Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 1166–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, K.J.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Jenkins, D.J.A.; Taylor, C.G.; Zahradka, P.; et al. Diets Enriched with Conventional or High-Oleic Acid Canola Oils Lower Atherogenic Lipids and Lipoproteins Compared to a Diet with a Western Fatty Acid Profile in Adults with Central Adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Bozzetto, L.; Costabile, G.; Cipriano, P.; Mangione, A.; Patti, L.; Riccardi, G.; Rivellese, A.A.; et al. Effects of a Diet Naturally Rich in Polyphenols on Lipid Composition of Postprandial Lipoproteins in High Cardiometabolic Risk Individuals: An Ancillary Analysis of a Randomized Controlled Trial. Eur. J. Clin. Nutr. 2020, 74, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The Beneficial Effects of Mediterranean Diet over Low-Fat Diet May Be Mediated by Decreasing Hepatic Fat Content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Kondo-Ando, M.; Seino, Y.; Morikawa, R.; Negi, K.; Todoroki, H.; Kawakami, T.; Asada, Y.; Yoshimoto, R.; Tanaka, C.; Okamoto, K.; et al. Low-Carbohydrate Diet by Staple Change Attenuates Postprandial GIP and CPR Levels in Type 2 Diabetes Patients. J. Diabetes Its Complicat. 2019, 33, 107415. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A Dietary Intervention with Functional Foods Reduces Metabolic Endotoxaemia and Attenuates Biochemical Abnormalities by Modifying Faecal Microbiota in People with Type 2 Diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Porter Starr, K.N.; Connelly, M.A.; Orenduff, M.C.; McDonald, S.R.; Sloane, R.; Huffman, K.M.; Kraus, W.E.; Bales, C.W. Impact on Cardiometabolic Risk of a Weight Loss Intervention with Higher Protein from Lean Red Meat: Combined Results of 2 Randomized Controlled Trials in Obese Middle-Aged and Older Adults. J. Clin. Lipidol. 2019, 13, 920–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, C.W.; Hauser, M.E.; Aronica, L.; Rigdon, J.; Gardner, C.D. Changes in Blood Lipid Concentrations Associated with Changes in Intake of Dietary Saturated Fat in the Context of a Healthy Low-Carbohydrate Weight-Loss Diet: A Secondary Analysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) Trial. Am. J. Clin. Nutr. 2019, 109, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Petersen, K.S.; Skulas-Ray, A.C.; Richter, C.K.; Proctor, D.N.; Kris-Etherton, P.M. Replacing Saturated Fat With Walnuts or Vegetable Oils Improves Central Blood Pressure and Serum Lipids in Adults at Risk for Cardiovascular Disease: A Randomized Controlled-Feeding Trial. JAHA 2019, 8, e011512. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Wong, L.H.; Tay, S.L.; Jacoby, J.J.; He, P.; Osman, F.; Ponnalagu, S.; Jiang, Y.R.; Lian, H.P.R.; Henry, C.J. Two Blends of Refined Rice Bran, Flaxseed, and Sesame Seed Oils Affect the Blood Lipid Profile of Chinese Adults with Borderline Hypercholesterolemia to a Similar Extent as Refined Olive Oil. J. Nutr. 2020, 150, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-A.; Shin, S.; Ha, K.; Hwang, Y.; Park, Y.-H.; Kang, M.-S.; Joung, H. Effect of a Balanced Korean Diet on Metabolic Risk Factors among Overweight/Obese Korean Adults: A Randomized Controlled Trial. Eur. J. Nutr. 2020, 59, 3023–3035. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Rai, S.K.; Lu, N.; Curhan, G.; Schwarzfuchs, D.; Shai, I.; Choi, H.K. Effects of Low-Fat, Mediterranean, or Low-Carbohydrate Weight Loss Diets on Serum Urate and Cardiometabolic Risk Factors: A Secondary Analysis of the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Care 2020, 43, 2812–2820. [Google Scholar] [CrossRef]
- Folwaczny, A.; Waldmann, E.; Altenhofer, J.; Henze, K.; Parhofer, K.G. Postprandial Lipid Metabolism in Normolipidemic Subjects and Patients with Mild to Moderate Hypertriglyceridemia: Effects of Test Meals Containing Saturated Fatty Acids, Mono-Unsaturated Fatty Acids, or Medium-Chain Fatty Acids. Nutrients 2021, 13, 1737. [Google Scholar] [CrossRef]
- Shah, M.; Franklin, B.; Adams-Huet, B.; Mitchell, J.; Bouza, B.; Dart, L.; Phillips, M. Effect of Meal Composition on Postprandial Glucagon-like Peptide-1, Insulin, Glucagon, C-Peptide, and Glucose Responses in Overweight/Obese Subjects. Eur. J. Nutr. 2017, 56, 1053–1062. [Google Scholar] [CrossRef]
- El Khoury, D.; Anderson, G.H. Recent Advances in Dietary Proteins and Lipid Metabolism. Curr. Opin. Lipidol. 2013, 24, 207–213. [Google Scholar] [CrossRef]
- Bladé, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic Effects of Proanthocyanidins and Their Underlying Biochemical and Molecular Mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef]
- Mårtensson, A.; Stomby, A.; Tellström, A.; Ryberg, M.; Waling, M.; Otten, J. Using a Paleo Ratio to Assess Adherence to Paleolithic Dietary Recommendations in a Randomized Controlled Trial of Individuals with Type 2 Diabetes. Nutrients 2021, 13, 969. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Jenkins, D.J.A.; et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018, 61, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Bitok, E.; Sabaté, J. Nuts and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 33–37. [Google Scholar] [CrossRef]
- Abbaspour, N.; Roberts, T.; Hooshmand, S.; Kern, M.; Hong, M.Y. Mixed Nut Consumption May Improve Cardiovascular Disease Risk Factors in Overweight and Obese Adults. Nutrients 2019, 11, 1488. [Google Scholar] [CrossRef] [Green Version]
- Ghanavati, M.; Alipour Parsa, S.; Nasrollahzadeh, J. A Calorie-restricted Diet with Nuts Favourably Raises Plasma High-density Lipoprotein-cholesterol in Overweight and Obese Patients with Stable Coronary Heart Disease: A Randomised Controlled Trial. Int. J. Clin. Pract. 2021, 75. [Google Scholar] [CrossRef]
- Julibert, A.; del Mar Bibiloni, M.; Gallardo-Alfaro, L.; Abbate, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Metabolic Syndrome Features and Excess Weight Were Inversely Associated with Nut Consumption after 1-Year Follow-Up in the PREDIMED-Plus Study. J. Nutr. 2020, 150, 3161–3170. [Google Scholar] [CrossRef]
- Coates, A.M.; Morgillo, S.; Yandell, C.; Scholey, A.; Buckley, J.D.; Dyer, K.A.; Hill, A.M. Effect of a 12-Week Almond-Enriched Diet on Biomarkers of Cognitive Performance, Mood, and Cardiometabolic Health in Older Overweight Adults. Nutrients 2020, 12, 1180. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hill, A.M.; West, S.G.; Gabauer, R.M.; McCrea, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Acute Peanut Consumption Alters Postprandial Lipids and Vascular Responses in Healthy Overweight or Obese Men. J. Nutr. 2017, 147, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Misra, A.; Pandey, R.M. Effect of Almond Supplementation on Glycemia and Cardiovascular Risk Factors in Asian Indians in North India with Type 2 Diabetes Mellitus: A 24-Week Study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Kong, Y.; Hu, X.; Li, K.; Guo, X.; Liu, C.; Han, L.; Li, D. Defatted Flaxseed Flour Improves Weight Loss and Lipid Profile in Overweight and Obese Adults: A Randomized Controlled Trial. Food Funct. 2020, 11, 8237–8247. [Google Scholar] [CrossRef] [PubMed]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the Effects of Flaxseed Oil and Sunflower Seed Oil Consumption on Serum Glucose, Lipid Profile, Blood Pressure, and Lipid Peroxidation in Patients with Metabolic Syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yari, Z.; Cheraghpour, M.; Hekmatdoost, A. Flaxseed and/or Hesperidin Supplementation in Metabolic Syndrome: An Open-Labeled Randomized Controlled Trial. Eur. J. Nutr. 2021, 60, 287–298. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Farhadi, P.; Attar, A.; Mosleh, A.; Amirmoezi, F.; Azimi, A. Effects of Walnut Oil on Lipid Profiles in Hyperlipidemic Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutr. Diabetes 2017, 7, e259. [Google Scholar] [CrossRef]
- Costa e Silva, L.M.; Pereira de Melo, M.L.; Faro Reis, F.V.; Monteiro, M.C.; dos Santos, S.M.; Quadros Gomes, B.A.; Meller da Silva, L.H. Comparison of the Effects of Brazil Nut Oil and Soybean Oil on the Cardiometabolic Parameters of Patients with Metabolic Syndrome: A Randomized Trial. Nutrients 2019, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Zibaeenezhad, M.J.; Ostovan, P.; Mosavat, S.H.; Zamirian, M.; Attar, A. Almond Oil for Patients with Hyperlipidemia: A Randomized Open-Label Controlled Clinical Trial. Complementary Ther. Med. 2019, 42, 33–36. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Mah, E.; Schulz, J.A.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew Consumption Reduces Total and LDL Cholesterol: A Randomized, Crossover, Controlled-Feeding Trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Tor-Roca, A.; Garcia-Aloy, M.; Mattivi, F.; Llorach, R.; Andres-Lacueva, C.; Urpi-Sarda, M. Phytochemicals in Legumes: A Qualitative Reviewed Analysis. J. Agric. Food Chem. 2020, 68, 13486–13496. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerra-Tomás, N.; Papandreou, C.; Salas-Salvadó, J. Legume Consumption and Cardiometabolic Health. Adv. Nutr. 2019, 10, S437–S450. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, R.; Ji, Y.-C.; Hao, J.-Y.; Ma, W.-W.; Chen, X.-D.; Xiao, R.; Yu, H.-L. Soy Milk Powder Supplemented with Phytosterol Esters Reduced Serum Cholesterol Level in Hypercholesterolemia Independently of Lipoprotein E Genotype: A Random Clinical Placebo-Controlled Trial. Nutr. Res. 2016, 36, 879–884. [Google Scholar] [CrossRef]
