How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes?
Abstract
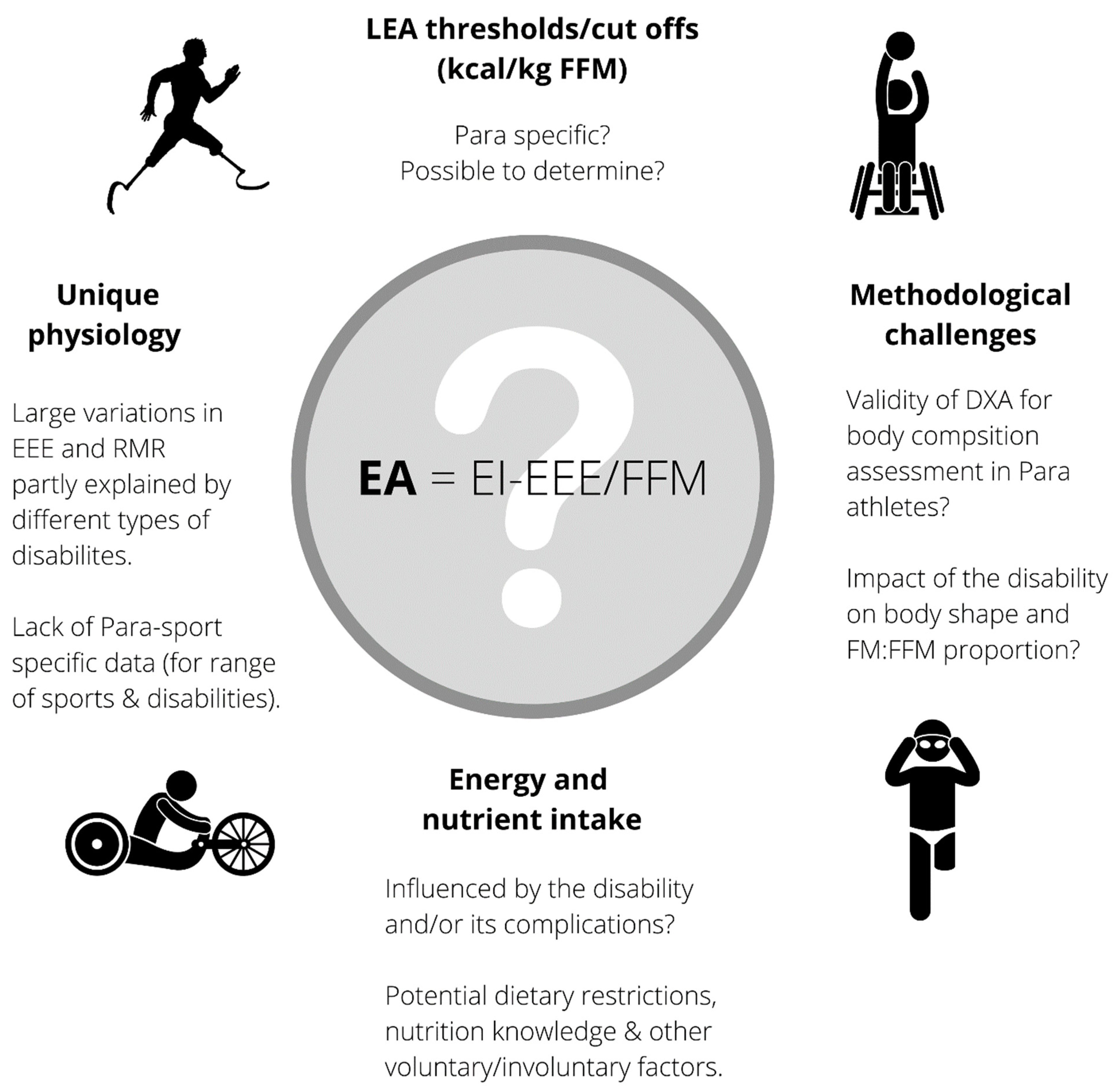
:1. Introduction
2. Energy Availability
2.1. Total Energy Intake
2.2. Exercise Energy Expenditure
2.3. Fat-Free Mass
3. Other Factors of RED-S
3.1. Questionnaires
3.2. Body Image and Eating Disorders
3.3. Bone Health
3.4. Resting Metabolic Rate
3.5. Hormones
4. How Can We Assess LEA and RED-S in the Future?
- Support the individual athlete by embedding experienced sports nutrition, medical and exercise science practitioners into Para sports programs. These practitioners require time to develop a deep understanding of each athlete, their impairment, sport and medical history. This will improve their ability to observe factors that may raise ‘red flags’ and understand the best assessment procedures to implement. Where feasible, Para athletes should be screened early on in their sporting careers for factors such as menstrual (dys)function (female), hormonal status, BMD and body composition, dietary intake and eating behavior, and RMR measured. If concerns regarding LEA/RED-S arise, having comparative data over time will aid in the assessment relative to the current issues and the impairment, sport and medical history of the athlete. The importance of having skilled practitioners in Para sport programs cannot be overstated, since these individuals will be the first to identify a problem. Other factors to consider are potential changes in mood state, performance, immune function, sleep, training capacity, recovery and general well-being.
