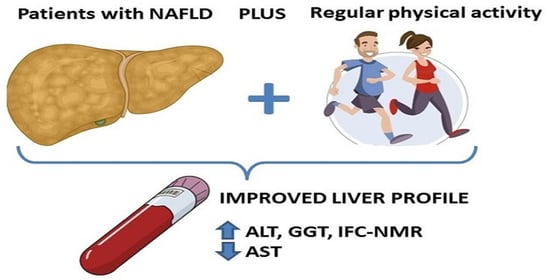
Association between Physical Activity and Non-Alcoholic Fatty Liver Disease in Adults with Metabolic Syndrome: The FLIPAN Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Ethics
2.2. Anthropometrics, Dietary Intake, and Fitness and Physical Activity
2.3. NAFLD Diagnosis
2.4. Blood Collections Analysis
2.5. Other Health Outcomes
2.6. Statistics
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Clinical Trials Registration
Abbreviations
| ALT | alanine aminotransaminase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CI | confidence interval |
| FFQ | food frequency questionnaire |
| FLI | fatty liver index |
| GGT | gamma-glutamyl transferase |
| HDL-cholesterol | high density lipoprotein cholesterol |
| HSI | hepatic steatosis index |
| IFC-NMR | intrahepatic fat content by nuclear magnetic resonance |
| IQR | interquartile range |
| MET | metabolic equivalent task of task |
| MetS | metabolic syndrome |
| MRI | magnetic resonance imaging |
| NAFLD | non-alcoholic fatty liver disease |
| OR | odds ratio |
| PA | physical activity |
| P50 | 50th percentile |
| SD | standard deviations |
References
- Cho, J.; Lee, I.; Park, D.H.; Kwak, H.B.; Min, K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2021, 18, 1892. [Google Scholar] [CrossRef] [PubMed]
- Tuyama, A.C.; Chang, C.Y. Non-alcoholic fatty liver disease. J. Diabetes 2012, 4, 266–280. [Google Scholar] [CrossRef]
- Orkin, S.; Brokamp, C.; Yodoshi, T.; Trout, A.T.; Liu, C.; Meryum, S.; Taylor, S.; Wolfe, C.; Sheridan, R.; Seth, A.; et al. Community Socioeconomic Deprivation and Nonalcoholic Fatty Liver Disease Severity. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Lee, K. Relationship between Handgrip Strength and Nonalcoholic Fatty Liver Disease: Nationwide Surveys. Metab. Syndr. Relat. Disord. 2018, 16, 497–503. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Cai, X.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Therap. Adv. Gastroenterol. 2017, 10, 701–713. [Google Scholar] [CrossRef]
- Medrano, M.; Arenaza, L.; Migueles, J.H.; Rodríguez-Vigil, B.; Ruiz, J.R.; Labayen, I. Associations of physical activity and fitness with hepatic steatosis, liver enzymes, and insulin resistance in children with overweight/obesity. Pediatr. Diabetes 2020, 21, 565–574. [Google Scholar] [CrossRef]
- Mascaró, C.M.; Bouzas, C.; Tur, J.A. Association between Non-Alcoholic Fatty Liver Disease and Mediterranean Lifestyle: A Systematic Review. Nutrients 2021, 14, 49. [Google Scholar] [CrossRef]
- Jeznach-Steinhagen, A.; Ostrowska, J.; Czerwonogrodzka-Senczyna, A.; Boniecka, I.; Shahnazaryan, U.; Kuryłowicz, A. Dietary and Pharmacological Treatment of Nonalcoholic Fatty Liver Disease. Medicina 2019, 55, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The International Diabetic Federation (IDF). The IDF Consensus Worldwide Definition of Definition of the Metabolic Syndrome. Available online: http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf (accessed on 22 January 2022).
- NCT04442620. Prevention and Reversion of NAFLD in Obese Patients with Metabolic Syndrome by Mediterranean Diet and Physical Activity (FLIPAN) [Internet]. ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04442620 (accessed on 20 January 2022).
- Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Gómez, C.; Ugarriza, L.; Tejada, S.; Abete, I.; Zulet, M.Á.; Sureda, A.; et al. Albuminuria Is Associated with Hepatic Iron Load in Patients with Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome. J. Clin. Med. 2021, 10, 3187. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Moreiras, O.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos (Spanish Food Composition Tables), 17th ed.; Pirámide: Madrid, Spain, 2015. [Google Scholar]
- Mataix-Verdú, J.; García-Diz, L.; Mañas-Almendros, M.; Martinez de Victoria, E.; Llopis-González, J. Tablas de Composición de Alimentos, 5th ed.; Universidad de Granada: Granada, Spain, 2013. [Google Scholar]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Investigators of the MARATDON Group. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef]
- Abbate, M.; Mascaró, C.M.; Montemayor, S.; Barbería-Latasa, M.; Casares, M.; Gómez, C.; Angullo-Martinez, E.; Tejada, S.; Abete, I.; Zulet, M.A.; et al. Energy Expenditure Improved Risk Factors Associated with Renal Function Loss in NAFLD and MetS Patients. Nutrients 2021, 13, 629. [Google Scholar] [CrossRef]
- Tur, J.A.; Serra-Majem, L.; Romaguera, D.; Pons, A. Profile of overweight and obese people in a Mediterranean region. Obes. Res. 2005, 13, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Bibiloni, M.D.M.; Karam, J.; Bouzas, C.; Aparicio-Ugarriza, R.; Pedrero-Chamizo, R.; Sureda, A.; González-Gross, M.; Tur, J.A. Association between Physical Condition and Body Composition, Nutrient Intake, Sociodemographic Characteristics, and Lifestyle Habits in Older Spanish Adults. Nutrients 2018, 10, 1608. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.K.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Xia, M.F.; Yan, H.M.; Lin, H.D.; Bian, H.; Pan, B.S.; Yao, X.Z.; Li, R.-K.; Zeng, M.S.; Gao, X. Elevation of liver enzymes within the normal limits and metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2011, 38, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013, 267, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konerman, M.A.; Walden, P.; Joseph, M.; Jackson, E.A.; Lok, A.S.; Rubenfire, M. Impact of a structured lifestyle programme on patients with metabolic syndrome complicated by non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2019, 49, 296–307. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.S.; Kim, D.; Chung, G.E.; Kim, W.; Kim, J.S. The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int. 2017, 37, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Villalobos, F.; Pedret, R.; Vinuesa, A.; Jovani, D.; Pascual, G.; Basora, J. Physical activity, cardiovascular health, quality of life and blood pressure control in hypertensive subjects: Randomized clinical trial. Health Qual. Life Outcomes 2018, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Carobene, A.; Braga, F.; Roraas, T.; Sandberg, S.; Bartlett, W.A. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin. Chem. Lab. Med. 2013, 51, 1997–2007. [Google Scholar] [CrossRef]
- Yu, A.S.; Keeffe, E.B. Elevated AST or ALT to nonalcoholic fatty liver disease: Accurate predictor of disease prevalence? Am. J. Gastroenterol. 2003, 98, 955–956. [Google Scholar] [CrossRef]