- Oliveira Godoy Ilha, A.; Sutti Nunes, V.; Silva Afonso, M.; Regina Nakandakare, E.; da Silva Ferreira, G.; de Paula Assis Bombo, R.; Rodrigues Giorgi, R.; Marcondes Machado, R.; Carlos Rocha Quintão, E.; Lottenberg, A.M. Phytosterols Supplementation Reduces Endothelin-1 Plasma Concentration in Moderately Hypercholesterolemic Individuals Independently of Their Cholesterol-Lowering Properties. Nutrients 2020, 12, 1507. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-M.; Chiu, H.-F.; Chen, Y.-M.; Shen, Y.-C.; Han, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Effect of a Balanced Nutrition Meal Replacement Diet with Altered Macromolecular Composition along with Caloric Restriction on Body Weight Control. Food Funct. 2019, 10, 3581–3588. [Google Scholar] [CrossRef]
- Haddad Tabrizi, S.; Haddad, E.; Rajaram, S.; Oda, K.; Kaur, A.; Sabaté, J. The Effect of Soybean Lunasin on Cardiometabolic Risk Factors: A Randomized Clinical Trial. J. Diet. Suppl. 2020, 17, 286–299. [Google Scholar] [CrossRef]
- Rodrigues, A.P.D.S.; Rosa, L.P.D.S.; Noll, M.; Silveira, E.A. Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients 2020, 12, 1413. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; Valls, R.-M.; Castañer, O.; Rubió, L.; Catalán, Ú.; Pedret, A.; Macià, A.; Sampson, M.L.; Covas, M.-I.; Fitó, M.; et al. Polyphenol Rich Olive Oils Improve Lipoprotein Particle Atherogenic Ratios and Subclasses Profile: A Randomized, Crossover, Controlled Trial. Mol. Nutr. Food Res. 2016, 60, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Seo, J.A.; Yoon, T.; Seo, I.; Lee, J.H.; Im, D.; Lee, J.H.; Bahn, K.-N.; Ham, H.S.; Jeong, S.A.; et al. Effects of Low-Fat Milk Consumption on Metabolic and Atherogenic Biomarkers in Korean Adults with the Metabolic Syndrome: A Randomised Controlled Trial. J. Hum. Nutr. Diet 2016, 29, 477–486. [Google Scholar] [CrossRef]
- Petersen, K.S.; Rogers, C.J.; West, S.G.; Proctor, D.N.; Kris-Etherton, P.M. The Effect of Culinary Doses of Spices in a High-Saturated Fat, High-Carbohydrate Meal on Postprandial Lipemia and Endothelial Function: A Randomized, Controlled, Crossover Pilot Trial. Food Funct. 2020, 11, 3191–3200. [Google Scholar] [CrossRef]
- Suganya, S.; Natarajan, S.; Chamundeeswari, D.; Anbarasu, A.; Balasubramanian, K.A.; Schneider, L.C.; Nandagopal, B. Clinical Evaluation of a Polyherbal Nutritional Supplement in Dyslipidemic Volunteers. J. Diet. Suppl. 2017, 14, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two Apples a Day Lower Serum Cholesterol and Improve Cardiometabolic Biomarkers in Mildly Hypercholesterolemic Adults: A Randomized, Controlled, Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; de Toro-Martín, J.; Garneau, V.; Guay, V.; Kearney, M.; Pilon, G.; Roy, D.; Couture, P.; Couillard, C.; Marette, A.; et al. Effects of Daily Raspberry Consumption on Immune-Metabolic Health in Subjects at Risk of Metabolic Syndrome: A Randomized Controlled Trial. Nutrients 2020, 12, 3858. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly Consuming a Green/Roasted Coffee Blend Reduces the Risk of Metabolic Syndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Han, K.A.; Yu, J.; Lee, S.H.; Jeon, H.K.; Kim, S.H.; Kim, S.Y.; Han, K.H.; Won, K.; Kim, D.-B.; et al. Efficacy and Safety of Adding Omega-3 Fatty Acids in Statin-Treated Patients with Residual Hypertriglyceridemia: ROMANTIC (Rosuvastatin-OMAcor iN Residual HyperTrIglyCeridemia), a Randomized, Double-Blind, and Placebo-Controlled Trial. Clin. Ther. 2018, 40, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Ballantyne, C.M.; Bays, H.E.; Granowitz, C.; Doyle, R.T.; Juliano, R.A.; Philip, S. Effects of Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester) on Atherogenic Lipid/Lipoprotein, Apolipoprotein, and Inflammatory Parameters in Patients With Elevated High-Sensitivity C-Reactive Protein (from the ANCHOR Study). Am. J. Cardiol. 2019, 124, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Su, T.-C.; Hwang, J.-J.; Huang, K.-C.; Chiang, F.-T.; Chien, K.-L.; Wang, K.-Y.; Charng, M.-J.; Tsai, W.-C.; Lin, L.-Y.; Vige, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Assess the Efficacy and Safety of Ethyl-Ester Omega-3 Fatty Acid in Taiwanese Hypertriglyceridemic Patients. J. Atheroscler. Thromb. 2017, 24, 275–289. [Google Scholar] [CrossRef] [Green Version]
- Tóth, Š.; Šajty, M.; Pekárová, T.; Mughees, A.; Štefanič, P.; Katz, M.; Spišáková, K.; Pella, J.; Pella, D. Addition of Omega-3 Fatty Acid and Coenzyme Q10 to Statin Therapy in Patients with Combined Dyslipidemia. J. Basic Clin. Physiol. Pharmacol. 2017, 28. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Ghavipisheh, M.; Attar, A.; Aslani, A. Comparison of the Effect of Omega-3 Supplements and Fresh Fish on Lipid Profile: A Randomized, Open-Labeled Trial. Nutr. Diabetes 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, Z.; Wang, P.; Zhang, B.; Chen, C.; Zhang, C.; Su, Y. EPA + DHA, but Not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2019, 63, 1801157. [Google Scholar] [CrossRef]
- Stroes, E.S.G.; Susekov, A.V.; de Bruin, T.W.A.; Kvarnström, M.; Yang, H.; Davidson, M.H. Omega-3 Carboxylic Acids in Patients with Severe Hypertriglyceridemia: EVOLVE II, a Randomized, Placebo-Controlled Trial. J. Clin. Lipidol. 2018, 12, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, T.; Shibata, H.; Suzaki, Y.; Matsui, S.; Uemura, N.; Tomiyama, H.; Yamashina, A. Discrepancy Between Fasting Flow-Mediated Dilation and Parameter of Lipids in Blood: A Randomized Exploratory Study of the Effect of Omega-3 Fatty Acid Ethyl Esters on Vascular Endothelial Function in Patients with Hyperlipidemia. Adv. Ther. 2020, 37, 2169–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, Y.; Hyun, T.; Song, S.; Yang, W.; Kim, Y.-S.; You, H.-S.; Chang, Y.-C.; Shin, S.-H.; Kang, H.-T. Beneficial Effects of a Specially Designed Home Meal Replacement on Cardiometabolic Parameters in Individuals with Obesity: Preliminary Results of a Randomized Controlled Clinical Trial. Nutrients 2021, 13, 2171. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhu, Y.; Liu, X.; Xia, H.; Yang, X.; Sun, G. Treatment for 6 Months with Fish Oil-Derived n-3 Polyunsaturated Fatty Acids Has Neutral Effects on Glycemic Control but Improves Dyslipidemia in Type 2 Diabetic Patients with Abdominal Obesity: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Nutr. 2017, 56, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.J.; Lamarche, B.; Hogue, J.-C.; Couture, P. N-3 Polyunsaturated Fatty Acid Supplementation Has No Effect on Postprandial Triglyceride-Rich Lipoprotein Kinetics in Men with Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2909210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.C.; Pang, J.; Barrett, P.H.R.; Sullivan, D.R.; Mori, T.A.; Burnett, J.R.; van Bockxmeer, F.M.; Watts, G.F. Effect of Omega-3 Fatty Acid Supplementation on Arterial Elasticity in Patients with Familial Hypercholesterolaemia on Statin Therapy. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C.; Pang, J.; Barrett, P.H.R.; Sullivan, D.R.; Burnett, J.R.; van Bockxmeer, F.M.; Watts, G.F. ω-3 Fatty Acid Ethyl Esters Diminish Postprandial Lipemia in Familial Hypercholesterolemia. J. Clin. Endocrinol. Metab. 2016, 101, 3732–3739. [Google Scholar] [CrossRef] [Green Version]
- Brinton, E.A.; Ballantyne, C.M.; Guyton, J.R.; Philip, S.; Doyle, R.T.; Juliano, R.A.; Mosca, L. Lipid Effects of Icosapent Ethyl in Women with Diabetes Mellitus and Persistent High Triglycerides on Statin Treatment: ANCHOR Trial Subanalysis. J. Women’s Health 2018, 27, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, K.; Szerlip, H.M.; Ballantyne, C.M.; Bays, H.E.; Philip, S.; Doyle, R.T.; Juliano, R.A.; Granowitz, C. Icosapent Ethyl Reduces Atherogenic Markers in High-Risk Statin-Treated Patients with Stage 3 Chronic Kidney Disease and High Triglycerides. Postgrad. Med. 2019, 131, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.; Ballantyne, C.M.; Bays, H.E.; Guyton, J.R.; Philip, S.; Doyle, R.T.; Juliano, R.A. Usefulness of Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester) in Women to Lower Triglyceride Levels (Results from the MARINE and ANCHOR Trials). Am. J. Cardiol. 2017, 119, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraswathi, V.; Heineman, R.; Alnouti, Y.; Shivaswamy, V.; Desouza, C.V. A Combination of Omega-3 PUFAs and COX Inhibitors: A Novel Strategy to Manage Obesity-Linked Dyslipidemia and Adipose Tissue Inflammation. J. Diabetes Its Complicat. 2020, 34, 107494. [Google Scholar] [CrossRef] [PubMed]
