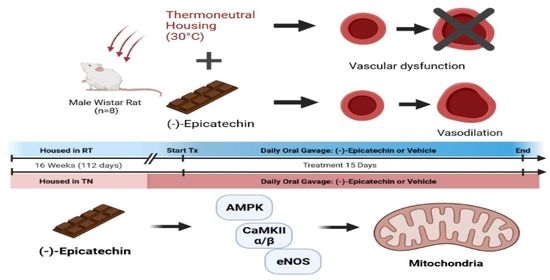
(–)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antibodies
2.3. In Vivo Experiments
2.4. Vasoreactivity
2.5. Respiration
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Metabolic Parameters
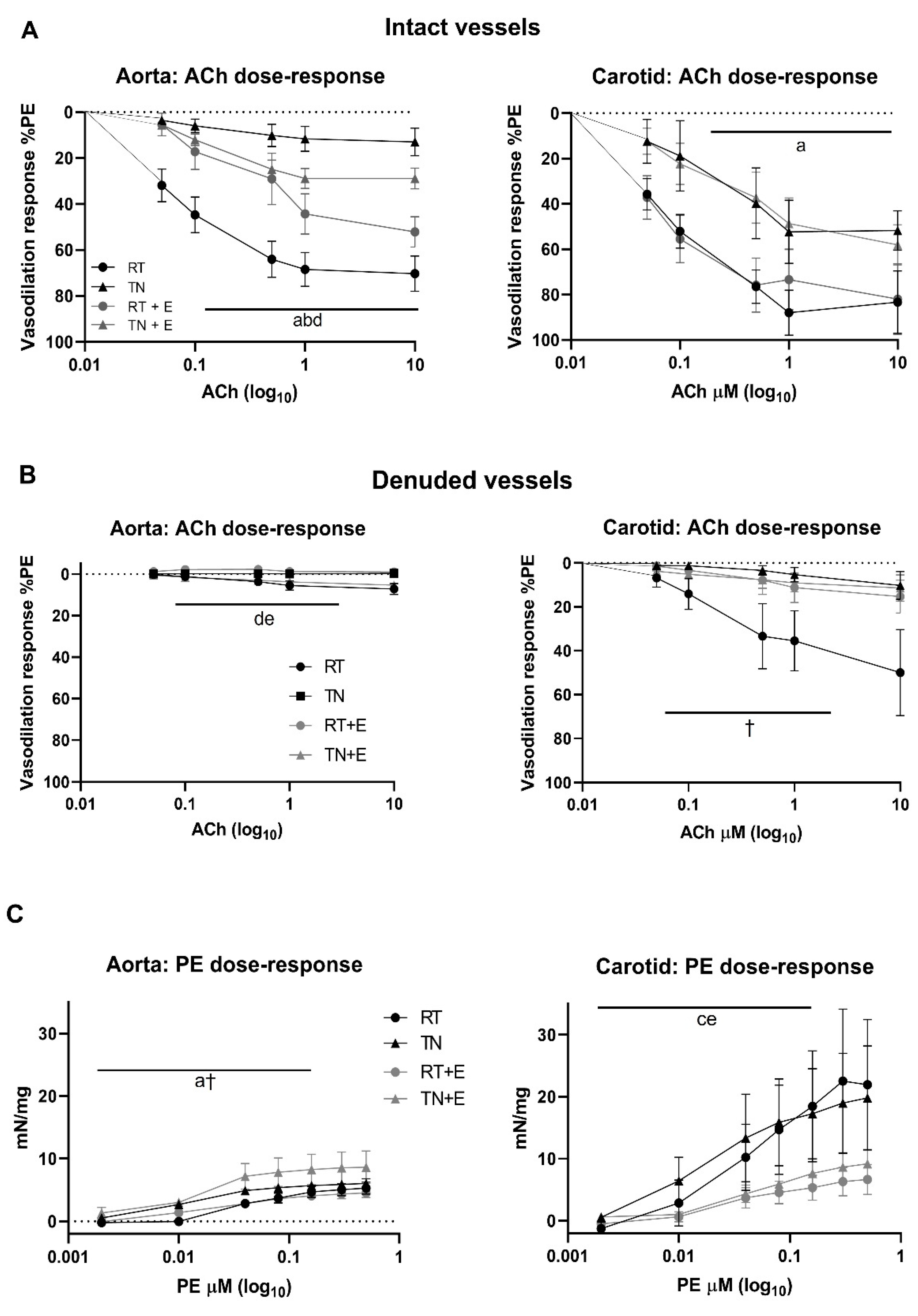