- Noakes, T.D. Effect of exercise on serum enzyme activities in humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copaci, I.; Lupescu, I.; Caceaune, E.; Chiriac, G.; Ismail, G. Noninvasive Markers of Improvement of Liver Steatosis Achieved by Weight Reduction in Patients with Nonalcoholic Fatty Liver Disease. Rom. J. Intern. Med. 2015, 53, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, B.; Schrauwen-Hinderling, V.B.; Jelenik, T.; Gemmink, A.; Sparks, L.M.; Havekes, B.; Bruls, Y.; Dahlmans, D.; Roden, M.; Hesselink, M.K.C.; et al. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E165–E173. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L.; et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016, 130, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo-Alfaro, L.; Bibiloni, M.D.M.; Mateos, D.; Ugarriza, L.; Tur, J.A. Leisure-Time Physical Activity and Metabolic Syndrome in Older Adults. Int. J. Environ. Res. Public Health 2019, 16, 3358. [Google Scholar] [CrossRef] [Green Version]
- Kühn, T.; Nonnenmacher, T.; Sookthai, D.; Schübel, R.; Quintana Pacheco, D.A.; von Stackelberg, O.; Graf, M.E.; Johnson, T.; Schlett, C.L.; Kirsten, R.; et al. Anthropometric and blood parameters for the prediction of NAFLD among overweight and obese adults. BMC Gastroenterol. 2018, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Cheung, B.; Yi, G.H.; Oh, B.; Oh, Y.H. Mobile health, physical activity, and obesity: Subanalysis of a randomized controlled trial. Medicine 2018, 97, e12309. [Google Scholar] [CrossRef]
- Brown, A.F.; Prado, C.M.; Ghosh, S.; Leonard, S.M.; Arciero, P.J.; Tucker, K.L.; Ormsbee, M.J. Higher-protein intake and physical activity are associated with healthier body composition and cardiometabolic health in Hispanic adults. Clin. Nutr. ESPEN 2019, 30, 145–151. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241, Erratum in N. Engl. J. Med. 2009, 361, 2681. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; O’Connor, L.E.; Zhou, J.; Campbell, W.W. Exercise patterns, ingestive behaviors, and energy balance. Physiol. Behav. 2014, 134, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.A.; Carels, R.A. Does when you eat and exercise matter? Differences in eating and physical activity patterns in overweight and obese adults. Eat. Weight. Disord. 2016, 21, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.C.; Liu, Y.; Rector, R.S.; Parks, E.J.; Ibdah, J.A.; Kanaley, J.A. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—A randomized trial. Metabolism 2018, 78, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.C.; Butler, G.; Garriguet, D.; Prince, S.A.; Roberts, K.C. Comparison of self-reported and accelerometer-measured physical activity in Canadian adults. Health Rep. 2018, 29, 3–15. [Google Scholar] [PubMed]
- Skender, S.; Ose, J.; Chang-Claude, J.; Paskow, M.; Brühmann, B.; Siegel, E.M.; Steindorf, K.; Ulrich, C.M. Accelerometry and physical activity questionnaires—a systematic review. BMC Public Health 2016, 16, 515. [Google Scholar] [CrossRef] [Green Version]
- Roldan-Valadez, E.; Favila, R.; Martínez-López, M.; Uribe, M.; Méndez-Sánchez, N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann. Hepatol. 2008, 7, 212–220. [Google Scholar] [CrossRef]