- Sezai, A.; Unosawa, S.; Taoka, M.; Osaka, S.; Obata, K.; Kanno, S.; Sekino, H.; Tanaka, M. Long-Term Comparison of Ethyl Icosapentate vs. Omega-3-Acid Ethyl in Patients With Cardiovascular Disease and Hypertriglyceridemia (DEFAT Trial). Circ. J. 2019, 83, 1368–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, W.A.M.; Koppenol, W.P.; Hiemstra, H.; Stojakovic, T.; Scharnagl, H.; Trautwein, E.A. A Low-Fat Spread with Added Plant Sterols and Fish Omega-3 Fatty Acids Lowers Serum Triglyceride and LDL-Cholesterol Concentrations in Individuals with Modest Hypercholesterolaemia and Hypertriglyceridaemia. Eur. J. Nutr. 2019, 58, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; She, Y.; Kaur, R.; Guo, N.; Zhang, X.; Zhang, R.; Gou, X. Is Plant Sterols a Good Strategy to Lower Cholesterol? J. Oleo Sci. 2019, 68, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Ho, S.; Gahler, R.; Wood, S. Effect on Insulin, Glucose and Lipids in Overweight/Obese Australian Adults of 12 Months Consumption of Two Different Fibre Supplements in a Randomised Trial. Nutrients 2017, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Soto, A.; Chavez-Santoscoy, R.A.; Lara-Jacobo, L.R.; Chavez-Santoscoy, A.V.; Gonzalez-Cobian, L.N. Daily Consumption of Chocolate Rich in Flavonoids Decreases Cellular Genotoxicity and Improves Biochemical Parameters of Lipid and Glucose Metabolism. Molecules 2018, 23, 2220. [Google Scholar]
- Gutiérrez-Salmeán, G.; Meaney, E.; Lanaspa, M.A.; Cicerchi, C.; Johnson, R.J.; Dugar, S.; Taub, P.; Ramírez-Sánchez, I.; Villarreal, F.; Schreiner, G.; et al. A Randomized, Placebo-Controlled, Double-Blind Study on the Effects of (−)-Epicatechin on the Triglyceride/HDLc Ratio and Cardiometabolic Profile of Subjects with Hypertriglyceridemia: Unique in Vitro Effects. Int. J. Cardiol. 2016, 223, 500–506. [Google Scholar] [CrossRef]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A Nutritive Dose of Pure (−)-Epicatechin Does Not Beneficially Affect Increased Cardiometabolic Risk Factors in Overweight-to-Obese Adults—A Randomized, Placebo-Controlled, Double-Blind Crossover Study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef]
- Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Oral Intake of a Combination of Glucosyl Hesperidin and Caffeine Elicits an Anti-Obesity Effect in Healthy, Moderately Obese Subjects: A Randomized Double-Blind Placebo-Controlled Trial. Nutr. J. 2015, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, R.; Parandoosh, M.; Khorsandi, H.; Hosseinzadeh, N.; Madani Tonekaboni, M.; Saidpour, A.; Babaei, H.; Ghorbani, A. Grape Seed Extract Supplementation along with a Restricted-calorie Diet Improves Cardiovascular Risk Factors in Obese or Overweight Adult Individuals: A Randomized, Placebo-controlled Trial. Phytother. Res. 2021, 35, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Fatmah Effects of High-Fiber Biscuits on Lipid and Anthropometric Profile of Patients with Type 2 Diabetes. J. Nutr. Sci. Vitaminol. 2020, 66, S391–S397. [CrossRef] [PubMed]
- Rakvaag, E.; Fuglsang-Nielsen, R.; Bach Knudsen, K.E.; Landberg, R.; Johannesson Hjelholt, A.; Søndergaard, E.; Hermansen, K.; Gregersen, S. Whey Protein Combined with Low Dietary Fiber Improves Lipid Profile in Subjects with Abdominal Obesity: A Randomized, Controlled Trial. Nutrients 2019, 11, 2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, P.M.; Hegele, R.A. Functional Foods and Dietary Supplements for the Management of Dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Jeong, I.-K.; Yu, J.M.; Kim, S.R.; Lee, I.K.; Han, K.-A.; Choi, S.H.; Kim, S.-K.; Park, H.K.; Mok, J.-O.; et al. Efficacy and Safety of Omega-3 Fatty Acids in Patients Treated with Statins for Residual Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Metab. J. 2020, 44, 78. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Oh, P.C.; Sakuma, I.; Lee, Y.; Han, S.H.; Shin, E.K. Vascular and Metabolic Effects of Omega-3 Fatty Acids Combined with Fenofibrate in Patients with Hypertriglyceridemia. Int. J. Cardiol. 2016, 221, 342–346. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef]
- Tariq, S.; Imran, M.; Mushtaq, Z.; Asghar, N. Phytopreventive Antihypercholesterolmic and Antilipidemic Perspectives of Zedoary (Curcuma Zedoaria Roscoe.) Herbal Tea. Lipids Health Dis. 2016, 15, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.; Helfrich, H.-P.; Ellinger, S. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rynarzewski, J.; Dicks, L.; Zimmermann, B.; Stoffel-Wagner, B.; Ludwig, N.; Helfrich, H.-P.; Ellinger, S. Impact of a Usual Serving Size of Flavanol-Rich Cocoa Powder Ingested with a Diabetic-Suitable Meal on Postprandial Cardiometabolic Parameters in Type 2 Diabetics—A Randomized, Placebo-Controlled, Double-Blind Crossover Study. Nutrients 2019, 11, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The Effect of Hesperidin Supplementation on Metabolic Profiles in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- Vetrani, C.; Costabile, G.; Luongo, D.; Naviglio, D.; Rivellese, A.A.; Riccardi, G.; Giacco, R. Effects of Whole-Grain Cereal Foods on Plasma Short Chain Fatty Acid Concentrations in Individuals with the Metabolic Syndrome. Nutrition 2016, 32, 217–221. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A. A Multifunctional Bread Rich in Beta Glucans and Low in Starch Improves Metabolic Control in Type 2 Diabetes: A Controlled Trial. Nutrients 2017, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Dodevska, M.S.; Sobajic, S.S.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S.; Spasojevic-Kalimanovska, V.V.; Djordjevic, B.I. Effects of Total Fibre or Resistant Starch-Rich Diets within Lifestyle Intervention in Obese Prediabetic Adults. Eur. J. Nutr. 2016, 55, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Babiker, R.; Elmusharaf, K.; Keogh, M.B.; Saeed, A.M. Effect of Gum Arabic (Acacia Senegal) Supplementation on Visceral Adiposity Index (VAI) and Blood Pressure in Patients with Type 2 Diabetes Mellitus as Indicators of Cardiovascular Disease (CVD): A Randomized and Placebo-Controlled Clinical Trial. Lipids Health Dis. 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Anggadiredja, K.; Ufamy, N.; Amalia, L.; Fisheri Kurniati, N.; Bahana Maulida Reyaan, I. Ameliorating Effects of Four-Week Fiber-Multivitamin Combination Treatment on Low-Density Lipoprotein Cholesterol, Total Cholesterol, and Apolipoprotein B Profiles in Hypercholesterolemic Participants. J. Diet. Suppl. 2020, 17, 173–183. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Raimondi de Souza, S.; Moraes de Oliveira, G.M.; Raggio Luiz, R.; Rosa, G. Effects of Oat Bran and Nutrition Counseling on the Lipid and Glucose Profile and Anthropometric Parameters of Hypercholesterolemia Patients. Nutr. Hosp. 2016, 33, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of Soluble Fiber from Oats on Lipid Levels of Asian Indians—A Randomized Controlled, Parallel Arm Study. Lipids Health Dis. 2017, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and InTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef] [Green Version]
- Schioldan, A.G.; Gregersen, S.; Hald, S.; Bjørnshave, A.; Bohl, M.; Hartmann, B.; Holst, J.J.; Stødkilde-Jørgensen, H.; Hermansen, K. Effects of a Diet Rich in Arabinoxylan and Resistant Starch Compared with a Diet Rich in Refined Carbohydrates on Postprandial Metabolism and Features of the Metabolic Syndrome. Eur. J. Nutr. 2018, 57, 795–807. [Google Scholar] [CrossRef]
- de Luis, D.A.; Aller, R.; Izaola, O.; Romero, E. Effect of -55CT Polymorphism of UCP3 on Insulin Resistance and Cardiovascular Risk Factors after a High Protein/Low Carbohydrate versus a Standard Hypocaloric Diet. Ann. Nutr. Metab. 2016, 68, 157–163. [Google Scholar] [CrossRef]