3.2. EPICAT Restored TN-Induced Vasodilation Impairment in Aortae
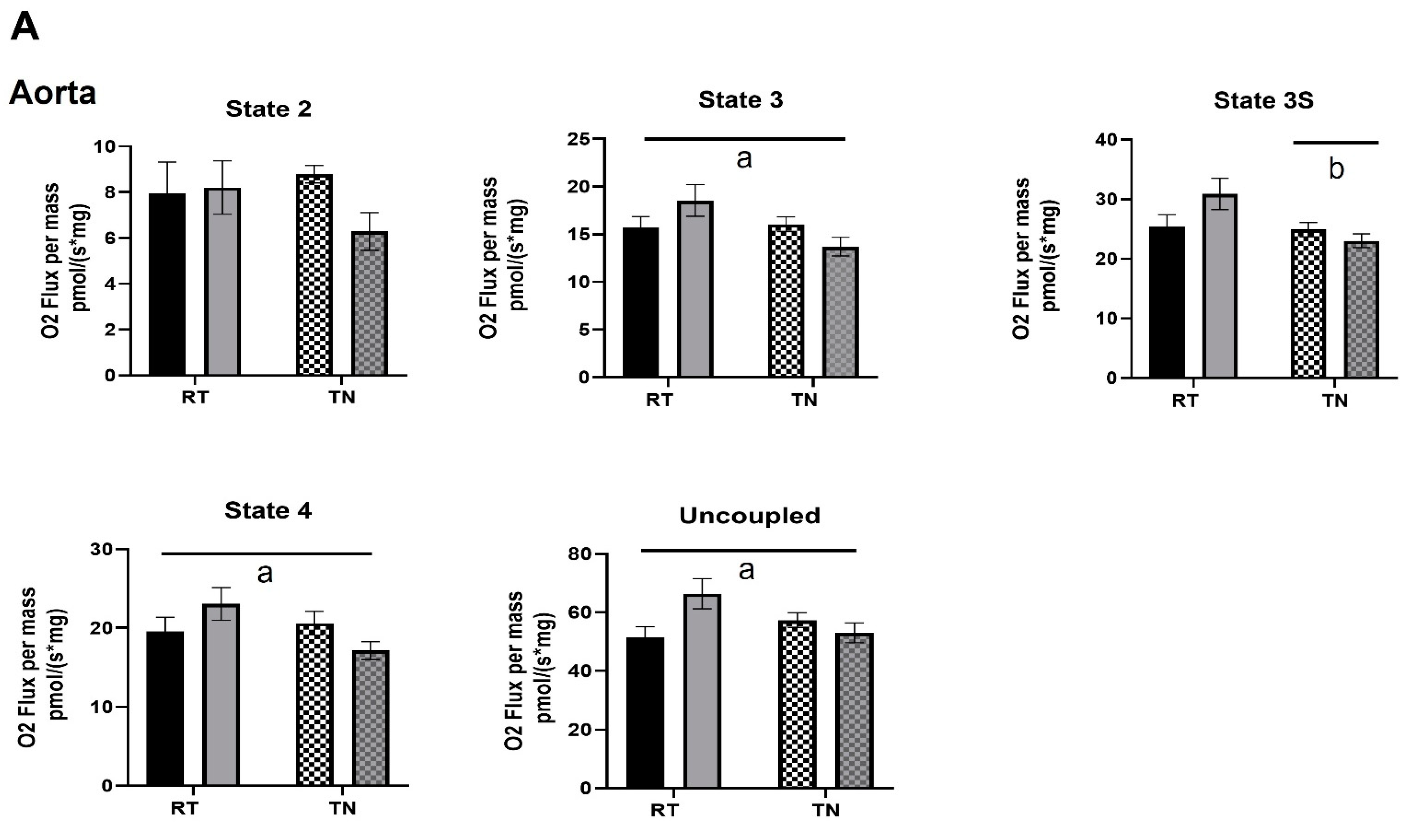
3.3. EPICAT Modulates Mitochondrial Respiration in Aortae and Carotids
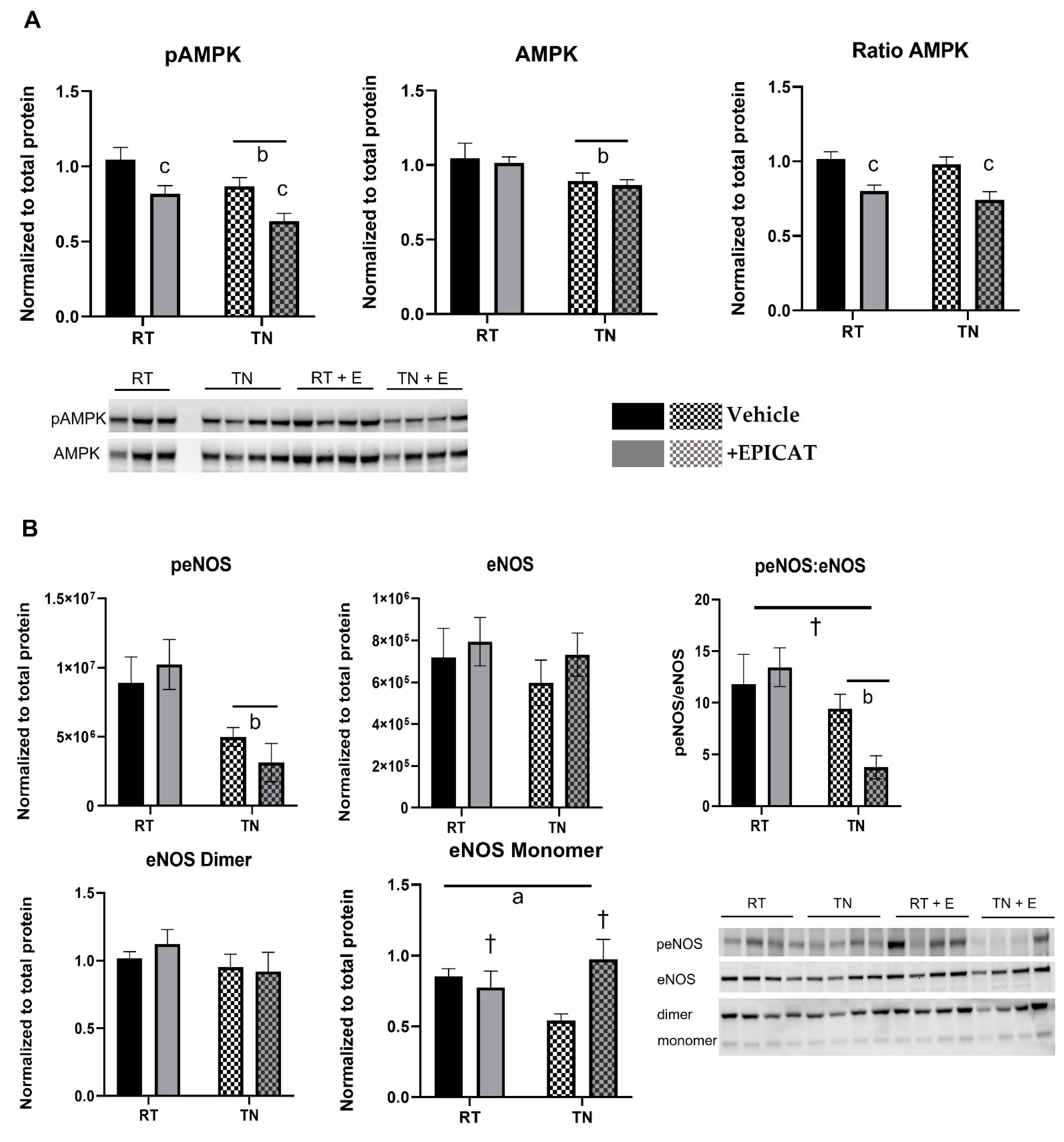