- Connect research institutions and funding sources with Para sports, athletes and practitioners to create opportunities for the collection, analyses and publishing of more physiological data on Para athletes. This includes, but is not limited to:
- (a)
- The expansion of EEE measurements across all Para sports and disciplines in a meaningful and comparative manner. For example, more research needs to be reported in kcal/kg/h rather than just watts or kcal/kJ.
- (b)
- Assessment of dietary intake of Para athletes in free-living situations combined with assessments of measured energy expenditure (either via DLW, or a combination of RMR and EEE measures using indirect calorimetry).
- (c)
- Best-practice protocols for BMD assessments and normative ranges in Para athletes with different disabilities.
- (d)
- Identify factors associated with negative body image and disordered eating behavior that are specific to Para athletes, including tools and programs to improve them.
- (e)
- Incidence, cause and potential treatment of disrupted reproductive hormones in male and female Para athletes.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patricios, J.; Webborn, N. Prioritising Para athlete care. Br. J. Sports Med. 2021, 55, 529–530. [Google Scholar] [CrossRef]
- Madden, R.F.; Shearer, J.; Parnell, J.A. Evaluation of Dietary Intakes and Supplement Use in Paralympic Athletes. Nutrients 2017, 9, 1266. [Google Scholar]
- Scaramella, J.; Kirihennedige, N.; Broad, E. Key Nutritional Strategies to Optimize Performance in Para Athletes. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 283–298. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Blauwet, C.A.; Brook, E.M.; Tenforde, A.S.; Broad, E.; Hu, C.H.; Abdu-Glass, E.; Matzkin, E.G. Low Energy Availability, Menstrual Dysfunction, and Low Bone Mineral Density in Individuals with a Disability: Implications for the Para Athlete Population. Sports Med. 2017, 47, 1697–1708. [Google Scholar] [CrossRef]
- Taleporos, G.; McCabe, M.P. Body image and physical disability—Personal perspectives. Soc. Sci. Med. 2002, 54, 971–980. [Google Scholar] [CrossRef]
- Deguchi, M.; Yokoyama, H.; Hongu, N.; Watanabe, H.; Ogita, A.; Imai, D.; Suzuki, Y.; Okazaki, K. Eating Perception, Nutrition Knowledge and Body Image among Para-Athletes: Practical Challenges in Nutritional Support. Nutrients 2021, 13, 3120. [Google Scholar] [CrossRef]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand. J. Med. Sci. Sports 2019, 29, 678–685. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Szollar, S.M.; Martin, E.M.; Sartoris, D.J.; Parthemore, J.G.; Deftos, L.J. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am. J. Phys. Med. Rehabil. 1998, 77, 28–35. [Google Scholar] [CrossRef]
- Gerrish, H.R.; Broad, E.; Lacroix, M.; Ogan, D.; Pritchett, R.C.; Pritchett, K. Nutrient Intake of Elite Canadian and American Athletes with Spinal Cord Injury. Int. J. Exerc. Sci. 2017, 10, 1018–1028. [Google Scholar]
- Goosey-Tolfrey, V.L.; Crosland, J. Nutritional practices of competitive British wheelchair games players. Adapt. Phys. Activ. Q. 2010, 27, 47–59. [Google Scholar] [CrossRef]
- Krempien, J.L.; Barr, S.I. Risk of nutrient inadequacies in elite Canadian athletes with spinal cord injury. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 417–425. [Google Scholar] [CrossRef]
- Egger, T.; Flueck, J.L. Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutrients 2020, 12, 3262. [Google Scholar] [CrossRef]
- Pritchett, K.; DiFolco, A.; Glasgow, S.; Pritchett, R.; Williams, K.; Stellingwerff, T.; Roney, P.; Scaroni, S.; Broad, E. Risk of Low Energy Availability in National and International Level Paralympic Athletes: An Exploratory Investigation. Nutrients 2021, 13, 979. [Google Scholar] [CrossRef]
- Gee, C.M.; Lacroix, M.A.; Stellingwerff, T.; Gavel, E.H.; Logan-Sprenger, H.M.; West, C.R. Physiological Considerations to Support Podium Performance in Para-Athletes. Front. Rehabil. Sci. 2021, 2, 84. [Google Scholar] [CrossRef]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.L.; De Souza, M.J.; Wagstaff, D.A.; Williams, N.I. Menstrual Disruption with Exercise Is Not Linked to an Energy Availability Threshold. Med. Sci. Sports Exerc. 2018, 50, 551–561. [Google Scholar] [CrossRef]
- Ida, A.H.; Arja, L.T.U.; Trent, S.; Dan, B.; Antti, A.M.; Louise, M.B. Low Energy Availability Is Difficult to Assess but Outcomes Have Large Impact on Bone Injury Rates in Elite Distance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar]