- de Luis, D.A.; Izaola, O.; Primo, D.; Aller, R. Polymorphism Rs16147 of the Neuropeptide Y Gene Modifies the Response of Cardiovascular Risk Biomarkers and Adipokines to Two Hypocaloric Diets. J. Nutrigenet. Nutr. 2017, 10, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dergunov, A.D. Role of ApoE in Conformation-Prone Diseases and Atherosclerosis. Biochemistry 2006, 71, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.; Walker, C.; Jebb, S.; Moore, C.; Frost, G.; Goff, L.; Sanders, T.; Lewis, F.; Griffin, M.; Gitau, R.; et al. APOE4 Genotype Exerts Greater Benefit in Lowering Plasma Cholesterol and Apolipoprotein B than Wild Type (E3/E3), after Replacement of Dietary Saturated Fats with Low Glycaemic Index Carbohydrates. Nutrients 2018, 10, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luis, D.A.; Izaola, O.; Primo, D.; Aller, R. Dietary-Fat Effect of the Rs10830963 Polymorphism in MTNR1B on Insulin Resistance in Response to 3 Months Weight-Loss Diets. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2020, 67, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Primo, D.; Izaola, O.; de Luis, D.A. Common Polymorphism in the Cannabinoid Receptor Gene Type 2 (CB2R) Rs3123554 Are Associated with Metabolic Changes after Two Different Hypocaloric Diets with Different Dietary Fatty Profiles. Clin. Nutr. 2019, 38, 2632–2638. [Google Scholar] [CrossRef]
- Primo, D.; Izaola, O.; de Luis, D. Efectos de una dieta hipocalórica rica en proteína/baja de hidratos de carbono vs. una estándar sobre los parámetros antropométricos y factores de riesgo cardiovascular, papel del polimorfismo rs3123554 del gen del receptor canabinoide tipo 2 (CB2R). Endocrinol. Diabetes Nutr. 2020, 67, 446–453. [Google Scholar] [CrossRef]
- de Luis, D.A.; Romero, E.; Izaola, O.; Primo, D.; Aller, R. Cardiovascular Risk Factors and Insulin Resistance after Two Hypocaloric Diets with Different Fat Distribution in Obese Subjects: Effect of the Rs10767664 Gene Variant in Brain-Derived Neurotrophic Factor. J. Nutrigenet. Nutr. 2017, 10, 163–171. [Google Scholar] [CrossRef]
- de Luis, D.A.; Fernández Ovalle, H.; Izaola, O.; Primo, D.; Aller, R. RS 10767664 Gene Variant in Brain Derived Neurotrophic Factor (BDNF) Affect Metabolic Changes and Insulin Resistance after a Standard Hypocaloric Diet. J. Diabetes Complicat. 2018, 32, 216–220. [Google Scholar] [CrossRef]
- Merino, J.; Jablonski, K.A.; Mercader, J.M.; Kahn, S.E.; Chen, L.; Harden, M.; Delahanty, L.M.; Araneta, M.R.G.; Walford, G.A.; Jacobs, S.B.R.; et al. Interaction Between Type 2 Diabetes Prevention Strategies and Genetic Determinants of Coronary Artery Disease on Cardiometabolic Risk Factors. Diabetes 2020, 69, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Corella, D.; Asensio, E.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK Gene Variation Is Associated with Incidence of Type-2 Diabetes and Cardiovascular Diseases in Type-2 Diabetic Subjects: Dietary Modulation in the PREDIMED Randomized Trial. Cardiovasc. Diabetol. 2016, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Arpón, A.; Milagro, F.; Razquin, C.; Corella, D.; Estruch, R.; Fitó, M.; Marti, A.; Martínez-González, M.; Ros, E.; Salas-Salvadó, J.; et al. Impact of Consuming Extra-Virgin Olive Oil or Nuts within a Mediterranean Diet on DNA Methylation in Peripheral White Blood Cells within the PREDIMED-Navarra Randomized Controlled Trial: A Role for Dietary Lipids. Nutrients 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sone, H.; Tanaka, S.; Tanaka, S.; Iimuro, S.; Oida, K.; Yamasaki, Y.; Oikawa, S.; Ishibashi, S.; Katayama, S.; Ohashi, Y.; et al. Serum Level of Triglycerides Is a Potent Risk Factor Comparable to LDL Cholesterol for Coronary Heart Disease in Japanese Patients with Type 2 Diabetes: Subanalysis of the Japan Diabetes Complications Study (JDCS). J. Clin. Endocrinol. Metab. 2011, 96, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, H.; Yi, R.; Tan, F.; Zhao, X. Anti-obesity Effect of Liupao Tea Extract by Modulating Lipid Metabolism and Oxidative Stress in High-fat-diet-induced Obese Mice. J. Food Sci. 2021, 86, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional Kefir Reduces Weight Gain and Improves Plasma and Liver Lipid Profiles More Successfully than a Commercial Equivalent in a Mouse Model of Obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Gao, J.; Ding, G.; Li, Q.; Gong, L.; Huang, J.; Sang, Y. Tibet Kefir Milk Decreases Fat Deposition by Regulating the Gut Microbiota and Gene Expression of Lpl and Angptl4 in High Fat Diet-Fed Rats. Food Res. Int. 2019, 121, 278–287. [Google Scholar] [CrossRef]
- Serisier, S.; Leray, V.; Poudroux, W.; Magot, T.; Ouguerram, K.; Nguyen, P. Effects of Green Tea on Insulin Sensitivity, Lipid Profile and Expression of PPARα and PPARγ and Their Target Genes in Obese Dogs. Br. J. Nutr. 2008, 99, 1208–1216. [Google Scholar] [CrossRef]
- Keirns, B.H.; Sciarrillo, C.M.; Koemel, N.A.; Emerson, S.R. Fasting, Non-Fasting and Postprandial Triglycerides for Screening Cardiometabolic Risk. J. Nutr. Sci. 2021, 10, e75. [Google Scholar] [CrossRef]
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main Results on Triglyceride Levels |
|---|---|---|---|---|
| Kim, 2016 [30] | 1. HMD 2. HWD | 1. White bread, leg ham, and orange juice 2. Whole grain bread, chicken, milk, yogurt, and nuts−5, 0, 10, 20, 30, 40, 50, 60, 75, 90, 120, 150, and 180 min | Randomized crossover study, 64 overweight and obese adults (43 average age) | ↓TG (iAUC)(−0.2, 0.5 mU/L/3 h for red meat/refined grain diet vs. 0.0, 0.4 mU/L/3 h for 12 of 17 dairy/chicken/nuts/wholegrain diet, p < 0.001) |
| Le, 2016 [29] | 1. LFHC 2. LCHF 3. WRHFLC. | 1. 20% fat, 65% carbohydrate, 15% protein 2. 35% fat, 45% carbohydrate, 20% protein 3. 35% fat, 45% carbohydrate, 20% protein 1 year | Unblinded clinical trial, 81 obese adults (mean age 43 years) | ↓ TG in all study arms at 6 months (p < 0.05) |
| Johansen, 2017 [20] | 1. Isocaloric diet 2. Regular diet | 1. 55% carbohydrate (added sugars were less than 10%), 15% protein, and 30% fat 2. Consume their regular diet 2 days | Randomized trial, 11 overweight and obese men (aged 35–65 years) | ↓ TG in diet group between the 2 study days 106.29 mg/dL (35.43–239.15) a 1.2 mmol/L (0.4–2.7) vs. 97.43 mg/dL (35.43–230) a 1.1 mmol/L (0.4–2.6) |
| Kim, 2017 [31] | 1. HMD 2. HWD | 1. Red meat, processed meat, refined grain diet, potato, indulgence food, vegetables, and fruits 2. Dairy, chicken, fish, legumes, nuts, whole grain, vegetables, and fruits 4 weeks per period | Randomized crossover study, 64 overweight and obese adults (43 average age) | ↑ TG concentrations TG (p < 0.05) after HMD than after HWD |
| Maki, 2017 [32] | 1. Combination of egg protein and UFA 2. Refined starches and added sugars | 1. 42% carbohydrate, 23% protein and 35% fat 2. 58% carbohydrate, 15% protein and 27% fat >3 servings/d of study test foods (includes muffins, waffles, yogurt, cookies) 3 weeks intervention; 2 weeks washout period; 3 weeks intervention | Randomized, double-blind, controlled-feeding, crossover trial 25 subjects overweight or obese with HTG (21–70 years) | ↓ TG in the egg protein and UFA intervention than during the refined-carbohydrate condition (−18.5% (−35.7, −6.9) c vs. −2.5 (−13.4,17.0) c; p < 0.002) |
| Mateo-Gallego, 2017 [33] | 1. Calorie-reduced diet 1 2. Calorie-reduced diet 2 3. Calorie-reduced diet 3 | 1. Protein 20%, carbohydrates 50%, fat 30% 2. Protein 27%, carbohydrates 43%, fat 30% 3. Protein 35% (80% from animal protein); carbohydrates 35%; fat 30%, 6 months | Randomized trial, 91 obese women (18–80 years) | ↓ Levels TG in 35%-protein group at 3 months (p < 0.0001) |
| Saslow, 2017 [34] | 1. VLCKD 2. PMD (ADA) | 1. 20–50 g of non-fiber carbohydrates per day (eaten ad libitum) 2. A 9-inch plate: half the plate is filled with non-starchy vegetables, one-quarter with carbohydrates, and one-quarter with lean proteins, 32 weeks | RCT, 56 obese and type 2 diabetes adults (53 average age) | ↓TG levels (EMM −60.1 mg/dL, 95% CI −91.3 to −28.9 mg/dL) in the intervention group, only at the 32-week time point (p = 0.01) |
| Veum, 2017 [28] | 1. VHFLC 2. LFHC | 1. 73% fat, 10% carbohydrate 2. 30% fat, 53% carbohydrate 12 weeks | Randomized trial, 56 overweight and obese men (aged 30–50 years) | Both groups improved dyslipidemia, with reduced circulating TG |
| Wright, 2017 [35] | 1. LFPB 2. CD | 1. 7% fat, 12% protein, and 81% carbohydrate (whole grains, legumes, vegetables, and fruits). Until satiation 2. Standard diet and general recommendations 12 months | RCT, 65 obese and type 2 diabetes adults (30–70 years) | Nonsignificant reduction in total TG |
| Bowen, 2018 [17] | 1. DER 2. ADF + DER | 1. 31% carbohydrate, 38% protein, 28% total fat (52% monounsaturated fat and 17% polyunsaturated fat). 2. Same macronutrient distribution as DER, fasting days −700 kJ/day, compared to the DER, and meal replacement Both groups were given meal replacements (approximately 1000 kJ, 25 g protein, 4 g fat, 27 g) 16 weeks | Randomized trial, 162 obese adults (25–60 years) | Both high-protein ADF + DER and DER programs were well accepted; TG improved (p < 0.05) |
| Moszak, 2018 [23] | Energy restricted diet | 25–30% reduction in caloric dietary intake. Diet with an identical composition of macronutrients (especially proteins) derived from the same products. 20% protein, 25–30% from fat, and 50–55% from carbohydrates. 3 weeks | Unblinded clinical trial, 24 obese hospitalized adults (24–66 years) | ↓ TG, −12.5% postintervention Not statistically significant |
