The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. In Vitro Batch Fermentations
2.3. Genomic DNA Extraction
2.4. Quantification of Bacterial Load and 16S rRNA Sequencing
2.5. SCFA Analysis
2.6. Faecal Calprotectin and Water Content
2.7. Bioinformatics
2.8. Statistical Analysis
2.9. Ethical Considerations
3. Results
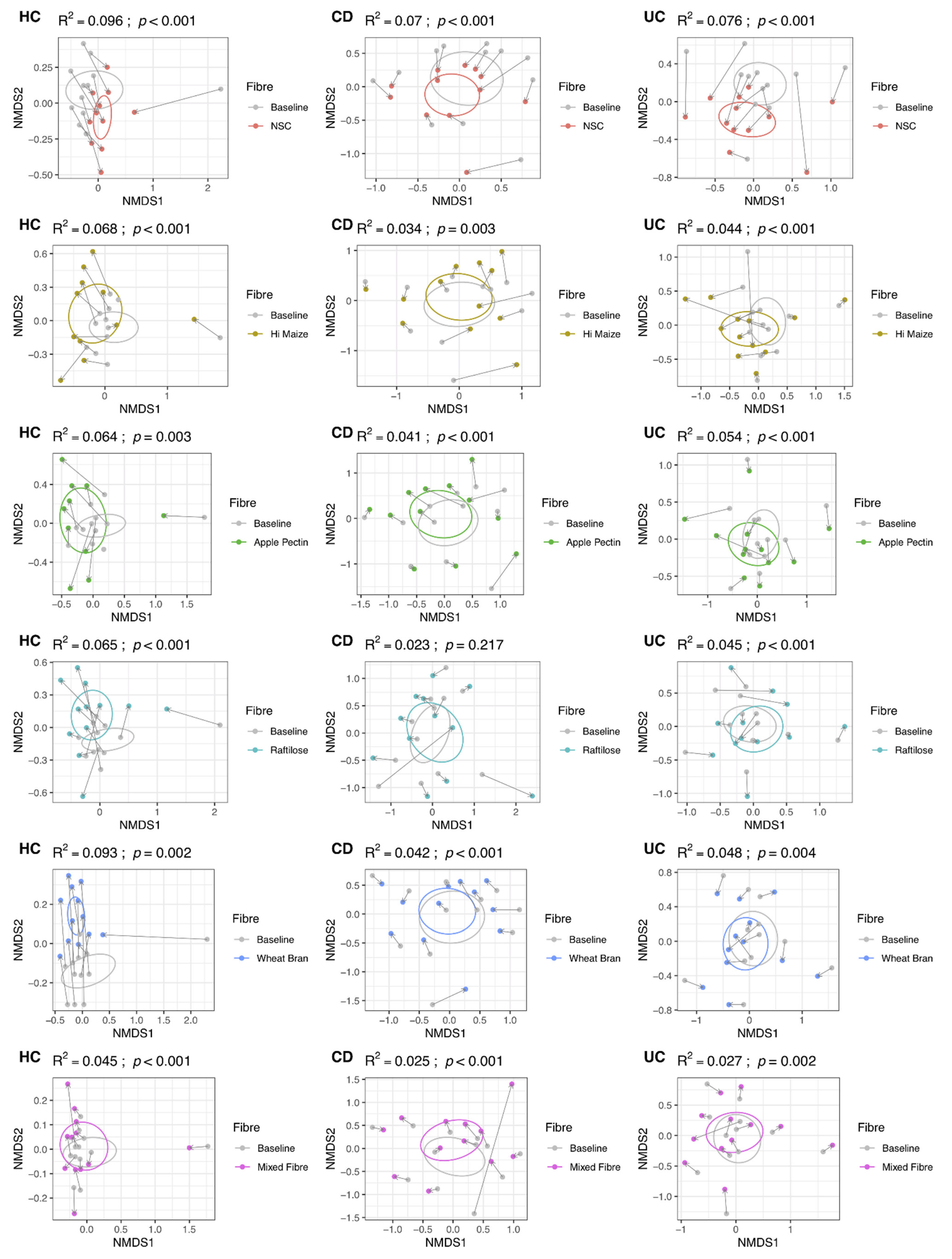
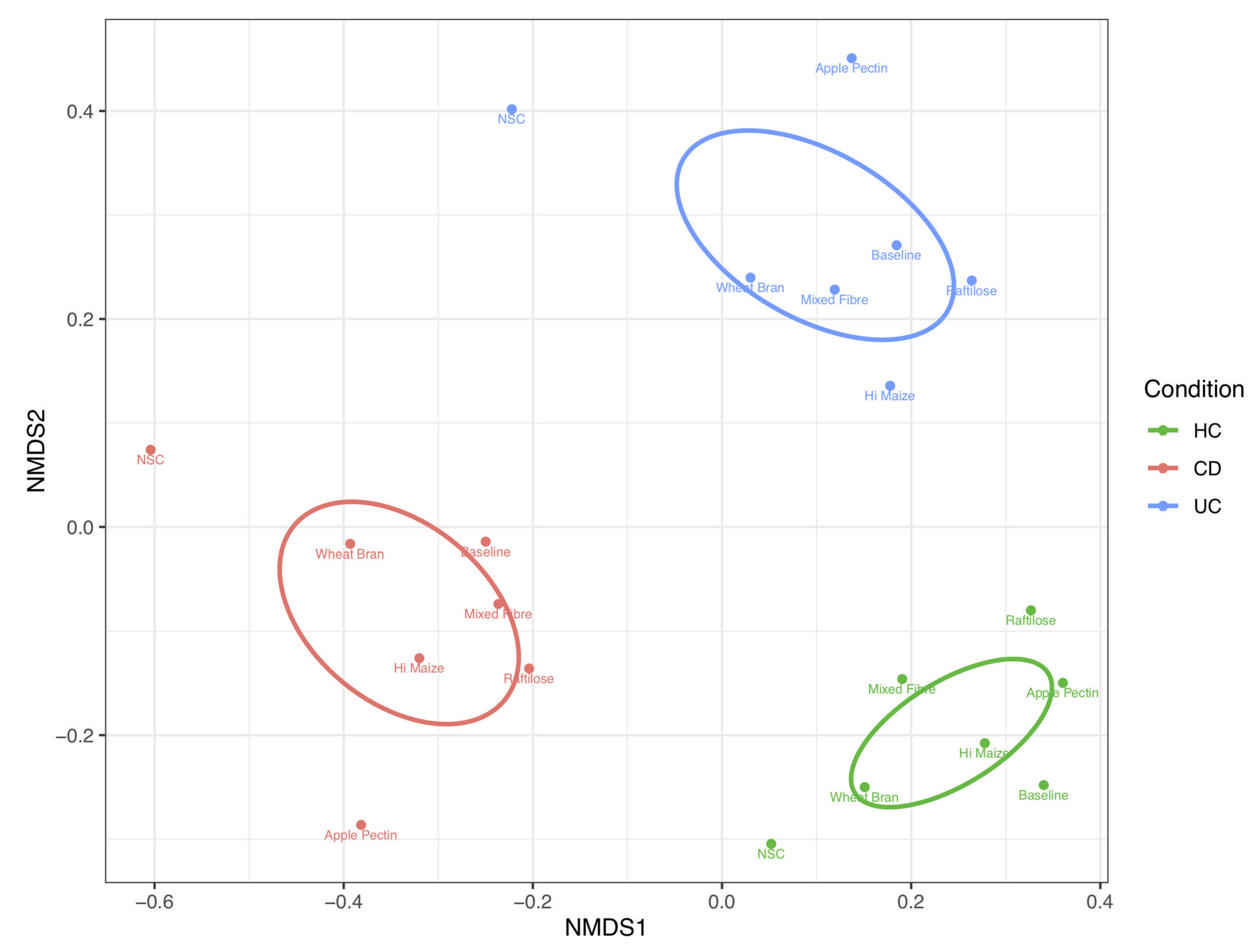
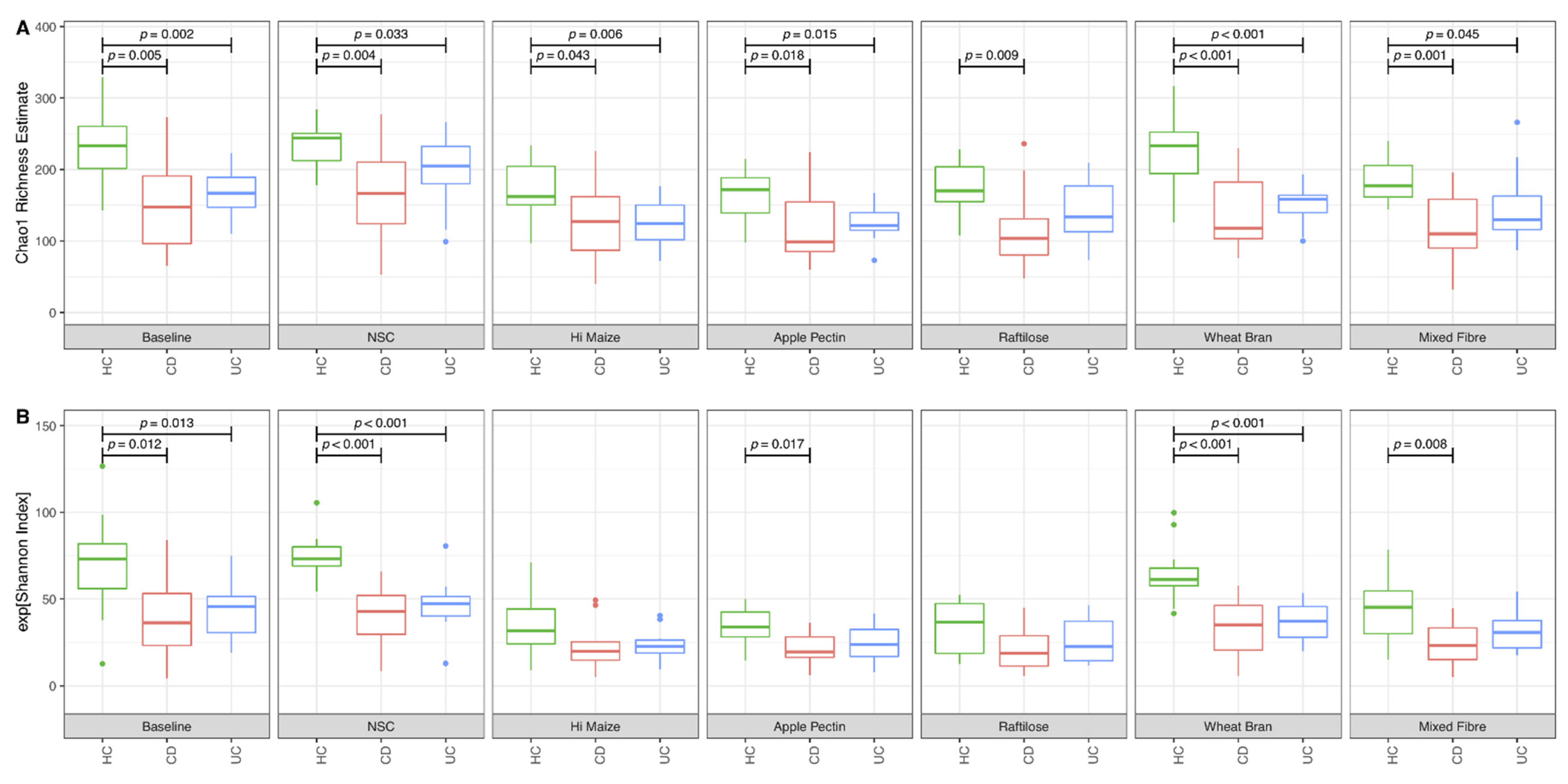
3.1. Effect of Fibre on Microbiome Composition
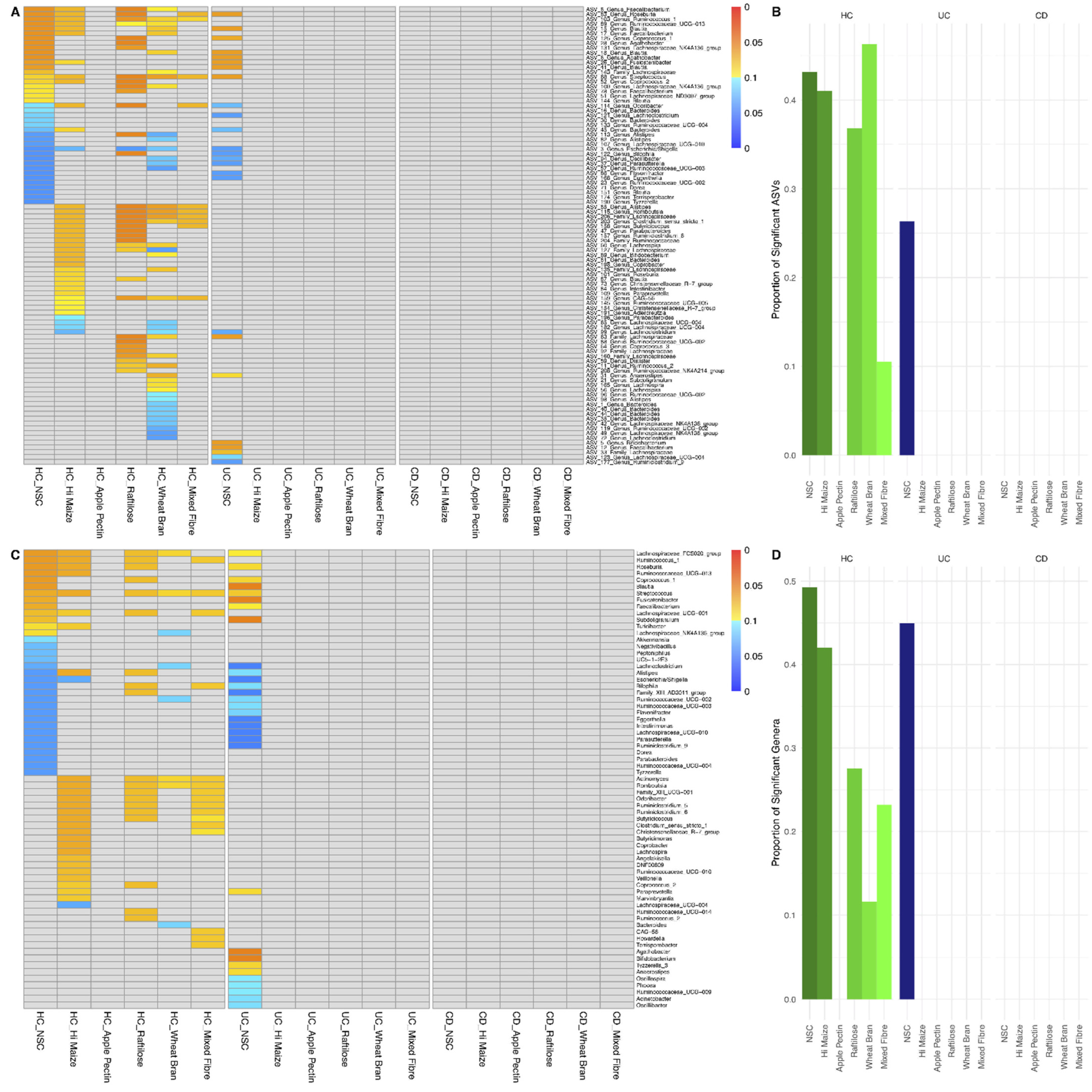
3.2. Effect of Fibre Fermentation on Taxon Relative Abundance
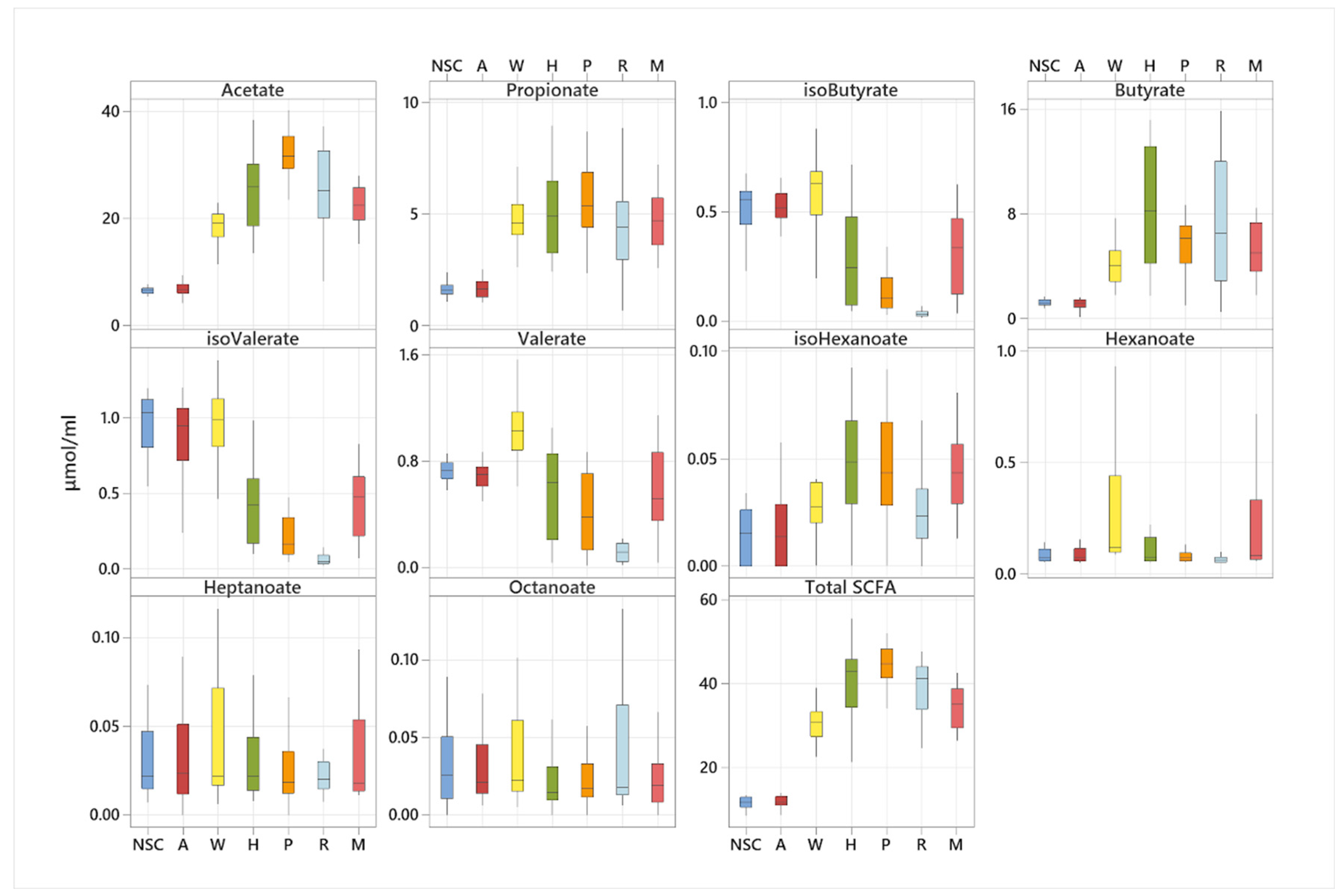
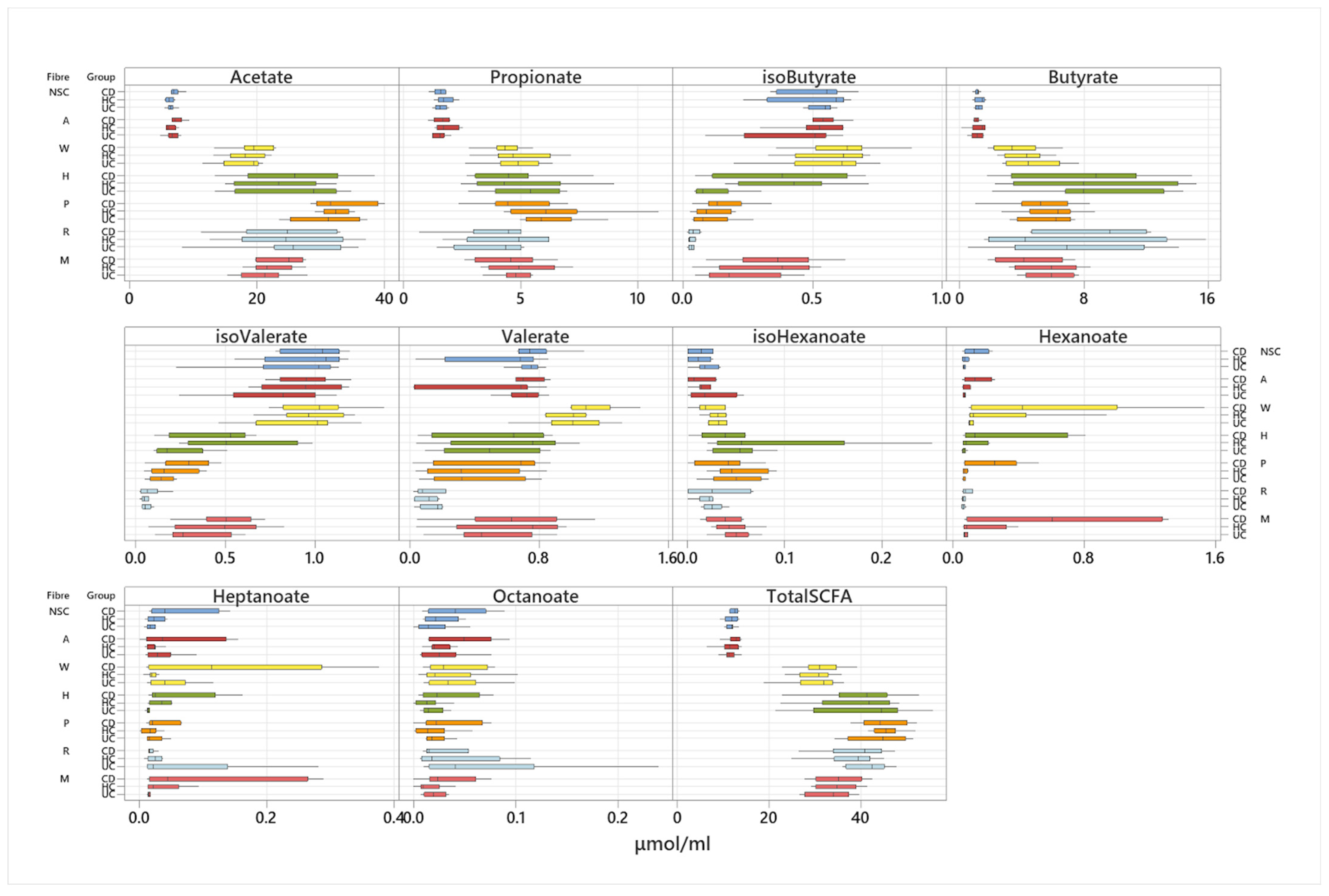
3.3. Effect of Fibre Fermentation on Fibre Fermentation and Production of SCFA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerasimidis, K.; Godny, L.; Sigall-Boneh, R.; Svolos, V.; Wall, C.; Halmos, E. Current recommendations on the role of diet in the aetiology and management of IBD. Frontline Gastroenterol. 