Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent
2.2. Participants and Study Design
- High NO3−: two 70 mL shots of concentrated beetroot juice per day (approximately ~400 mg of NO3− per shot, as reported by manufacturer (James White Company, Ashbocking, Suffolk, UK)), one every morning (~8 am) and one every evening (~9 pm).
- Medium NO3−: one shot of concentrated beetroot juice every evening (~9 pm).
- Low NO3−: one shot of concentrated beetroot juice every other evening (~9 pm).
- Placebo: one shot of NO3−-depleted beetroot juice (~0.001 mg of NO3−) every other evening (~9 pm).
2.3. Study Setting
2.4. Data Collection Procedures
2.5. Compliance with Intervention
2.6. Measurements
2.6.1. Anthropometry and Body Composition
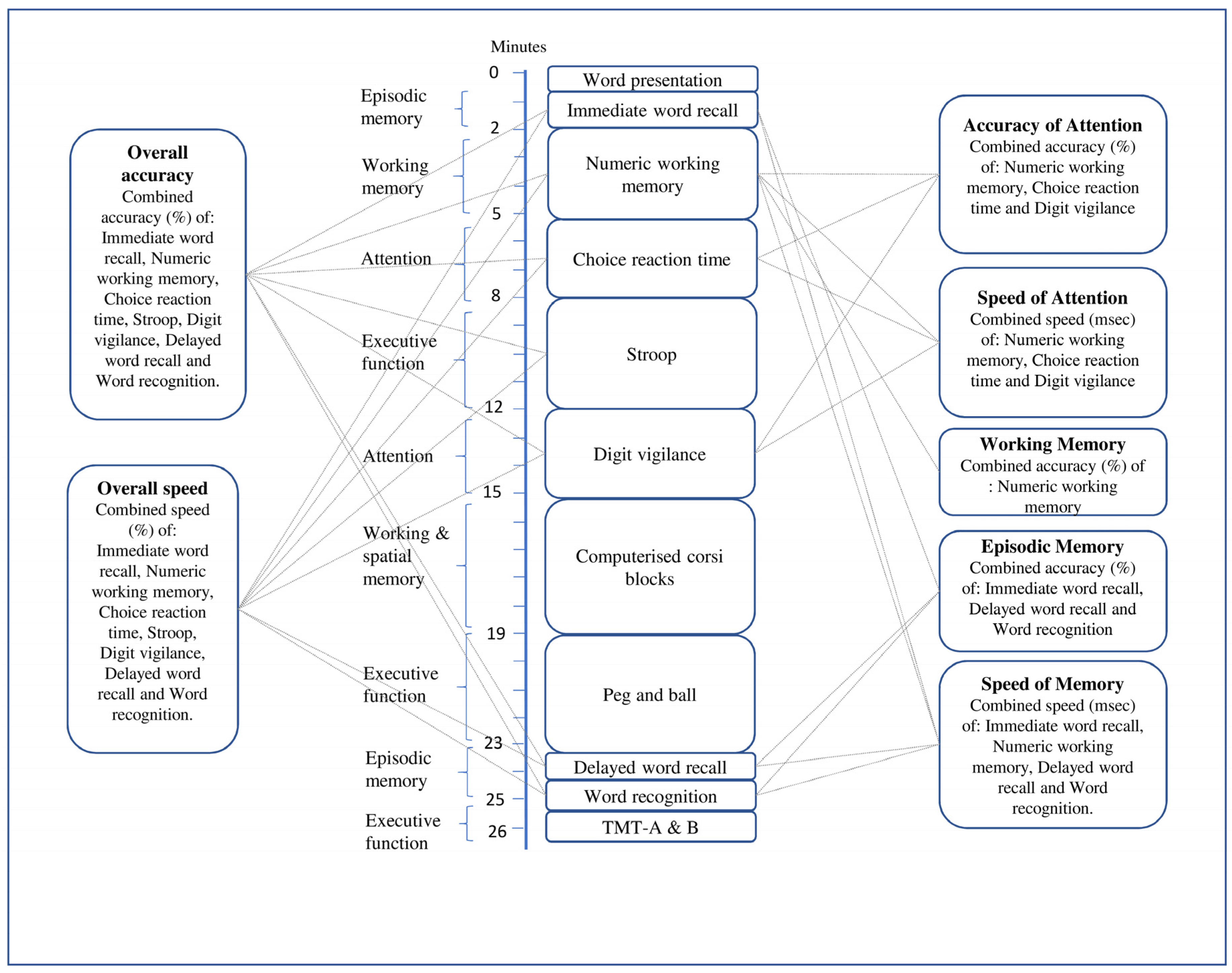
2.6.2. Cognitive Function
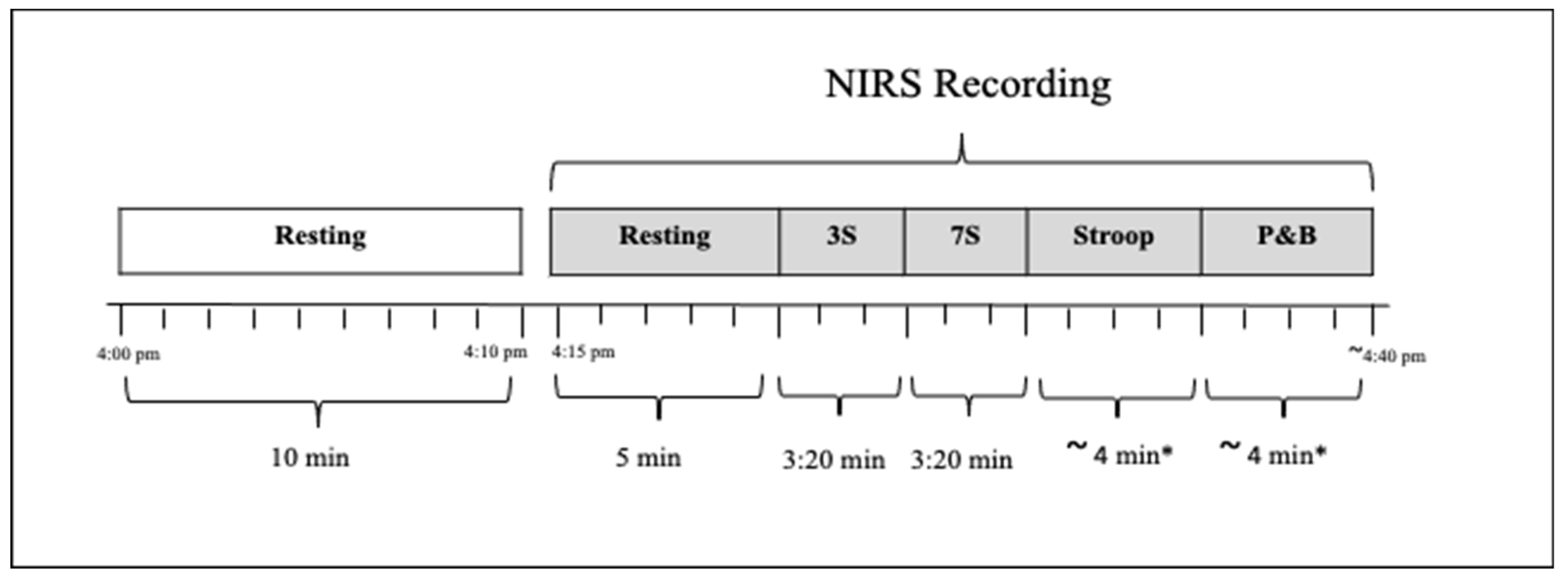
2.6.3. Quantitative Near-Infrared Spectroscopy (qNIRS)
2.7. Sample Size Calculation
2.8. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Effects of Nitrate Supplementation on Measures of Cognitive Function
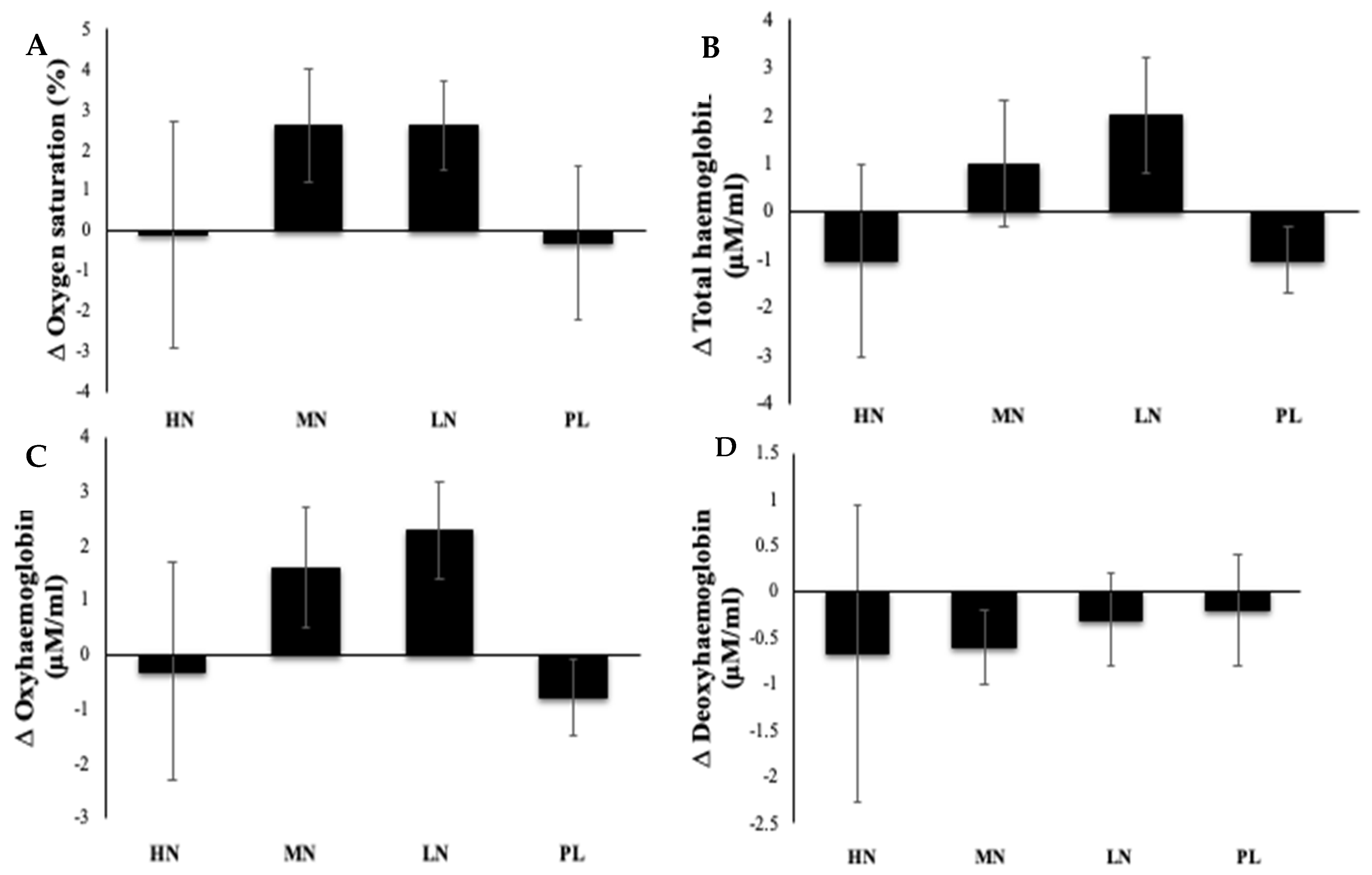
3.3. Effects of Nitrate Supplementation on Measures of qNIRS Parameters Used to Estimate Cerebral Blood Flow
3.4. Plasma Nitrate and Nitrite Concentrations
4. Discussion
4.1. Summary of Main Findings
4.2. Effects of Prolonged Beetroot Juice Consumption on Cognition and on CBF
4.3. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deary, I.J.; Corley, J.; Gow, A.; Harris, S.E.; Houlihan, L.M.; Marioni, R.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-associated cognitive decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strait, J.B.; Lakatta, E.G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitz, C.; Luchsinger, A.J.; Mayeux, R. Vascular disease and cognitive impairment. Expert Rev. Neurother. 2008, 8, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Leeuwis, A.E.; Benedictus, M.R.; Kuijer, J.P.; Binnewijzend, M.A.; Hooghiemstra, A.M.; Verfaillie, S.C.; Koene, T.; Scheltens, P.; Barkhof, F.; Prins, N.D.; et al. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimer’s Dement. 2017, 13, 531–540. [Google Scholar] [CrossRef]