| Øyri, 2018 [36] | 1. Muffin 2. Muffin | 1. 60 g/day (70% of fat, 40% SFA) 2. 60 g/day (70% of fat, 40% PUFA) 1 meal—3 to 5 weeks of washout | Randomized controlled double-blind crossover study 13 hypercholesterolaemic subjects with FH and 14 normolipidemic controls (18–30 years) | ↔ Postprandial TG AUC with SFA meal 901.25 (612.5–1032.5) a mg/dL (10.3 (7.0–11.8) mmol/L compared with PUFA meal 586.25 (533.75–796.25)) a 6.7 (6.1–9.1) a in subjects with FH (p = 0.72) ↔ Postprandial TG iAUC with SFA meal 175 (78.75–288.75) mg/dL a (2.0 (0.9–3.3) mmol/L compared with PUFA meal 166.25 (87.5–253.75)) a 1.9 (1.0–2.9) a in subjects with FH (p = 0.36) ↔ Postprandial TG AUC and iAUC between groups (p = 0.56, p = 0.25) |
| Shah, 2018 [16] | 1. HP 2. HMF | 1. 840 kcal for men, 700 kcal for women (31.9% of protein, 15.5% of fat, 4.3% of MUFA, 9.9% of SFA, and 52.6% of carbohydrates) 2. 840 kcal for men, 700 kcal for women (35.2% of fat, 20.7% of MUFA, 12.6% of protein, 8.7% SFA, and 52.3% from carbohydrates) Postprandial (3 h) states | Randomized trial 24 overweight and obese adults (18–65 years) | ↓ TG HP post-intervention at 120 min (geometric mean (95% CI): 148 (125–175) vs. 194 (164–230) mg/dL) and 180 min (167 (138–203) vs. 230 (189–278) mg/dL) HMF condition |
| Wade, 2018 [37] | 1. MedDairy 2. LF | 1. 3–4 servings daily of dairy foods, ≤1 serving of cheese, abundant use of extra virgin olive oil, ≥2–3 daily servings of fresh fruits, ≥3 weekly servings of legumes, ≥3 weekly servings of fish and seafood, ≥5 weekly servings of nuts or seeds, ad libitum consumption of whole-grain cereal 2. ≤20 mL oil/day and ≤2 teaspoons butter or margarine/day. Restricted foods: oil, butter, margarine, full-fat dairy products, processed and high-fat meats, nuts, chocolate, cakes, biscuits, pastry, and ice cream 8 weeks, and an 8-week washout period separating the interventions | Randomized, controlled, crossover trial, 41 overweight and obese adults (45–75 years) | ↓ TG (mean difference: = −0.05 mmol/L; 95% CI: −0.08, −0.01 mmol/L; p < 0.01) after MedDairy intervention |
| Bowen, 2019 [38] | 1. HOCO 2. Control oil 3. Canola oil | 1. 19.1% MUFA, 7.0% PUFAs, 6.4% SFA 2. 10.5% MUFA, 10.0% PUFAs, 12.3% SFA 3. 17.5% MUFA, 9.2% PUFAs, 6.6% SFA 6 weeks (per period) | Double-blind, randomized, 3-period crossover, controlled feeding trial, 119 obese adults (aged 25–60 years) | ↓TG levels (canola: 128.43 ± 3.54 mg/dL (1.45 ± 0.04 mmol/L) b p = 0.0182, HOCO: 127.55 ± 3.54 mg/dL (1.44 ± 0.04 mmol/L) b p = 0.0053, control: 124 ± 3.54 mg/dL (1.40 ± 0.04 mmol/L) b p = 0.0002) |
| Della Pepa, 2019 [39] | 1. HPN2. LPN | 1. Low in omega-3 (1.4 g/day) and rich in polyphenols (2.903 mg/day)Rich in omega-3 (4 g/day) and polyphenols (2.861 mg/day).2. Low in omega-3 (1.5 g/day) and polyphenols (365 mg/day)Rich in omega-3 (4 g/day) and low in polyphenols (363 mg/day)8 weeks | Randomized trial 88 overweight and obese men (35–70 years) | ↓ TG decreased in HPM group (3.27 ± 2.20 vs. 2.55 ± 2.14 mmol/L × 6 h) b |
| Gepner, 2019 [40] | 1. LF 2. MED/LC | 1. 30% of total fat, 10% SFA, 300 mg/day of cholesterol (whole grains, vegetables, fruits, and legumes and limited consumption of additional fats, sweets, and high-fat snacks) 2. Carbohydrate intake less than 40 g/day in the first 2 months, and thereafter a gradual increase up to 70 g/day (rich in vegetables and legumes and low in red meat, with poultry and fish and 28 g of walnuts/day) 18 months | Randomized controlled trial 278 dyslipidemia and obese adults (mean age 48 years) | The reduction in TG was similar between physical activity groups and MED/LC not statistically significant |
| Hutchinson, 2019 [22] | 1. TRFe 2. TRFd | 1. Eating window between 8 a.m. and 5 p.m. 2. Eating window between 12 p.m. and 9 p.m. Participants were asked to maintain their usual lifestyles, including sleep patterns, and to consume their habitual diet 1 week | Randomized, controlled, crossover trial, 50 overweight and T2DM risk adults (mean age 55 years) | ↓ Fasting TG (p = 0.003) on day 7 versus day 0 in both TRF interventions |
| Kondo-Ando, 2019 [41] | 1. LB 2. NB | 1. 187.8 kcal: 20.7 g carbohydrate (dietary fiber 11.1 g); protein, 17.4 g; fat, 6.0 g 2. 185 kcal: 32.7 g carbohydrate (dietary fiber, 1.6 g); protein, 6.5 g; fat, 3.1 g. 7 days (1 and 2 h after the breakfast) | Unblinded clinical trial 41 T2DM hospitalized patients (20–80 years) | ↓ TG 2 h after breakfast in the LB group compared with those in the NB group (p < 0.01) |
| Medina, 2019 [42] | 1. DP 2. P | 1. 500 kcal/day deficit 45–55% carbohydrate, 15–20% protein, 25–35% fat, < 7% SFA, 200 mg/day of cholesterol, 20–35 g fiber. Comprising high-fiber, polyphenol-rich and vegetable-protein functional foods: 14 g of dehydrated nopal, 4 g of chia seeds, 30 g of soy protein and 4 g of inulin) 2. Dehydrated sachets similar in kcal (28 g of calcium caseinate and 15 g of 137 maltodextrin) 3 months | Placebo-controlled, randomized, double-blind trial 81 overweight and T2DM adults (30–60 years) | ↓ TG (p < 0.01) vs. the p group |
| Starr, 2019 [43] | 1. HP 2. CG | 1. 30% protein, 30% fat, 40% carbohydrate, with a protein intake of 1.2 g/kg 2. 15% protein, 30% fat, 55% carbohydrate diet with a protein intake meeting the RDA of 0.8 g/kg. 6 months | Randomized trial, 80 obese adults (64 average age) | ↓ TG at endpoint; protein group had significant TG reduction (p ≤ 0.05) (−17.3 ± 50.2 mg/dL vs. 11.5 ± 34.7 mg/dL) b |
| Shih, 2019 [44] | 1. HLC 2. HLF | 1. With respect to basal (−111.9 ± 76.1 carbohydrates, −506.7 ± 616.5 energy, 2.9 ± 4.4 change in saturated fatty acids) 2. With respect to basal (−36.1 ± 81.3 carbohydrates, −484.3 ± 625.8, −2.6 ± 3.6 change in saturated fatty acids) 12 months | Randomized trial, 609 obese adults (18–50 years) | ↓TG in the context of a weight-loss study in which participants simultaneously decreased carbohydrate intake (p < 0.0001) |
| Tindall, 2019 [45] | 1. WD 2. WFAMD 3. ORAD | 1. 7% SFA, 16% PUFA, 3% ALA, 9% MUFA 2. 7% SFA, 16% PUFA, 3% ALA, 9% MUFA 3. 7% SFA, 14% PUFA, 0.5% ALA, 12% MUFA 6-week intervention, 22.8 c days washout period, 6-week intervention, 22.8 c days washout period, 6-week intervention | Randomized, controlled, 3-period, crossover, feeding trial, 36 subjects with overweight or obesity, LDL cholesterol between 128–177 mg/dL for men and 121–172 mg/dL for women and/or elevated brachial blood pressure (30–65 years) | ↔ TG between-diet differences compared baseline 117.7 ± 8.1 mg/dL (1.34 ± 0.09 mmol/L) b to after intervention WD 116.5 ± 8.5 (1.33 ± 0.01) WFAMD 117.4 ± 8.1 (1.34 ± 0.09) b ORAD 118.1 ± 8.3 (1.35 ± 0.09) b; p = 0.70 |
| Haldar, 2020 [46] | 1. Oil blend made with refined rice bran, flaxseed, and sesame oils 2. Oil blend made with refined rice bran, flaxseed, and sesame oils 3. Refined olive oil (control group) | 1. 30 g/day (14.9% SFA, 52.2% PUFA, 32.9% MUFA) 2. 30 g/day (13.6% SFA, 58.4% PUFA, 28.1% MUFA) 3. 30 g/day (16.6% SFA, 12.5% PUFA, 70.8% MUFA) 8 weeks | Parallel-design, randomized, single-blind, controlled trial 131 borderline hypercholesterolemic (50–70 years) | ↓ TG post-intervention time points (−10.3% d; p < 0.0001) for all 3 intervention oils compared with baseline ↔ between the 3 treatments across time |
| Kim, 2020 [47] | 1. BKD 2. TAD 3. 2010DGA | 1. 64% carbohydrates, 15% protein, 21% fat 2. 48% carbohydrates, 15% protein, 37% fat 3. 53% carbohydrates, 16% protein, 31% fat 4 weeks per period | Three-period, randomized, crossover-controlled trial, in 62 overweight or obese adults | ↓ TG significantly in three diets after 4 weeks (changes in TG levels) BKD: −33.11 ± 7.99 mg/dL b (0.37 ± 0.09 mmol/L) b (p < 0.001) TAD: −27.63 ± 7.99 md/dL (−0.31 ± 0.09 mmol/L) b (p = 0.001) 2010DGA: −20.69 ± 8.06 mg/dL (−0.23 ± 0.09 mmol/L) b (p = 0.012) No difference between treatment groups |
| Yokose, 2020 [48] | 1. LFRC 2. MedRC 3. LCNRC | 1. 1500 kcal per day for women and 1800 kcal per day for men (30% of fat, 10% of saturated fat, 300 mg of cholesterol) 2. 1500 kcal per day for women and 1800 kcal per day for men (no more than 35% of fat, 30 to 45 g of olive oil and nuts (5–7 nuts) per/day) 3. 20 g of carbohydrates per/day for the 2-month induction phase with a gradual increase to a maximum of 120 g per/day. The intakes of total calories, protein, and fat were not limited 24 months | RCT, 205 obese adults (40–65 years) | ↓ TG in all three groups (p < 0.05 at 6 months) ↓ TG at both (MedRC and LCNRC) 6 and 24 months (all p for within-group comparison, 0.01) |