- Burke, L.M.; Lundy, B.; Fahrenholtz, I.L.; Melin, A.K. Pitfalls of Conducting and Interpreting Estimates of Energy Availability in Free-Living Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 350–363. [Google Scholar] [CrossRef]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef] [Green Version]
- O’Driscoll, R.; Turicchi, J.; Beaulieu, K.; Scott, S.; Matu, J.; Deighton, K.; Finlayson, G.; Stubbs, J. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br. J. Sports Med. 2020, 54, 332–340. [Google Scholar]
- Braakhuis, A.J.; Meredith, K.; Cox, G.R.; Hopkins, W.G.; Burke, L.M. Variability in estimation of self-reported dietary intake data from elite athletes resulting from coding by different sports dietitians. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 152–165. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47 (Suppl. 1), 51–63. [Google Scholar] [CrossRef] [Green Version]
- Edwards, T.; Barfield, J.P.; Niemiro, G.M.; Beals, J.W.; Broad, E.M.; Motl, R.W.; De Lisio, M.; Burd, N.A.; Pilutti, L.A. Physiological responses during a 25-km time trial in elite wheelchair racing athletes. Spinal Cord Ser. Cases 2018, 4, 77. [Google Scholar] [CrossRef]
- Broad, E.; Juzwiak, C. Sports Nutrition in Para Athletes: Determining Energy Requirements. Sports Med. 2018, 170–175. [Google Scholar]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef]
- Ndahimana, D.; Kim, E.-K. Measurement methods for physical activity and energy expenditure: A review. Clin. Nutr. Res. 2017, 6, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Hajj-Boutros, G.; Landry-Duval, M.A.; Comtois, A.S.; Gouspillou, G.; Karelis, A.D. Wrist-worn devices for the measurement of heart rate and energy expenditure: A validation study for the Apple Watch 6, Polar Vantage V and Fitbit Sense. Eur. J. Sport Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Nightingale, T.E.; Rouse, P.C.; Thompson, D.; Bilzon, J.L.J. Measurement of Physical Activity and Energy Expenditure in Wheelchair Users: Methods, Considerations and Future Directions. Sports Med. Open 2017, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Conger, S.A.; Bassett, D.R. A compendium of energy costs of physical activities for individuals who use manual wheelchairs. Adapt. Phys. Activ. Q. 2011, 28, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.H.; Meyers, M.C. Exercise performance of lower-extremity amputees. Sports Med. 1995, 20, 207–214. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Schwabacher, I.J.; Kim, Y.; Welk, G.J.; Cook, D.B. Validity of an Integrative Method for Processing Physical Activity Data. Med. Sci. Sports Exerc. 2016, 48, 1629–1638. [Google Scholar] [CrossRef]
- Flueck, J.L. Body Composition in Swiss Elite Wheelchair Athletes. Front Nutr. 2020, 7, 1. [Google Scholar] [CrossRef]
- Goosey-Tolfrey, V.; Keil, M.; Brooke-Wavell, K.; de Groot, S. A Comparison of Methods for the Estimation of Body Composition in Highly Trained Wheelchair Games Players. Int. J. Sports Med. 2016, 37, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjödin, A.; Sundgot-Borgen, J. The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014, 48, 540–545. [Google Scholar] [CrossRef]
- Rogers, M.A.; Drew, M.K.; Appaneal, R.; Lovell, G.; Lundy, B.; Hughes, D.; Vlahovich, N.; Waddington, G.; Burke, L.M. The Utility of the Low Energy Availability in Females Questionnaire to Detect Markers Consistent With Low Energy Availability-Related Conditions in a Mixed-Sport Cohort. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 427–437. [Google Scholar] [CrossRef]
- Deans, S.; Burns, D.; McGarry, A.; Murray, K.; Mutrie, N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: A review of the literature. Prosthet. Orthot. Int. 2012, 36, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, A.V.; Edwardson, C.L.; Dawkins, N.P.; Maylor, B.D.; Metcalf, K.M.; Janz, K.F. Physical Activity for Bone Health: How Much and/or How Hard? Med. Sci. Sports Exerc. 2020, 52, 2331–2341. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Daniels, J.A.; Adler, R.A.; Moore, J.R.; Gater, D.R., Jr. The effects of spinal cord injury and exercise on bone mass: A literature review. NeuroRehabilitation 2011, 29, 261–269. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Brook, E.M.; Broad, E.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Braun, P.W.; Blauwet, C.A. Prevalence and Anatomical Distribution of Bone Stress Injuries in the Elite Para Athlete. Am. J. Phys. Med. Rehabil. 2019, 98, 1036–1040. [Google Scholar] [CrossRef]