2021, 13, 160–167. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Dijkstra, G.; Campmans-Kuijpers, M.J.E. Are all dietary fibers equal for patients with inflammatory bowel disease? A systematic review of randomized controlled trials. Nutr. Rev. 2021, 062. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Beards, E.; Tuohy, K.; Gibson, G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe 2010, 16, 420–425. [Google Scholar] [CrossRef]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404–3414. [Google Scholar] [CrossRef]
- Stephen, A.M.; Wiggins, H.S.; Cummings, J.H. Effect of changing transit time on colonic microbial metabolism in man. Gut 1987, 28, 601–609. [Google Scholar] [CrossRef]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in UC in remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef]
- Patz, J.; Jacobsohn, W.Z.; Gottschalk-Sabag, S.; Zeides, S.; Braverman, D.Z. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am. J. Gastroenterol. 1996, 91, 731–734. [Google Scholar] [PubMed]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; Di Paolo, M.C.; Paoluzi, P.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, M.J. Measuring Crohn’s disease activity. Lancet 1980, 1, 1134–1135. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef]
- Cummings, J.H.; Bingham, S.A.; Heaton, K.W.; Eastwood, M.A. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber). Gastroenterology 1992, 103, 1783–1789. [Google Scholar] [CrossRef]
- Gibson, R.; Eriksen, R.; Chambers, E.; Gao, H.; Aresu, M.; Heard, A.; Chan, Q.; Elliott, P.; Frost, G. Intakes and Food Sources of Dietary Fibre and Their Associations with Measures of Body Composition and Inflammation in UK Adults: Cross-Sectional Analysis of the Airwave Health Monitoring Study. Nutrients 2019, 11, 1839. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Quince, C.; Brunner, K.; Bruce, A.; Combet, E.; Calus, S.; Loman, N.; Ijaz, U.Z. The effect of DNA extraction methodology on gut microbiota research applications. BMC Res. Notes 2016, 9, 365. [Google Scholar] [CrossRef]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.G.F.; Kindt, R.M.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.M.; Stevens, M.H.; Szoecs, E.; et al. Vegan Community Ecology Package. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 17 January 2022).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, J.; Ianiro, G.; Mukhopadhya, I.; Hansen, R.; Hold, G.L. Review article: The gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol. Ther. 2018, 47, 26–42. [Google Scholar] [CrossRef]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2018, 11, Cd012774. [Google Scholar] [CrossRef]
- Hallert, C.; Björck, I.; Nyman, M.; Pousette, A.; Grännö, C.; Svensson, H. Increasing fecal butyrate in ulcerative colitis patients by diet: Controlled pilot study. Inflamm. Bowel Dis. 2003, 9, 116–121. [Google Scholar] [CrossRef]
- Simpson, E.J.; Chapman, M.A.; Dawson, J.; Berry, D.; Macdonald, I.A.; Cole, A. In vivo measurement of colonic butyrate metabolism in patients with quiescent ulcerative colitis. Gut 2000, 46, 73–77. [Google Scholar] [CrossRef][Green Version]
- Takaishi, H.; Matsuki, T.; Nakazawa, A.; Takada, T.; Kado, S.; Asahara, T.; Kamada, N.; Sakuraba, A.; Yajima, T.; Higuchi, H.; et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int. J. Med. Microbiol. 2008, 298, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Kataoka, K.; Ishikawa, H.; Ikata, K.; Arimochi, H.; Iwasaki, T.; Ohnishi, Y.; Kuwahara, T.; Yasutomo, K. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig. Dis. Sci. 2012, 57, 2955–2964. [Google Scholar] [CrossRef]