3.4. EPICAT and Housing Temperature Dampen AMPK Expression and Modulate eNOS Expression
3.5. EPICAT Treatment Modulates CaMKII Expression
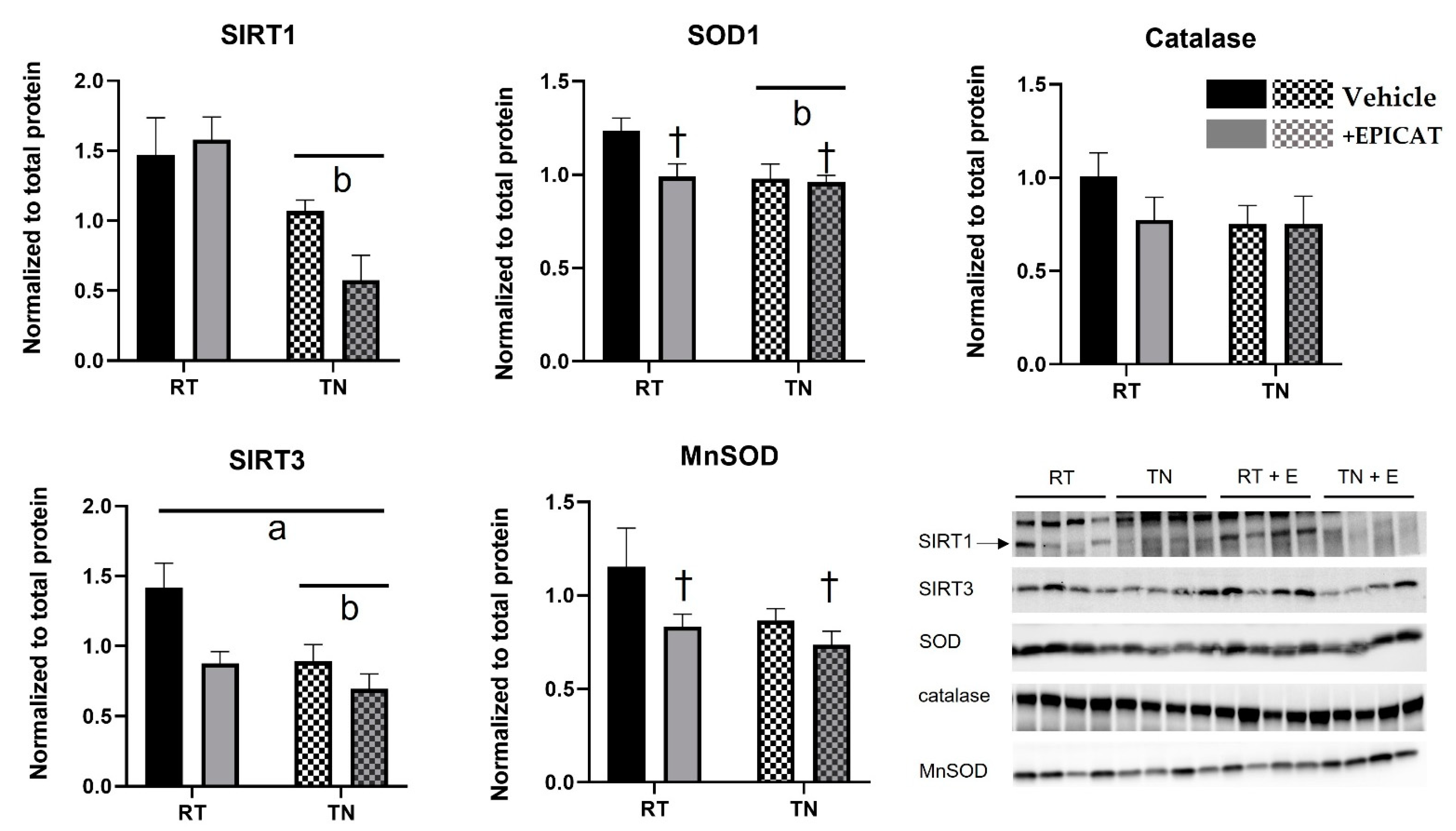
3.6. Both TN Housing and EPICAT Treatment Modify Broad and Local Endogenous Antioxidant Defenses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The National Heart, Lung, and Blood Institute. Know the Difference Fact Sheet. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/know-differences-cardiovascular-disease-heart-disease-coronary-heart-disease (accessed on 14 April 2021).