- Wolters, F.J.; de Bruijn, R.F.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Cerebral vasoreactivity, apolipoprotein E, and the risk of dementia: A population-based study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Bangen, K.J.; Nation, D.A.; Clark, L.R.; Harmell, A.L.; Wierenga, C.E.; Dev, S.I.; Edelano-Wood, L.; Zlatar, Z.Z.; Salmon, D.P.; Liu, T.; et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front. Aging Neurosci. 2014, 6, 159. [Google Scholar] [CrossRef] [Green Version]
- Wolters, F.J.; Ikram, M.A. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Mokhber, N.; Shariatzadeh, A.; Avan, A.; Saber, H.; Babaei, G.S.; Chaimowitz, G.; Azarpazhooh, M.R. Cerebral blood flow changes during aging process and in cognitive disorders: A review. Neuroradiol. J. 2021, 34, 300–307. [Google Scholar] [CrossRef]
- Ruitenberg, A.; den Heijer, T.; Bakker, S.L.; van Swieten, J.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam Study. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 57, 789–794. [Google Scholar] [CrossRef]
- Leeuwis, A.E.; Smith, L.A.; Melbourne, A.; Hughes, A.; Richards, M.; Prins, N.D.; Sokolska, M.; Atkinson, D.; Tillin, T.; Jäger, H.R.; et al. Cerebral blood flow and cognitive functioning in a community-based, multi-ethnic cohort: The SABRE Study. Front. Aging Neurosci. 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Novel Aspects of Dietary Nitrate and Human Health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, M.; Pedrinolla, A.; Boscolo Galazzo, I.; Fonte, C.; Smania, N.; Tamburin, S.; Muti, E.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; et al. Impact of Nitric Oxide Bioavailability on the Progressive Cerebral and Peripheral Circulatory Impairments During Aging and Alzheimer’s Disease. Front. Physiol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. JGC 2011, 8, 230. [Google Scholar]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.D.; Mingorance, C.; Rodriguez-Rodriguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2010, 9, 142–152. [Google Scholar] [CrossRef]