| Microbiome Study | Fermentative Capacity Study | |||||
|---|---|---|---|---|---|---|
| Crohn’s Disease (n = 12) | Ulcerative Colitis (n = 12) | Healthy Controls (n = 12) | Crohn’s Disease (n = 8) | Ulcerative Colitis (n = 8) | Healthy Controls (n = 8) | |
| Gender, F/M | 5/7 | 6/6 | 5/7 | 5/3 | 6/2 | 6/2 |
| BMI, kg/m2 | 25.4 (5.6) | 26.0 (5.3) | 26.4 (5.8) | 25.3 (3.6) | 24.7 (3.6) | 26.3 (4.9) |
| Age, years | 35.5 (9.0) | 50.6 (18.0) | 40.0 (13.8) | 36.6 (13.9) | 45.3 (18.8) | 34.3 (10.4) |
| Disease duration, years | 5.7 (7.9) | 9.9 (13.3) | n/a | 8.7 (4.1) | 4.6 (2.1) | n/a |
| Raised calprotectin, n (%) | 5 (42%) | 0 (0%) | 0 (0%) | 1 (12.5%) | 3 (37.5%) | 0 (0%) |
| Raised CRP, n (%) | 4 (33%) | 1 (8.3%) | n/a | 0 (0%) | 0 (0%) | n/a |
| Disease location | ||||||
| Ileocolonic | 4 (33%) | n/a | n/a | 5 (62.5%) | n/a | n/a |