| Low PA | High PA | ||
|---|---|---|---|
| Median (IQR) | Median (IQR) | p Value | |
| Age (years) | 56.0 (10.0) | 52.0 (12.0) | <0.001 |
| Anthropometrics | |||
| Weight (Kg) | 93.8 (15.6) | 94.8 (19.2) | 0.034 |
| BMI (Kg/m2) | 32.5 (4.4) | 33.6 (5.5) | 0.094 |
| WC (cm) | 111.1 (14.4) | 112.6 (12.5) | 0.466 |
| HC (cm) | 110.9 (9.6) | 113.4 (14.2) | 0.006 |
| NC (cm) | 42.6 (3.4) | 40.7 (6.1) | 0.002 |
| Diet | |||
| Carbohydrates (g/day) | 248.4 (122.3) | 236.9 (131.5) | 0.693 |
| Protein (g/day) | 91.4 (36.6) | 113.6 (48.3) | 0.003 |
| Lipids (g/day) | 100.0 (42.4) | 93.9 (43.1) | 0.011 |
| Ingested calories (Kcal/day) | 2311.0 (960.6) | 2321.2 (1013.6) | 0.442 |
| Expended calories (Kcal/day) | 1700.2 (708.8) | 2515.3 (856.8) | <0.001 |
| Ingested–expended calories (Kcal/day) | 642.5 (1256.3) | −143.9 (1174.4) | <0.001 |
| Energy expenditure | |||
| Measured accelerometer (MET/day) | 1.8 (0.2) | 2.1 (0.4) | <0.001 |
| Reported Minnesota (MET/day) | 0.3 (0.4) | 0.4 (0.5) | <0.001 |
| Measured-reported (MET/day) | 1.5 (0.3) | 1.9 (0.5) | <0.001 |
| Education (years) | 15.0 (4.0) | 15.0 (6.0) | 0.607 |
| n (%) | n (%) | ||
| Gender | 0.002 | ||
| Female | 12 (30.8) | 19 (47.5) | |
| Socioeconomic status according job | 0.068 | ||
| Low | 20 (62.5) | 17 (77.3) | |
| Medium | 10 (31.2) | 4 (18.2) | |
| High | 2 (6.2) | 1 (4.5) | |
| Smoking habit ≥ 1 cigarette/day | 2 (5.1) | 7 (17.5) | 0.001 |
| Alcohol consumption ≥ 7 drinks/week | 5 (12.8) | 9 (22.5) | 0.048 |
| Low PA | High PA | ||
|---|---|---|---|
| Median (IQR) | Median (IQR) | p Value | |
| Liver profile | |||
| AST (U/L) | 24.0 (12.5) | 23.0 (8.0) | <0.001 |
| ALT (U/L) | 30.5 (29.5) | 24.0 (18.0) | <0.001 |
| GGT (U/L) | 33.0 (28.5) | 32.0 (25.0) | 0.001 |
| FLI | 91.0 (10.0) | 92.0 (10.7) | 0.457 |
| HSI | 45.3 (6.0) | 44.3 (7.9) | 0.958 |
| IFC-NMR (%) | 12.3 (12.1) | 10.3 (6.5) | <0.001 |
| n (%) | n (%) | ||
| NAFLD according to | |||
| AST > 40 U/L | 2 (5.4) | 3 (7.7) | 0.421 |
| ALT > 40 U/L | 15 (38.5) | 8 (20.0) | <0.001 |
| GGT > 50 U/L men; > 32 U/L women | 16 (41.0) | 13 (32.5) | 0.116 |
| FLI ≥ 60 | 38 (100.0) | 38 (95.0) | 0.005 |
| HSI > 36 | 35 (94.6) | 38 (97.4) | 0.203 |
| IFC-NMR > 6.4% | 33 (84.6) | 30 (75.0) | 0.033 |
| Metabolic syndrome parameters | |||
| Abdominal obesity (WC ≥ 94 cm men; ≥ 80 cm women) | 37 (94.9) | 39 (97.5) | 0.222 |
| High triglyceridemia ≥150 mg/dL | 27 (69.2) | 23 (57.5) | 0.031 |
| Low HDL-C <40 mg/dL men; <50 mg/dL women | 23 (59.0) | 25 (62.5) | 0.521 |
| Hypertension (systolic pressure ≥ 130 and/or diastolic pressure ≥ 85 mmHg) | 30 (78.9) | 26 (66.7) | 0.016 |
| High fasting glycemia ≥ 100 mg/dL | 25 (64.1) | 18 (45.0) | 0.001 |
| Low PA | High PA | |||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | p-Value | ||
| Abdominal obesity | Crude OR | 1.00 (ref.) | 2.11 (0.62–7.15) | 0.231 |
| OR Adjusted 1 | 1.00 (ref.) | 1.79 (0.48–6.68) | 0.387 | |
| OR Adjusted 2 | 1.00 (ref.) | 1.42 (0.38–5.28) | 0.601 | |
| High triglyceridemia | Crude OR | 1.00 (ref.) | 0.60 (0.38–0.96) | 0.031 |
| OR Adjusted 1 | 1.00 (ref.) | 0.48 (0.29–0.82) | 0.007 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.67 (0.37–1.19) | 0.172 | |
| Low HDL-C | Crude OR | 1.00 (ref.) | 1.16 (0.74–1.82) | 0.521 |