- Van Der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.; Quaschning, T.; Malinski, T.; et al. Enhanced Peroxynitrite Formation Is Associated with Vascular Aging. J. Exp. Med. 2000, 192, 1731–1744. [Google Scholar] [CrossRef] [Green Version]
- Torreilles, F.; Salman-Tabcheh, S.; Guérin, M.-C.; Torreilles, J. Neurodegenerative disorders: The role of peroxynitrite. Brain Res. Rev. 1999, 30, 153–163. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Adan, R.A.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional psychiatry: Towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Scialò, F.; Shannon, O.M.; Stephan, B.C.; Ashor, A.W. Does dietary nitrate say NO to cardiovascular ageing? Current evidence and implications for research. Proc. Nutr. Soc. 2018, 77, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samieri, C.; Perier, M.-C.; Gaye, B.; Proust-Lima, C.; Helmer, C.; Dartigues, J.-F.; Berr, C.; Tzourio, C.; Empana, J.-P. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 2018, 320, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral blood flow measurements in adults: A review on the effects of dietary factors and exercise. Nutrients 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.; Blackwell, J.R.; Winyard, P.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [PubMed] [Green Version]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar]
- Clifford, T.; Babateen, A.; Shannon, O.M.; Capper, T.; Ashor, A.; Stephan, B.; Robinson, L.; O’Hara, J.P.; Mathers, J.C.; Stevenson, E.; et al. Effects of inorganic nitrate and nitrite consumption on cognitive function and cerebral blood flow: A systematic review and meta-analysis of randomised clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2400–2410. [Google Scholar] [CrossRef]
- Justice, J.N.; Johnson, L.C.; DeVan, A.; Cruickshank-Quinn, C.; Reisdorph, N.; Bassett, C.J.; Evans, T.D.; Brooks, F.A.; Bryan, N.S.; Chonchol, M.B.; et al. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: A pilot trial in middle-aged and older adults. Aging 2015, 7, 1004. [Google Scholar] [CrossRef] [Green Version]