| Colonic | 3 (25%) | n/a | n/a | 1 (12.5%) | n/a | n/a |
| Ileitis | 5 (42%) | n/a | n/a | 1 (12.5%) | n/a | n/a |
| Perianal | 0 (0%) | n/a | n/a | 3 (37.5%) | n/a | n/a |
| Disease behaviour | ||||||
| Inflammatory | 8 (67%) | n/a | n/a | 3 (37.5%) | n/a | n/a |
| Stricturing | 3 (25%) | n/a | n/a | 4 (50.0%) | n/a | n/a |
| Penetrating | 1 (8%) | n/a | n/a | 1 (12.5%) | n/a | n/a |
| Proctitis | n/a | 2 (17%) | n/a | n/a | 2 (25.0%) | n/a |
| Left-side | n/a | 3 (25%) | n/a | n/a | 2 (25.0%) | n/a |
| Extensive | n/a | 7 (58%) | n/a | n/a | 4 (50.0%) | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimidis, K.; Nichols, B.; McGowan, M.; Svolos, V.; Papadopoulou, R.; Kokkorou, M.; Rebull, M.; Bello Gonzalez, T.; Hansen, R.; Russell, R.K.; et al. The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission. Nutrients 2022, 14, 1053. https://doi.org/10.3390/nu14051053
Gerasimidis K, Nichols B, McGowan M, Svolos V, Papadopoulou R, Kokkorou M, Rebull M, Bello Gonzalez T, Hansen R, Russell RK, et al. The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission. Nutrients. 2022; 14(5):1053. https://doi.org/10.3390/nu14051053
Chicago/Turabian StyleGerasimidis, Konstantinos, Ben Nichols, Mhairi McGowan, Vaios Svolos, Rodanthi Papadopoulou, Margarita Kokkorou, Martina Rebull, Teresita Bello Gonzalez, Richard Hansen, Richard Kay Russell, and et al. 2022. "The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission" Nutrients 14, no. 5: 1053. https://doi.org/10.3390/nu14051053
APA StyleGerasimidis, K., Nichols, B., McGowan, M., Svolos, V., Papadopoulou, R., Kokkorou, M., Rebull, M., Bello Gonzalez, T., Hansen, R., Russell, R. K., & Gaya, D. R. (2022). The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission. Nutrients, 14(5), 1053. https://doi.org/10.3390/nu14051053