- Morse, L.R.; Biering-Soerensen, F.; Carbone, L.D.; Cervinka, T.; Cirnigliaro, C.M.; Johnston, T.E.; Liu, N.; Troy, K.L.; Weaver, F.M.; Shuhart, C.; et al. Bone Mineral Density Testing in Spinal Cord Injury: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 554–566. [Google Scholar] [CrossRef]
- Abdelrahman, S.; Ireland, A.; Winter, E.M.; Purcell, M.; Coupaud, S. Osteoporosis after spinal cord injury: Aetiology, effects and therapeutic approaches. J. Musculoskelet. Neuronal Interact. 2021, 21, 26–50. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Broad, E.M.; Newsome, L.J.; Dew, D.A.; Barfield, J.P. Measured and predicted resting energy expenditure in wheelchair rugby athletes. J. Spinal Cord Med. 2020, 43, 388–397. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.J. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am. J. Clin. Nutr. 1980, 33, 2372–2374. [Google Scholar] [CrossRef]
- Pelly, F.E.; Broad, E.M.; Stuart, N.; Holmes, M.A. Resting energy expenditure in male athletes with a spinal cord injury. J. Spinal Cord Med. 2018, 41, 208–215. [Google Scholar] [CrossRef]
- Slater, G.; Goosey-Tolfrey, V. Assessing body composition of athletes. In Sports Nutrition for Paralympic Athletes; CRC Press: Boca Raton, FL, USA, 2019; pp. 245–264. [Google Scholar]
- Chun, S.M.; Kim, H.R.; Shin, H.I. Estimating the Basal metabolic rate from fat free mass in individuals with motor complete spinal cord injury. Spinal Cord 2017, 55, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, T.E.; Gorgey, A.S. Predicting Basal Metabolic Rate in Men with Motor Complete Spinal Cord Injury. Med. Sci. Sports Exerc. 2018, 50, 1305–1312. [Google Scholar] [CrossRef]
- Juzwiak, C.R.; Winckler, C.; Joaquim, D.P.; Silva, A.; de Mello, M.T. Comparison of Measured and Predictive Values of Basal Metabolic Rate in Brazilian Paralympic Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 330–337. [Google Scholar] [CrossRef]
- Owen, O.E.; Holup, J.L.; D’Alessio, D.A.; Craig, E.S.; Polansky, M.; Smalley, K.J.; Kavle, E.C.; Bushman, M.C.; Owen, L.R.; Mozzoli, M.A. A reappraisal of the caloric requirements of men. Am. J. Clin. Nutr. 1987, 46, 875–885. [Google Scholar] [CrossRef]
- Owen, O.E.; Kavle, E.; Owen, R.S.; Polansky, M.; Caprio, S.; Mozzoli, M.A.; Kendrick, Z.V.; Bushman, M.C.; Boden, G. A reappraisal of caloric requirements in healthy women. Am. J. Clin. Nutr. 1986, 44, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- De Souza, M.J.; Hontscharuk, R.; Olmsted, M.; Kerr, G.; Williams, N.I. Drive for thinness score is a proxy indicator of energy deficiency in exercising women. Appetite 2007, 48, 359–367. [Google Scholar] [CrossRef]
- Joy, E.; De Souza, M.J.; Nattiv, A.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr. Sports Med. Rep. 2014, 13, 219–232. [Google Scholar] [CrossRef]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. The Role of Energy Availability in Reproductive Function in the Female Athlete Triad and Extension of its Effects to Men: An Initial Working Model of a Similar Syndrome in Male Athletes. Sports Med. 2019, 49 (Suppl. 2), 125–137. [Google Scholar] [CrossRef] [Green Version]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.M.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [Green Version]
- Otzel, D.M.; Lee, J.; Ye, F.; Borst, S.E.; Yarrow, J.F. Activity-Based Physical Rehabilitation with Adjuvant Testosterone to Promote Neuromuscular Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1701. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonvik, K.L.; Vardardottir, B.; Broad, E. How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes? Nutrients 2022, 14, 1068. https://doi.org/10.3390/nu14051068
Jonvik KL, Vardardottir B, Broad E. How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes? Nutrients. 2022; 14(5):1068. https://doi.org/10.3390/nu14051068
Chicago/Turabian StyleJonvik, Kristin L., Birna Vardardottir, and Elizabeth Broad. 2022. "How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes?" Nutrients 14, no. 5: 1068. https://doi.org/10.3390/nu14051068
APA StyleJonvik, K. L., Vardardottir, B., & Broad, E. (2022). How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes? Nutrients, 14(5), 1068. https://doi.org/10.3390/nu14051068