- Aliahmadi, M.; Amiri, F.; Bahrami, L.S.; Hosseini, A.F.; Abiri, B.; Vafa, M. Effects of raw red beetroot consumption on metabolic markers and cognitive function in type 2 diabetes patients. J. Diabetes Metab. Disord. 2021, 20, 673–682. [Google Scholar] [CrossRef]
- Williams, I.; Wheatcroft, S.; Shah, A.; Kearney, M. Obesity, atherosclerosis and the vascular endothelium: Mechanisms of reduced nitric oxide bioavailability in obese humans. Int. J. Obes. 2002, 26, 754–764. [Google Scholar] [CrossRef] [Green Version]
- Toda, N.; Okamura, T. Obesity impairs vasodilatation and blood flow increase mediated by endothelial nitric oxide: An overview. J. Clin. Pharmacol. 2013, 53, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Babateen, A.M.; Rubele, S.; Shannon, O.; Okello, E.; Smith, E.; McMahon, N.; O’Brien, G.; Wightman, E.; Kennedy, D.; Mathers, J.C.; et al. Protocol and recruitment results from a 13-week randomized controlled trial comparing the effects of different doses of nitrate-rich beetroot juice on cognition, cerebral blood flow and peripheral vascular function in overweight and obese older people. Contemp. Clin. Trials Commun. 2020, 18, 100571. [Google Scholar] [CrossRef]
- Babateen, A.; Shannon, O.; O’Brien, G.; Okello, E.; Khan, A.; Rubele, S.; Wightman, E.; Smith, E.; McMahon, N.; Olgacer, D.; et al. Acceptability and Feasibility of a 13-Week Pilot Randomised Controlled Trial Testing the Effects of Incremental Doses of Beetroot Juice in Overweight and Obese Older Adults. Nutrients 2021, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 14336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.; Lamport, D.J.; Field, D.T.; Butler, L.T.; Williams, C.M. Practice effects in nutrition intervention studies with repeated cognitive testing. Nutr. Healthy Aging 2018, 4, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, T.E.; Harvey, P.D.; Wesnes, K.; Snyder, P.; Schneider, L.S. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskell, C.F.; Robertson, B.; Jones, E.; Forster, J.; Jones, R.; Wilde, A.; Maggini, S.; Kennedy, D.O. Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 448–461. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.A.; Kennedy, D.O. The application of near infrared spectroscopy in nutritional intervention studies. Front. Hum. Neurosci. 2013, 7, 473. [Google Scholar] [CrossRef] [Green Version]