- Gibbons, G.H.; Dzau, V.J. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994, 19, 1431–1438. [Google Scholar]
- Kizhakekuttu, T.J.; Wang, J.; Dharmashankar, K.; Ying, R.; Gutterman, D.D.; Vita, J.A.; Widlansky, M.E.l. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2531–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S.; Alivon, M.; Beaussier, H.; Boutouyrie, P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Ann. Med. 2012, 44 (Suppl. 1), S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Knaub, L.A.; Olivera-Fragoso, L.F.; Keller, A.C.; Balasubramaniam, V.; Watson, P.A.; Reusch, J.E.-B. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1624–H1633. [Google Scholar] [CrossRef] [Green Version]
- Nisoli, E.; Clementi, E.; Paolucci, C.; Cozzi, V.; Tonello, C.; Sciorati, C.; Bracale, R.; Valerio, A.; Francolini, M.; Moncada, S.; et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science 2003, 299, 896–899. [Google Scholar] [CrossRef]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef]
- Salabei, J.K.; Hill, B.G. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol. 2013, 1, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Sward, K.; Dreja, K.; Lindqvist, A.; Persson, E.; Hellstrand, P. Influence of mitochondrial inhibition on global and local [Ca2+](I) in rat tail artery. Circ. Res. 2002, 90, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Taggart, M.J.; Wray, S. Hypoxia and smooth muscle function: Key regulatory events during metabolic stress. J. Physiol. 1998, 509 Pt 2, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Hull, S.E.; Elajaili, H.; Johnston, A.; Knaub, L.A.; Chun, J.H.; Walker, L.A.; Nozik-Grayck, E.; Reusch, J.E.-B. (–)-Epicatechin Modulates Mitochondrial Redox in Vascular Cell Models of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 6392629. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Bernatova, I.; Puzserova, A.; Balis, P.; Sestakova, N.; Pechanova, O.; Fraga, C.G. (–)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life 2013, 65, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Tamura, Y.; Lanaspa, M.A.; Miyazaki, M.; Suzuki, N.; Sato, W.; Maeshima, Y.; Schreiner, G.F.; Villarreal, F.; Johnson, R.J.; et al. Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am. J. Physiol. Ren. Physiol. 2012, 303, F1264–F1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litterio, M.C.; Vazquez Prieto, M.A.; Adamo, A.M.; Elesgaray, R.; Oteiza, P.I.; Galleano, M.; Fraga, C.G. (–)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015, 26, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (–)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef] [Green Version]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Perkins, G.; Murphy, A.N.; Naviaux, R.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: Effects of epicatechin rich cocoa. Clin. Transl. Sci. 2012, 5, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (–)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 2010, 55, 1398–1405. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Aguilar, H.; Ceballos, G.; Villarreal, F. (–)-epicatechin-induced calcium independent eNOS activation: Roles of HSP90 and AKT. Mol. Cell. Biochem. 2012, 370, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Ulloa, A.; Cid, A.; Rubio-Gayosso, I.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (–)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorg. Med. Chem. Lett. 2013, 23, 4441–4446. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, K.G.; Adreyev, A.Y.; Ortiz-Vilchis, P.; Petrosyan, S.; Divakaruni, A.S.; Wiley, S.E.; De La Fuente, C.; Perkins, G.; Ceballos, G.; Villarreal, F.; et al. Intravenous (–)-epicatechin reduces myocardial ischemic injury by protecting mitochondrial function. Int. J. Cardiol. 2014, 175, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, S.; Stephenson, J.D. Body temperature regulation and thermoneutrality in rats. Q. J. Exp. Physiol. Cogn. Med. Sci. 1977, 62, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingma, B.R.; Frijns, A.J.; Schellen, L.; van Marken Lichtenbelt, W.D. Beyond the classic thermoneutral zone: Including thermal comfort. Temperature 2014, 1, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol. Metab. 2018, 7, 161–170. [Google Scholar] [CrossRef]
- Overton, J.M. Phenotyping small animals as models for the human metabolic syndrome: Thermoneutrality matters. Int. J. Obes. 2010, 34 (Suppl. 2), S53–S58. [Google Scholar] [CrossRef] [Green Version]
- Swoap, S.J.; Overton, J.M.; Garber, G. Effect of ambient temperature on cardiovascular parameters in rats and mice: A comparative approach. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R391–R396. [Google Scholar] [CrossRef] [Green Version]
- Maloney, S.K.; Fuller, A.; Mitchell, D.; Gordon, C.; Overton, J.M. Translating animal model research: Does it matter that our rodents are cold? Physiology 2014, 29, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.C.; Knaub, L.A.; McClatchey, P.M.; Connon, C.A.; Bouchard, R.; Miller, M.W.; Geary, K.E.; Walker, L.A.; Klemm, D.J.; Reusch, J.E.-B. Differential mitochondrial adaptation in primary vascular smooth muscle cells from a diabetic rat model. Oxidative Med. Cell. Longev. 2016, 2016, 8524267. [Google Scholar] [CrossRef] [Green Version]
- Babu, G.J.; Pyne, G.J.; Zhou, Y.; Okwuchukuasanya, C.; Brayden, J.E.; Osol, G.; Paul, R.J.; Low, R.B.; Periasamy, M. Isoform switching from SM-B to SM-A myosin results in decreased contractility and altered expression of thin filament regulatory proteins. Am. J. Physiol.-Cell Physiol. 2004, 287, C723–C729. [Google Scholar] [CrossRef]
- Sutliff, R.L.; Paul, R.J. Smooth muscle studies using gene-altered mouse models: A users guide. In Cardiovascular Physiology in the Genetically Engineered Mouse; Hoit, B.D., Walsh, R.A., Eds.; Kluwer: Boston, MA, USA, 1998. [Google Scholar]
- Walker, L.A.; Gailly, P.; Jensen, P.E.; Somlyo, A.V.; Somlyo, A.P. The unimportance of being (protein kinase C) epsilon. FASEB J. 1998, 12, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.C.; Knaub, L.A.; Scalzo, R.L.; Hull, S.E.; Johnston, A.E.; Walker, L.A.; Reusch, J.E.-B. Sepiapterin Improves Vascular Reactivity and Insulin-Stimulated Glucose in Wistar Rats. Oxid. Med. Cell. Longev. 2018, 2018, 7363485. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Flavahan, S.; Flavahan, N.A. Potential pitfalls in analyzing structural uncoupling of eNOS: Aging is not associated with increased enzyme monomerization. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H80–H88. [Google Scholar] [CrossRef] [PubMed]
- Garate-Carrillo, A.; Navarrete-Yanez, V.; Ortiz-Vilchis, P.; Guevara, G.; Castillo, C.; Mendoza-Lorenzo, P.; Ceballos, G.; Ortiz-Flores, M.; Najera, N.; Bustamante-Pozo, M.M.; et al. Arginase inhibition by (–)-Epicatechin reverses endothelial cell aging. Eur. J. Pharmacol. 2020, 885, 173442. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Savickas, A.; Vetchy, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct effects of (–)-epicatechin and procyanidin B2 on the respiration of rat heart mitochondria. Biomed. Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef] [Green Version]