| Folwaczny, 2021 [49] | 1. Isocaloric fat shake (SFA meal) 2. Isocaloric fat shake (MUFA meal) 3. Isocaloric fat shake (MCFA meal) | 1. 80 g/day fat (68% SFA, 2% PUFA, 30% MUFA) 2. 80 g/day fat (8% SFA, 29% PUFA, 63% MUFA) 3. 80 g/day fat (99% SFA) 1 meal, 7 to 28 washout period, 1 meal, 7 to washout period | RCT 8 mildly hypertriglyceridemic (43 ± 12 y b) and 5 normolipidemic controls (35 ± 5 y b) | ↓ iAUC TG with MCFA meal 152 ± 271 mg/dL*h b (1.73 ± 3.09 mmol/L*h) compared to SFA meal 1006 ± 583 (11.47 ± 665) b; p = 0.03 and MUFA meal 962 ± 673 (10.97 ± 7.67) b; p <0.01 in hypertriglyceridemic patients ↓ iAUC TG in normolipidemic controls with SFA meal 259 ± 160 mg*h/dL b (2.95 ± 1.82 mmol*h/L) and MUFA meal 248 ± 298 (2.83 ± 3.4) b compared to hypertriglyceridemic patients SFA meal 1006 ± 583 (11.47 ± 665) b; p = 0.019, MUFA meal 962 ± 673 (10.97 ± 7.67) b; p = 0.05. |
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main Results on Triglyceride Levels |
|---|---|---|---|---|
| Abbaspour, 2019 [58] | Mixed Nuts Pretzels | 42.5 g/day 8 weeks | Randomized, parallel-arm controlled trial 54 overweight and obese subjects (18–55 years) | ↓ TG levels post intervention from 0 to 8 weeks (101.5 ± 58.63 mg/dL (1.16 ± 0.67 mmol/L) a vs. 110.25 ± 96.25 (1.26 ± 1.1); p < 0.05). ↔ between groups (p = 0.655). |
| Gulati, 2017 [63] | Almond | Almonds substituted for 20% of total energy of fat 24 weeks | Pre-post intervention study design 50 T2DM patients | ↓ TG post intervention with almond vs. baseline (170.5 mg/dL (72,499) (1.94 (0.68 mmol/L) c vs. 149.5 (72,499) (1.7 (0.83 mmol/L); p < 0.004). |
| Liu, 2017 [62] | Peanut shake Control | 85 g per visit 10 days | Randomized, controlled, crossover trial 15 overweight or obese men (20–65 years) | IG vs. CG ↓ TG after 120 min (16,528.75 ± 1697.5 mg/dL (188.9 ± 19.4 mmol/L) a vs. 17,281.25 ± 1811.25 (197.5 ± 20.7)) and 240 min (16,616.25 ± 2126.25 mg/dL (189.9 ± 24.3 mmol/L)) vs. 17,263.75 ± 1610 (197.3 ± 18.4) with peanut shake and 240 min compared with control (p < 0.005). |
| Mah, 2017 [71] | Cashew (roasted, salted) Potato chips | 28–64 g/day 32–64 g/day 4 weeks | Randomized, crossover, isocaloric, controlled-feeding study 51 hypercholesterolemic subjects (21–73 years) | ↔ between IG and CG for TG from baseline (7.7% (−8.8, 17.3) b vs. 15.7 (1.1, 25.3); p = 0.206. |
| Mohan, 2018 [70] | Cashew nut | 30 g/day 12 weeks | Parallel-arm, randomized controlled trial 269 T2DM subjects | ↔ between groups in TG mean change post intervention (IG 4.3 ± 51.1 mg/dL (0.05 ± 0.58 mmol/L) a vs. CG 0.4 ± 62.2 (0 ± 0.71); p = 0.57). |
| Zibaeenezhad, 2017 [67] | PWO | 15 mL 90 days | Randomized, double-blind, placebo-controlled clinical trial 100 hyperlipidemic and T2DM patients (35–75 years) | ↓ TG (−15.04 mg/dL(−0.17 mmol/L); p = 0.021). |
| Akrami, 2018 [65] | FO SFSO | 25 mL/day 7 weeks | Randomized controlled interventional trial 60 MS patients (30–60 years) | ↓ TG levels after treatment with both oils IG: −52.46 ± 74.32 mg/dL (−0.6 ± 0.85 mmol/L) a; p < 0.001 and CG: −53.46 ± 58.21 (0.61 ± 0.66 mmol/L; p < 0.001). ↔ compared between the two groups (p = 0.516). |
| Zibaeenezhad, 2019 [69] | Almond oil | 10 mL, two times daily 30 days | Randomized open-label controlled clinical trial 99 hyperlipidemic adults | ↔ post intervention with almond in TG (mean change): 6.01 ± 5.78 mg/dL (0.07 ± 0.07 mmol/L a; 224.90 ± 57.93 (2.56 ± 0.66) vs. 131.05 ± 17.84 (1.49 ± 0.2); p = 0.491). |
| Coates, 2020 [61] | AED NFD with carbohydrate-rich snack foods | Portion of snack foods equivalent to 15% of their EER 12 weeks | Two-arm, parallel group randomized dietary intervention 128 overweight and obese adults (50–80 years) | ↓ TG after AED compared to the CG (IG: 100.62 ± 5.25 mg/dL (1.15 ± 0.06 mmol/L) vs. 112.88 ± 6.13 (1.29 ± 0.07) a; CG: 101.5 ± 0.61 (1.16 ± 0.007) vs. 03.25 ± 0.53 (1.18 ± 0.006); p = 0.008). |
| Costa e Silva, 2020 [68] | EVBNO SO | 10 mL/day 10 mL/day 30 days | Randomized, double-blind, placebo-controlled clinical trial 31 MS patients (36–65 years) | ↑ 68.6 ± 83.6 mg/dL (0.78 ± 0.95 mmol/L) a in TG (p < 0.05) after EVBNO consumption ↓ TG (−4.5 ± −4.5 mg/dL (0.05 ± 0.05 mmol/L); p = 0.8501) after SO Significant difference between EVBNO and SO (p < 0.05). |
| Yari, 2021 [66] | WFP HE FHE | 30 g/day 1 g/day 30 g/ 1 g/day 12 weeks | RCT 173 adults with MS | ↓ TG after 12 weeks with WFP and FHE group intervention (211.0 ± 83.9 mg/dL (2.41 ± 0.96 mmol/L) vs. 145.0 ± 58.5 (1.65 ± 0.67) a; p < 0.001). ↓ TG in WFP compared to control (p < 0.002). |
| Ghanavati, 2021 [59] | NELCD NFLCD | 8 weeks | RCT 67 overweight and obese subjects (aged 58.8 ± 7.4 years) | ↓ TG in both groups compared with baseline (mean change) (IG: −39.7 mg/dL (−0.45 mmol/L) vs. CG −33.6 (0.38); p = 0.60). ↔ between groups. |
| Haldar, 2020 [46] | RFSSO | 30 g per day 8 weeks | Parallel-design randomized controlled trial 143 hypercholesterolemic subjects | ↓ TG after intervention (−10.3%; p < 0.001). |
| Julibert, 2020 [60] | MD enriched with nuts | MD + 30 g/d of nuts (almond, pistachios, walnuts) MD + 1 L/week EVOO 1 year | Randomized trial 5800 overweight, obese and MS adults (55–75 years) | ↓TG (−7.8 ± 68.9 mg/dL (0.09 ± 0.79 mmol/L) a after 1 year of nut consumption Greater TG ↓ in T3 compared with T1 (−10.6 ± 60.1 mg/dL (0.12 ± 0.69 mmol/L) vs. −4.1 ± 77.0 (0.05 ± 0.88); p < 0.005). |
| Kuang, 2020 [64] | DFF Biscuit | 100 g/day 60 days | Double-blind randomized controlled trial 53 overweight and obese patients (16–36 years) | Difference between groups after 60 days of intervention (p < 0.005). ↓ TG compared day 0 to day 60 DFF group (105 ± 51.63 mg/dL (1.20 ± 0.59 mmol/L) a vs. 101.5 ± 55.15 (1.16 ± 0.63); p < 0.005) |
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main Results on Triglyceride Levels |
|---|---|---|---|---|
| Dong, 2016 [75] | PSSMP NPSSMP | 30 g/day (2 g plant sterol) 30 g/day 6 months | RCT 137 subjects with mild to moderate hypercholesterolemia (55–65 years) | ↔ between the NPSSMP and PSSMP group in TG after 3 and 6 months. (Baseline, IG: 166.25 ± 85.75 mg/dL (1.90 ± 0.98 mmol/L) and CG: 171.5 ± 84 (1.96 ± 0.96); p = 0.704). After 6-month IG: 147 ± 89.25 mg/dL (1.68 ± 1.02 mmol/L) vs. CG: 163.63 ± 64.75 (1.87 ± 0.74); (p = 0.236). |
| Lu, 2019 [77] | Shake mix MRDSP-SF-CR | 2 times/day 29 g: 6.3 g from protein (21% calories) 15 g from carbohydrates (of which 5 g are dietary fiber, 50% calories) 3.9 g from fats (29% calorie) 8 weeks | RCT 50 obese subjects (20–80 years) | ↓ 11.5% after intervention compared with baseline (p < 0.05). |
| Tabrizi, 2019 [78] | SBL PC * | 4 capsules 335/50 mg/day 4 capsules (1240/72 mg) 8 weeks | Triple-blind, RCT crossover 31 participants with cardiovascular risk factors (dyslipidemia or obesity or hypertension or HTG) (33–75 years) | ↔ in TG after 8 weeks of SBL treatment. ↓ TG without significance after intervention in PC (8.0 (−17.00, 1.00) a; p = 0.089). |
| Oliveira, 2020 [76] | SMPS SM | 400 mL/day + 1.6 g 400 mL/day 4 weeks | Controlled, crossover study 38 moderately hypercholesterolemic subjects (38–77 years) | ↓ 8.3% on TG levels after SMPS (p < 0.05) Difference after 4 weeks with SMPS compared SM (133 ± 7 mg/dL (1.52 ± 0.08 mmol/L) vs. 154 ± 10 (1.76 ± 0.11); p = 0.008). |
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main results on Triglyceride Levels |
|---|---|---|---|---|
| Fernández-Castillejo, 2016 [80] | 1. VOO 2. FVOO 3. FVOOT | 1. (25 mL/day) 2. (25 mL/day 3. (25 mL/day) 3 weeks and 2 week washout period | Randomized, double-blind, crossover, controlled trial 33 hypercholesterolemic subjects | ↔ between groups in TG after 3 weeks and 2-week washout period (1) VOO −7.9 (−25 to 9.1 mg/dL) a, (2) FVOO 3.8 (−14 to 21), (3) FVOOT 3.8 (−14 to 22). |
| Lee, 2016 [81] | 1. LFM 2. CG | 1. Two packs of low-fat milk per day, 200 mL twice daily 2. Maintain habitual diet 6 weeks | Parallel-group dietary intervention study, 58 overweight and metabolic syndrome subjects | ↔ between groups in TG after 6 weeks IG: 35.9 ± 101.5 mg/dL b vs. CG 17.2 ± 156.8; p = 0.301. |
| Suganya, 2017 [83] | 1. Standard statin therapy 2. Nutritional supplement in addition to the standard statin therapy. | Nutritional supplement (mix spices: cuminum, piper nigrum, solanum nigrum) 9 months | Clinical trial two arms, 65 dyslipidemic subjects | ↓ TG levels after supplement intake along with standard therapy, showed significant decrease in TG (338.2 ± 256.1 mg/dL b to 216.9 ± 102.6 mg/dL); p < 0.005. |
| Sarriá, 2018 [86] | 1. IG 2. CG | 1. 3 servings/day of the blend (510.6 mg hydroxycinnamic acids and 121.2 mg caffeine/day) 2. Control drink 8 weeks | Crossover, randomized, controlled study, 52 hypercholesterolemic and normocholesterolaemic | ↓ TG levels (p = 0.017) and the reduction were much greater in the hypercholesterolemic subjects (group effect, p = 0.027). |
| Franck M, 2020 [85] | 1. IG 2. CG | 1. 280 g/day of frozen raspberries 2. Maintain usual diet 8 weeks | RCT, trial, 54 overweight/ obese subjects | No significant differences between follow-up and baseline values between CG and IG for triglycerides (Baseline: CG 1.42 ± 0.61 b, IG 1.47 ± 0.82 vs. wk8 CG 1.38 ± 0.61, IG 1.29 ± 0.61; p = 0.71). |