| OR Adjusted 1 | 1.00 (ref.) | 0.80 (0.48–1.32) | 0.374 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.76 (0.44–1.31) | 0.323 | |
| Hypertension | Crude OR | 1.00 (ref.) | 0.53 (0.32–0.89) | 0.016 |
| OR Adjusted 1 | 1.00 (ref.) | 0.75 (0.43–1.30) | 0.305 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.98 (0.52–1.85) | 0.957 | |
| High fasting glycemia | Crude OR | 1.00 (ref.) | 0.46 (0.29–0.72) | 0.001 |
| OR Adjusted 1 | 1.00 (ref.) | 0.55 (0.33–0.91) | 0.019 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.68 (0.39–1.17) | 0.161 |
| Low PA | High PA | |||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | p-Value | ||
| AST | Crude OR | 1.00 (ref.) | 1.46 (0.58–3.68) | 0.424 |
| OR Adjusted 1 | 1.00 (ref.) | 1.46 (0.56–3.78) | 0.439 | |
| OR Adjusted 2 | 1.00 (ref.) | 4.14 (1.26–13.58) | 0.019 | |
| OR Adjusted 3 | 1.00 (ref.) | 7.26 (1.79–29.40) | 0.005 | |
| ALT | Crude OR | 1.00 (ref.) | 0.40 (0.24–0.66) | <0.001 |
| OR Adjusted 1 | 1.00 (ref.) | 0.43 (0.26–0.73) | 0.002 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.24 (0.12–0.47) | <0.001 | |
| OR Adjusted 3 | 1.00 (ref.) | 0.24 (0.12–0.48) | <0.001 | |
| GGT | Crude OR | 1.00 (ref.) | 0.69 (0.44–1.10) | 0.117 |
| OR Adjusted 1 | 1.00 (ref.) | 0.76 (0.47–1.24) | 0.272 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.52 (0.29–0.94) | 0.031 | |
| OR Adjusted 3 | 1.00 (ref.) | 0.57 (0.31–1.04) | 0.066 | |
| HSI | Crude OR | 1.00 (ref.) | 2.17 (0.64–7.37) | 0.214 |
| OR Adjusted 1 | 1.00 (ref.) | 3.48 (0.95–12.80) | 0.061 | |
| OR Adjusted 2 | 1.00 (ref.) | 1.06 (0.29–3.93) | 0.927 | |
| OR Adjusted 3 | 1.00 (ref.) | 1.05 (0.15–7.15) | 0.963 | |
| IFC-NMR | Crude OR | 1.00 (ref.) | 0.55 (0.31–0.96) | 0.035 |
| OR Adjusted 1 | 1.00 (ref.) | 0.78 (0.42–1.42) | 0.412 | |
| OR Adjusted 2 | 1.00 (ref.) | 0.28 (0.14–0.55) | <0.001 | |
| OR Adjusted 3 | 1.00 (ref.) | 0.26 (0.12–0.56) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascaró, C.M.; Bouzas, C.; Montemayor, S.; Casares, M.; Gómez, C.; Ugarriza, L.; Borràs, P.-A.; Martínez, J.A.; Tur, J.A. Association between Physical Activity and Non-Alcoholic Fatty Liver Disease in Adults with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 1063. https://doi.org/10.3390/nu14051063
Mascaró CM, Bouzas C, Montemayor S, Casares M, Gómez C, Ugarriza L, Borràs P-A, Martínez JA, Tur JA. Association between Physical Activity and Non-Alcoholic Fatty Liver Disease in Adults with Metabolic Syndrome: The FLIPAN Study. Nutrients. 2022; 14(5):1063. https://doi.org/10.3390/nu14051063
Chicago/Turabian StyleMascaró, Catalina M., Cristina Bouzas, Sofia Montemayor, Miguel Casares, Cristina Gómez, Lucía Ugarriza, Pere-Antoni Borràs, José Alfredo Martínez, and Josep A. Tur. 2022. "Association between Physical Activity and Non-Alcoholic Fatty Liver Disease in Adults with Metabolic Syndrome: The FLIPAN Study" Nutrients 14, no. 5: 1063. https://doi.org/10.3390/nu14051063
APA StyleMascaró, C. M., Bouzas, C., Montemayor, S., Casares, M., Gómez, C., Ugarriza, L., Borràs, P.-A., Martínez, J. A., & Tur, J. A. (2022). Association between Physical Activity and Non-Alcoholic Fatty Liver Disease in Adults with Metabolic Syndrome: The FLIPAN Study. Nutrients, 14(5), 1063. https://doi.org/10.3390/nu14051063