- Nichols, M.; Zhang, J.; Polster, B.M.; Elustondo, P.A.; Thirumaran, A.; Pavlov, E.V.; Robertson, G.S. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 2015, 308, 75–94. [Google Scholar] [CrossRef]
- Li, C.; Reif, M.M.; Craige, S.M.; Kant, S.; Keaney, J.F., Jr. Endothelial AMPK activation induces mitochondrial biogenesis and stress adaptation via eNOS-dependent mTORC1 signaling. Nitric Oxide 2016, 55–56, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Lee, I. Regulation of Cytochrome c Oxidase by Natural Compounds Resveratrol, (–)-Epicatechin, and Betaine. Cells 2021, 10, 1346. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (–)-Epicatechin induces calcium and translocation independent eNOS activation in arterial endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C880–C887. [Google Scholar] [CrossRef] [Green Version]
- Proshkina, E.; Lashmanova, E.; Dobrovolskaya, E.; Zemskaya, N.; Kudryavtseva, A.; Shaposhnikov, M.; Moscalev, A. Geroprotective and Radioprotective Activity of Quercetin, (–)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front. Pharmacol. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Salmean, G.; Ortiz-Vilchis, P.; Vacaseydel, C.M.; Garduno-Siciliano, L.; Chamorro-Cevallos, G.; Meaney, E.; Villafana, S.; Villarreal, F.; Ceballos, G.; Ramirez-Sanchez, I. Effects of (–)-epicatechin on a diet-induced rat model of cardiometabolic risk factors. Eur. J. Pharmacol. 2014, 728, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; De los Santos, S.; Gonzalez-Basurto, S.; Canto, P.; Mendoza-Lorenzo, P.; Palma-Flores, C.; Ceballos-Reyes, G.; Villarreal, F.; Zentalla-Dehesa, A.; Coral-Vazquez, R.; et al. (–)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic delta-sarcoglycan null mouse striated muscle. FEBS J. 2014, 281, 5567–5580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, K.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. 2008, 32, 1289–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]



| 1 Week | ||||
| Housing | RT | TN | ||
| Weight (g) a,† | 136.7 ± 3.5 | 121.2 ± 1.8 | ||
| Glucose d,e (mg/dL) | 85.9 ± 1.7 | 85.4 ± 2.3 | ||
| Insulin a,b,c (µg/mL) | 0.671 ± 0.084 | 0.611 ± 0.113 | ||
| 16 Week | ||||
| Housing | RT | TN | ||
| Treatment | Vehicle | +EPICAT | Vehicle | +EPICAT |
| Weight (g) a,† | 571.0 ± 13.2 | 571.6 ± 22.6 | 508.3 ± 23.7 | 557.6 ± 23.7 |
| Glucose d,e (mg/dL) | 70.4 ± 2.6 | 66.8 ± 1.9 | 67.2 ± 2.3 | 70.9 ± 3.7 |
| Insulin a,b,c (µg/mL) | 1.142 ± 0.173 | 2.094 ± 0.186 | 0.886 ± 0.236 | 1.035 ± 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.H.; Henckel, M.M.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Walker, L.A.; Reusch, J.E.-B.; Keller, A.C. (–)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature. Nutrients 2022, 14, 1097. https://doi.org/10.3390/nu14051097
Chun JH, Henckel MM, Knaub LA, Hull SE, Pott GB, Walker LA, Reusch JE-B, Keller AC. (–)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature. Nutrients. 2022; 14(5):1097. https://doi.org/10.3390/nu14051097
Chicago/Turabian StyleChun, Ji Hye, Melissa M. Henckel, Leslie A. Knaub, Sara E. Hull, Greg B. Pott, Lori A. Walker, Jane E.-B. Reusch, and Amy C. Keller. 2022. "(–)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature" Nutrients 14, no. 5: 1097. https://doi.org/10.3390/nu14051097
APA StyleChun, J. H., Henckel, M. M., Knaub, L. A., Hull, S. E., Pott, G. B., Walker, L. A., Reusch, J. E.-B., & Keller, A. C. (2022). (–)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature. Nutrients, 14(5), 1097. https://doi.org/10.3390/nu14051097