| Koutsos, 2020 [84] | 1. WA 2. CB | 1. 2 apples/day 2. Sugar and energy-matched apple control beverage 8 weeks (4-week washout period) | Randomized, controlled, crossover, intervention study, 40 hypercholesterolemic subjects | ↓ TG levels WA (WA: 1.17 mmol/L; CB: 1.30 mmol/L; p = 0.021). |
| Petersen, 2020 [82] | HSFHC | 1. 0 g spices 2. 2 g of spices 3. 6 g of spices | randomized, controlled, crossover, pilot study, 13 overweight/ obese men | ↓ TG 2 g of spices vs. the no spice meal (−18 ± 6 mg/dL b; p = 0.015); no difference was observed between the meal with 6 g of spice and the no |
| Santos, 2020 [79] | 1. EVOO group 2. TBD 3. TBD + EVOO | 1. 52 mL/day of EVOO 2. Common Brazilian foods 3. 52 mL/day of EVOO 12 weeks | RCT 149 subjects with several obesity | ↔ between groups in TG after 12 weeks (1) EVOO group (160.46 ± 79.85 mg/dL b vs. 151.35 ± 65.53; p = 0.296), (2) TBD (154.75 ± 87.76 vs. 142.46 ± 64.96; p = 0.069), (3) TBD + EVOO (165.60 ± 67.72 vs. 160.83 ± 77.33; p = 0.286). |
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main results on Triglyceride Levels |
|---|---|---|---|---|
| Gutiérrez-Salméan, 2016 [110] | EPIC Placebo | Two capsules of 25 mg EPIC or placebo 4 weeks | Randomized, placebo-controlled, double-blind study 30 hypertriglyceridemia subjects (18–55 years) | ↓ TG by 75 mg/dL (0.86 mmol/L) (26.5%, p < 0.05), while in the placebo group it decreased by 40 mg/dL (0.46 mmol/L) (14.7% ns). ↓ TG after EPIC group compared baseline values (207 ± 73.2 mg/dL (2.36 ± 0.83 mmol/L) vs. 282 ± 94.4 (3.21 ± 1.08); p = 0.008). |
| Ohara, 2016 [112] | GH GHC Placebo | 3 tablets 500 mg/day 25, 50, 75 mg/day 12 weeks | Randomized double-blind placebo-controlled trial 75 overweight and obese subjects (25–65 years) | ↓ TG after 8 weeks of intervention with GH + 75 mg of caffeine 29.3 ± 66 mg/dL (0.33 ± 0.75 mmol/L). ↔ TG after 4-, 8- and 12-weeks post intervention in all groups. |
| Tariq, 2016 [123] | CZRHT | Twice daily G1: 500 mg G2: 1 g G3: 1.5 g in 200 mL water 2 months | Randomized trial 30 hypercholesterolemic male (25–40 years old) | ↓ TG of 12.5% after 60 days for G3 compared with day 0 (147.61 ± 2.56 mg/dL (1.68 ± 0.03 mmol/L) vs. 168.70 ± 2.92 (1.92 ± 0.03); p < 0.05). |
| Dicks, 2018 [124] | CP CFC | Capsules of 2.5 g/day of flavanol-rich cocoa (207.5 mg) or CFC 12 weeks | Double-blind randomized, placebo-controlled trial 42 hypertensive and T2DM patients (median of 64.2 years) | ↔ TG after CP in either group compared with baseline (IG: 122.5 (78.75, 157.5 mg/dL) 1.4 (0.9, 1.8 mmol/L) b vs. 113.75 (78.75, 166.25) 1.3 (0.9;1.9) and CFC: 131.25 (96.25, 175 mg/dL) 1.5 (1.1, 2.0 mmol/L) vs. 157.5 (113.75, 201.25) 1.8 (1.3; 2.3); ns). |
| Kirch, 2018 [111] | EPIC Placebo | 25 mg (encapsulated) 2 weeks intervention 2 weeks washout | Randomized, placebo-controlled, double-blind crossover study 48 overweight or obese and MS adults (20–65 years) | ↔ TG after intervention with EPIC compared with week 0 (195.13 ± 23.63 mg/dL (2.23 ± 0.27 mmol/L) a vs. 194.25 ± 16.62 (2.22 ± 0.19); ns) ↔ after intervention and time. |
| Leyva-Soto, 2018 [109] | DC MC | 2 g/day (70% cocoa or 2 g of MC) 6 months | Randomized, placebo-controlled, double-blind study 84 obese, dyslipidemia and T2DM young subjects (men and women, 23.8 and 23.6, respectively). | ↓ TG after 6 months with DC intervention compared with baseline (153.26 ± 18.95 mg/dL (1.75 ± 0.22 mmol/L) a vs. 228.25 ± 17.9 (2.6 ± 0.2); p < 0.05). |
| Braxas, 2019 [9] | GE Placebo | 2 capsules daily (54 mg of GE or placebo capsule) 12 weeks | Randomized, double-blind, placebo-controlled clinical trial 54 post-menopausal women with T2DM (47–69 years) | ↓ TG in genistein group compared with baseline values (−10.19%; p < 0.05). ↓ TG after intervention with genistein compared with placebo (IG: 162.14 ± 52.04 mg/dL (2.11 ± 0.59 mmol/L) vs. 197.42 ± 76.44 (2.25 ± 0.87) and CG: 194.46 ± 77.80 mg/dL (2.22 ± 0.89) vs. 195.69 ± 63.41 (2.23 ± 0.72); p < 0.05). |
| Rynarzewski, 2019 [125] | CP Placebo | 2.5 g of flavonoid-rich cocoa or microcrystalline cellulose Unique dose (postprandial) 2 weeks washout | Randomized, placebo-controlled, double-blind crossover study 12 T2DM, obese and hypertensive adults (>18 years) | ↔ TG postprandial after CP treatment compared with baseline (4 h: 168.88 ± 16.63 mg/dL (1.93 ± 0.19 mmol/L) a vs. 0 h: 168.88 ± 19.25 (1.93 ± 0.22); p = 0.184). |
| Yari, 2020 [126] | HE Placebo | 2 capsules daily of 500 mg or placebo 12 weeks | Randomized, double-blind, placebo-controlled clinical trial 49 MS patients (18–70 years) | ↓ TG after HE intervention compared with placebo (IG: 185.50 ± 84.34 mg/dL vs. 136.41 ± 72.86) (CG: 155.61 ± 54.12 vs. 164.44 ± 63.77; p < 0.05). |
| Yousefi, 2021 [113] | GSE + RCD Placebo | 3 capsules of 300 mg/day or placebo capsules 250 kcal lower than EER 12 weeks | Randomized, placebo-controlled trial 40 obese or overweight individuals (20–50 years) | ↓ TG after 12 weeks in GSE group compared with baseline (−33.73 ± 14.07 mg/dL (−0.38 ± 0.16 mmol/L); p < 0.05). |
| Author, year | Dietary Component | Dose/Time | Study Design n | Main Results on Triglyceride Levels |
|---|---|---|---|---|
| Dodevska, 2016 [134] | LFHF RSD | 25–35 g/day of fiber 25–35 g/day of fiber Plant foods replaced with RSD 12 months | Experimental Trial 47 overweight, obese and dyslipidemia adults | ↑ TG (2.8%) after 12 months of LFHF group. ↔ in TG on LFHF group compared with RSD (p = 0.717). |
| Li, 2016 [131] | WGO LFHFHD 7-day cyclical menu | 50 g 100 g 30 days | RCT 298 overweight and T2DM participants | ↓ TG after 50 g WGO compared with baseline, 180.25 mg/dL (92.75) (2.06 mmol/L (1.06)) a vs. 173.25 mg/dL (144.38) (1.98 mmol/L (1.65)). Change −7.88 mg/dL (−27.13, −11.38) (−0.09 mmol/L (−0.31, −0.13); p = 0.003) a,b. ↓ TG after 100 g WGO compared with baseline, 136.5 mg/dL (67.38) a (1.56 mmol/L (0.77)) a vs. 173.25 mg/dL (87.5) a (1.98 mmol/L (1.00) a). Change −37.63 mg/dL (−56.88, −18.38) a (−0.43 mmol/L (−0.65, −0.21) a; p = 0.003). p value between groups (change). |
| Raimondi de Souza, 2016 [138] | Oat bran Corn starch and rice flour 40 g (placebo group) | 40 g/day (3 g of beta-glucans) 40 g/day 90 days | Double-blind, placebo-controlled, block-randomized trial 132 hypercholesterolemic patients (>20 years) | ↓ TG in the oat bran group compared baseline to 30 days after intervention, 147 mg/dL (106–232) a 1.68 mmol/L (1.21–2.64) vs. 121 (93–178) (1.38 (1.06–2.03) a; p < 0.005) ↓ TG in the oat bran group compared with the placebo group; p < 0.005 |
| Tessari, 2016 [133] | FB RWB | Fiber (7 g/100 g) Beta glucan/starch ratio of (7.6:100, g/g) 6 months | Observational, controlled study with parallel groups T2DM (50–80 years). | ↓ TG at end of FB compared with RWB (140 ± 14 mg/dL (1.60 ± 0.16 mmol/L) d vs. 95.38 ± 10.5 (1.09 ± 0.12); p = 0.16). |
| Vetrani, 2016 [132] | Isoenergetic diet: WGCF c RCP | 52% carbohydrates 46% GI 72% GI 12 weeks | Randomized, controlled, parallel-group design 54 overweight, obese, and MS adults (men and women, median 58.4 and 57.2, respectively). | ↓ TG after 12 weeks of intervention on WGCF (−16.9 ± 2.8 mg/dL (0.19 ± 0.03 mmol/L); p = 0.538) d. ↓ TG at the end in the WGCF compared to the control (p < 0.005). |
| Gulati, 2017 [139] | Oats in the form of porridge and upma Routine diet and usual exercise habits (control group) | 70 g/day 4 weeks | Prospective, randomized, parallel, controlled study 69 subjects mildly hypercholesterolemic (20–50 years) | ↔ TG post intervention in both groups Oats group: 162.2 ± 54.7 mg/dL b (1.85 ± 0.62 mmol/L) to 172.7 ± 75.2 (1.97 ± 0.86) Control group: 163.6 ± 62.5 mg/dL (1.87 ± 0.71 mmol/L) to 165.8 ± 69.2 (1.89 ± 0.79) ↔ % reduction TG post intervention between groups 5.9 (−29.6, 41.5) a; p = 0.03. |
| Pal, 2017 [108] | Fiber Supplements: PSY PGY RF | 5 g/day each one 12 months | Randomized, double blind, parallel study 159 overweight and obese people (19–68 years) | ↓ TG in the PSY after 6 months (100.62 ± 0.53 mg/dL (1.15 ± 0.006 mmol/L) e vs. 108.5 ± 0.53 (1.24 ± 0.006); p < 0.05) compared to baseline. |
| Anggadiredja, 2018 [136] | FMC IF | Twice daily 4 g of soluble fiber 0.5 g of insoluble fiber 6 weeks | Double-blind, randomized parallel-group study 41 hypercholesterolemic participants (>18 years). | ↔ Between FMC and IF groups at start or at the end of the study (104.90 ± 9.9 mg/dL (1.24 ± 0.11 mmol/L) d vs. 113.95 ± 12.65 mg/dL (1.3 ± 0.14); p > 0.5 and 96.2 ± 9.99 mg/dL (1.1 ± 0.11 mmol/L) d vs. 102.4 ± 11.95 (1.17 ± 0.14) d; p > 0.05). |
| Babiker, 2018 [135] | GA Placebo (pectin) | 30 g 5 g 3 months | Randomized, double-blind, placebo-controlled clinical trial 91 T2DM subjects (mean 50.09 years) | ↔ TG post GA intervention vs. baseline (p = 0.17) ↔ between GA and control group after intervention (p = 0.958). |
| Schioldan, 2018 [141] | HCD WSD | 64 g/day dietary fiber 18 g/day dietary fiber 4 weeks intervention, 4 to 6 weeks washout period, 4 weeks intervention | Open-label, randomized, crossover study 19 subjects with MS (39–75 years) | ↔ TG compared baseline to post intervention in the diets: WSD: 118.13 (90.13–157.5 mg/dL) e (1.35 (1.03–1.8 mmol/L)) vs. 138.25 (97.13–175) (1.58 (1.11–2.0)); p = 0.58 HCD: 122.5 (90.13–164.5 mg/dL) e (1.4 (1.03–1.88 mmol/L)) vs. 136.5 (88.38–173.25) (1.56 (1.01–1.98)); p = 0.67. |
| Fatmah, 2020 [114] | HFB TB | 100 g/day 100 g/day 4 weeks | Quasi-experimental design, single-blind, randomized controlled trial 66 T2DM participants (50–60 years) | ↓ TG after caromma biscuit intervention (123.67 ± 73.69 mg/dL (1.41 ± 0.84) e vs. 175.09 ± 112.64 (2 ± 1.28) e; p <0.001). |
| Rakvaag, 2019 [115] | WPLF MDLF WPHF MDHF | 2 daily servings with 300 mL of water + 10 g/day from fiber 2 daily servings with 300 mL of water + 30 g/day from fiber 12 weeks | Double-blind, randomized parallel-group study 41 hypercholesterolemic adults (men and women, median 42 and 38.6, respectively). | Fasting TG ↓ TG after intervention with WPLF compared with baseline (10,237.5 ± 2887.5 mg/dL (117 ± 33 mmol/L) e vs. 10,237.5 ± 1925 (117 ± 22); p < 0.05). Postprandial TG WPLF ↓ TG tAUC (360 min) after 12 weeks compared with baseline (19,512.5 ± 8662.5 mg/dL (223 ± 99 mmol/L) e vs. 24,150 ± 11,550 (276 ± 132); p <0.05). WPHF ↓ TG tAUC after intervention vs. baseline (18,200 ± 5687.5 mg/dL (208 ± 65 mmol/L) e vs. 20,300 ± 8312.5 (232 ± 95); p < 0.05). ↔ between WPHF and the two MD groups. |
| Cicero, 2020 [140] | Proprietary formulation of beta-glucans Oat-based isocaloric placebo without beca-glucan | 3 g/day beta-glucans of oat 2 months intervention-4 weeks wash-out period- 2 months intervention | Double-blind, placebo-controlled, crossover randomized clinical trial 83 free-living subjects, adherent to Mediterranean diet, with moderate hypercholesterolemia (20–65 years) | ↔ TG compared baseline to post intervention in oat with beta-glucan intervention 129.5 ± 70.88 mg/dL e,b (1.48 ± 0.81 mmol/L) vs. 141.75 ± 96.25 e (1.62 ± 1.10); p = 0.385. |
| Author, Year | Dietary Component | Dose/Time | Study Design n | Main Results on Triglyceride Levels |
|---|---|---|---|---|
| Corella, 2016 [152] | Mediterranean diet + Nuts Mediterranean diet + EVOO LF diet | 30 g/day 1 L per week 4.8 years a | Large, multicenter, randomized and controlled clinical trial 7098 with T2DM or cardiovascular risk genotyped for CLOCK rs4580704 C>G (55–80 years) | ↔ TG in any genotype or diet. |
| De Luis, 2016 [142] | HP diet S diet | 1050 kcal/day, 33% fats, 33% carbohydrates, and 34% proteins 1093 kcal/day, 27% fats, 53% carbohydrates, and 20% proteins 9 months | RCT 283 obese subjects genotyped for UCP3 rs1800849 C>T (52.9 ± 11.2 y a and 52.3 ± 10.4) | ↓ TG with HP diet and only in C/C genotype, 122.1 ± 31.1 mg/dL a (1.39 ± 0.35 mmol/L) to 106.6 ± 13.2 (1.22 ± 0.15) at 9 months to baseline—15.5 ± 3.9 (0.15 ± 0.04) a; p < 0.05. |
| De Luis, 2017 [143] | Low in carbohydrates and hypocaloric diet LF and hypocaloric diet | 1507 kcal/day 36% fats, 38% carbohydrates, 26% proteins 1500 kcal/day, 27% fats, 53% carbohydrates, 20% proteins 3 months | RCT 283 obese subjects genotyped for NPY rs16147 G>A (46.8 ± 10.1 y a). | ↔ TG in any genotype or diet. |
| De Luis, 2017 [149] | High-PUFA hypocaloric diet High-MUFA hypocaloric diet | 1448 kcal/day 45.9% carbohydrates, 34.3% lipids (21.8% SFA, 55.3% MUFA, 22.9% PUFA), and 19.8% proteins 1442 kcal/day 46.0% carbohydrates, 34.4% lipids (21.6% SFA, 67.7% MUFA, 10.7% PUFA), and 19.6% proteins. | RCT 361 Obese subjects BDNF rs10767664 A>T (20–65 years). | ↔ TG in any genotype or diet. |
| Arpón, 2018 [153] | Mediterranean diet + Nuts Mediterranean diet + EVOO LF diet | 30 g/day 1 L per week 5 years | RCT 36 participants with MS (60 and 70 years) | ↔ TG with any diet. |
| De Luis, 2018 [150] | Standard hypocaloric diet | 1520 kcal/day 52% of carbohydrates, 25% of lipids and 23% of proteins (50.7% MUFA, 38.5% SFA and 11.8% PUFA) 3 months | Non-randomized interventional study 80 obese subjects with the genetic variant BDNF rs10767664 A>T (20–65 years) | ↓ TG in non T allele carriers −13.2 ± 7.5 mg/dL a (−0.15 ± 0.09 mmol/L) compared to T allele group +2.8 ± 1.2 a (0.03 ± 0.01) p = 0.02. |
| Griffin, 2018 [145] | High-MUFA and low-glycaemic diet High-MUFA and high-glycaemic diet LF and low-glycaemic diet LF and high-glycaemic diet High-SFA high-glycaemic diet (reference diet) | 38% fats (10% SFA), 45% carbohydrates 38% fats (10% SFA), 45% carbohydrates 28% fats (10% SFA), 55% carbohydrates 28% fats (10% SFA), 55% carbohydrates 38% fats (16% SFA), 45% carbohydrates All diets are iso-energetic 24 weeks | Secondary analysis of data from a five-arm, randomized controlled, parallel dietary intervention trial 389 subjects with increased risk of developing the metabolic syndrome and genotyped for APOE rs429358 (112) and rs7412 (158) (E2/E2, E2/E3, E4/E4, E3/E4 and E3/E3 genotypes) (30–70 years) | ↑ TG Among carriers of E2 40.25 mg/dL b (0.46 mmol/L) p = 0.001 and E4 28.88 (0.33); p = 0.01 compared with E3/E3 when SFA was replaced with MUFA and low glycemic index carbohydrates. |
| Aller, 2019 [147] | High-MUFA and hypocaloric diet High-PUFA and hypocaloric diet | 34.1% fats (21.7% SFA, 67.5% MUFA, 22.7% PUFA), 46.6% carbohydrates and 19.2% proteins 34.4% fats (21.8% SFA, 55.5% MUFA, 10.8% PUFA), 45.7% carbohydrates and 19.9% proteins 3 months | RCT 362 obese subjects genotyped for CB2R rs3123554 G>A (25–65 years) | ↓ TG after high MUFA diet in G/G genotype 129.8 ± 9.1 mg/dL a (1.48 ± 0.1 mmol/L) to 110.7 ± 10.0 (1.26 0.11); p = 0.03. |
| De Luis, 2020 [146] | High-MUFA and hypocaloric diet High-PUFA and hypocaloric diet | 34.1 fats, 46.6% carbohydrates and 19.2% proteins 34.4% fats, 45.7% carbohydrates and 19.9% proteins 400–500 kcal/day less than the individually estimated total energy expenditure in both diets 3 months | RCT 361 obese subjects genotyped for MTNR1B rs10830963 C>G (18–70 years) | ↔ TG in any genotype or diet. |
| Franck, 2020 [85] | Frozen raspberries Maintain usual diet (control group) | 240 g/day 8 weeks | RCT 54 subjects with overweight or abdominal obesity (18–45 years) | ↔ TG between groups. |
| Merino, 2020 [151] | Intensive lifestyle intervention Metformin Placebo | Healthy low-calorie, low-fat diet Started at a dose of 850 mg/day and increased to 1700 mg/day 1 year | Multicenter randomized controlled trial 2658 participants with glucose intolerant and polygenic risk score of 204 variants representative of 160 coronary artery disease loci (50.7 years a) | ↓ TG with the intensive lifestyle intervention −14.79 (−16.89, −12.69) c mg/dL (−0.169 (−0.193, −0.145)) mmol/L compared to placebo −5.25 (−7.35, −3.15) (−0.060 (−0.084, −0.036)) p < 0.001. |
| Primo, 2020 [148] | HP diet S diet | 1050 kcal/d, 33% fats, 33% carbohydrates, and 34% protein 1093 kcal/d, 27% fats, 53% carbohydrates, and 20% protein 9 months | RCT 238 obese subjects genotyped for CB2R rs3123554 G>A (53.9 ± 9.1 y a) | ↓ TG in G/G genotype with the HP diet 125.8 ± 23.1 mg/dL a (1.43 ± 0.26 mmol/L) to 104.1 ± 13.2 (1.19 ± 0.15) and with S diet 129.1 ± 22.6 (1.47 ± 0.26) to 114.1 ± 20.1 (1.3 ± 0.23) at 9 months from baseline (p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Castillo, K.P.; Olivares-Ochoa, X.C.; Hernández-Ruiz, R.G.; Llamas-Covarrubias, I.M.; Rodríguez-Reyes, S.C.; Betancourt-Núñez, A.; Vizmanos, B.; Martínez-López, E.; Muñoz-Valle, J.F.; Márquez-Sandoval, F.; et al. The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients 2022, 14, 1104. https://doi.org/10.3390/nu14051104
Luna-Castillo KP, Olivares-Ochoa XC, Hernández-Ruiz RG, Llamas-Covarrubias IM, Rodríguez-Reyes SC, Betancourt-Núñez A, Vizmanos B, Martínez-López E, Muñoz-Valle JF, Márquez-Sandoval F, et al. The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients. 2022; 14(5):1104. https://doi.org/10.3390/nu14051104
Chicago/Turabian StyleLuna-Castillo, Karla Paulina, Xochitl Citlalli Olivares-Ochoa, Rocío Guadalupe Hernández-Ruiz, Iris Monserrat Llamas-Covarrubias, Saraí Citlalic Rodríguez-Reyes, Alejandra Betancourt-Núñez, Barbara Vizmanos, Erika Martínez-López, José Francisco Muñoz-Valle, Fabiola Márquez-Sandoval, and et al. 2022. "The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence" Nutrients 14, no. 5: 1104. https://doi.org/10.3390/nu14051104
APA StyleLuna-Castillo, K. P., Olivares-Ochoa, X. C., Hernández-Ruiz, R. G., Llamas-Covarrubias, I. M., Rodríguez-Reyes, S. C., Betancourt-Núñez, A., Vizmanos, B., Martínez-López, E., Muñoz-Valle, J. F., Márquez-Sandoval, F., & López-Quintero, A. (2022). The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients, 14(5), 1104. https://doi.org/10.3390/nu14051104