- Kazui, H.; Kitagaki, H.; Mori, E. Cortical activation during retrieval of arithmetical facts and actual calculation: A functional magnetic resonance imaging study. Psychiatry Clin. Neurosci. 2000, 54, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, J.; D’Ostilio, K.; Phillips, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Salmon, E.; Collette, F. Modulation of brain activity during a Stroop inhibitory task by the kind of cognitive control required. PLoS ONE 2012, 7, e41513. [Google Scholar]
- Ruocco, A.C.; Rodrigo, A.H.; Lam, J.; Di Domenico, S.I.; Graves, B.; Ayaz, H. A problem-solving task specialized for functional neuroimaging: Validation of the Scarborough adaptation of the Tower of London (S-TOL) using near-infrared spectroscopy. Front. Hum. Neurosci. 2014, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Dormanns, K.; Brown, R.; David, T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016, 394, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Ayajiki, K.; Okamura, T. Cerebral blood flow regulation by nitric oxide in neurological disorders. Can. J. Physiol. Pharmacol. 2009, 87, 581–594. [Google Scholar] [CrossRef]
- Bor-Seng-Shu, E.; Kita, W.S.; Figueiredo, E.G.; Paiva, W.; Fonoff, E.T.; Teixeira, M.J.; Panerai, R.B. Cerebral hemodynamics: Concepts of clinical importance. Arq. Neuro-Psiquiatr. 2012, 70, 352–356. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar]
- Bangen, K.J.; Werhane, M.L.; Weigand, A.J.; Edmonds, E.C.; Delano-Wood, L.; Thomas, K.; Nation, D.A.; Evangelista, N.D.; Clark, A.L.; Liu, T.T.; et al. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes. Front. Aging Neurosci. 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Fulford, J.; Vanhatalo, A.; Blackwell, J.R.; French, O.; Bailey, S.J.; Gilchrist, M.; Winyard, P.G.; Jones, A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R73–R83. [Google Scholar] [CrossRef] [Green Version]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.G.; et al. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid. Redox Signal. 2015, 23, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, M.; Winyard, P.G.; Fulford, J.; Anning, C.; Shore, A.C.; Benjamin, N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: Development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide 2015, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Dahl, A.K.; Pedersen, N.L.; Johansson, B. Overweight in midlife is related to lower cognitive function 30 years later: A prospective study with longitudinal assessments. Dement. Geriatr. Cogn. Disord. 2010, 29, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 2005, 26, 11–16. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | All | HN | MN | LN | PL |
|---|---|---|---|---|---|
| Number | 62 | 16 | 17 | 14 | 15 |
| Gender, M/F | 24/38 | 10/6 | 5/12 | 4/10 | 5/10 |
| Age (years) | 66.3 ± 3.7 | 64.7 ± 3.5 | 66.7 ± 4.2 | 67.3 ± 2.7 | 65.7 ± 3.9 |
| Education (years) | 15.3 ± 3.0 | 16.0± 3 | 15.7± 2.6 | 15.0 ± 3.1 | 14.6 ± 3.1 |
| Body weight (kg) | 84.9 ± 12.6 | 90.9 ± 13.4 | 84.6 ± 10.5 | 80.1 ± 12.5 | 83.9 ± 12.6 |
| BMI (kg/m2) | 30.3 ± 3.7 | 30.5 ± 3.6 | 30.5 ± 3.2 | 29.9 ± 3.4 | 30.3 ± 4.8 |
| WC (cm) | 102.4 ± 9.2 | 104.5 ± 10.3 | 100.6 ± 9.6 | 102.1 ± 8.9 | 102.6 ± 8.2 |
| FM (kg) | 32.4 ± 8.7 | 32.1 ± 8.7 | 34.5 ± 9.0 | 31.3 ± 7.2 | 31.5± 10.0 |
| FFM (%) | 37.8 ± 7.8 | 35.2 ± 8.2 | 39.2 ± 7.8 | 39.3 ± 6.6 | 37.1 ± 8.1 |
| TBW (kg) | 38.6 ± 6.3 | 41.3 ± 7.4 | 38.6 ± 4.5 | 35.6 ± 6.3 | 38.8 ± 5.9 |
| SBP (mm Hg) | 135.1 ± 14.7 | 130.8 ± 12.0 | 136.1 ± 10.4 | 139.5 ± 13.2 | 134.1 ± 12.9 |
| DBP (mm Hg) | 76.9 ± 9.4 | 75.8 ± 9.7 | 77.3 ± 9.1 | 77.8 ± 8.1 | 76.9 ± 11.2 |
| PA (METs/wk) | 3667 ± 5604 | 2741 ± 1522 | 3257 ± 1845 | 2262 ± 1933 | 6280 ± 10,512 |
| Measure | High NO3− (n = 10) | Medium NO3− (n = 13) | Low NO3− (n = 14) | Placebo (n = 13) | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 13 Weeks (Change) | Baseline | 13 Weeks (Change) | Baseline | 13 Weeks (Change) | Baseline | 13 Weeks (Change) | ||
| IWR correct (number) | 5.4 ± 0.4 | 0.05 ± 0.5 | 6.5 ± 0.4 | 0.2 ± 0.3 | 5.6 ± 0.5 | −0.07 ± 0.4 | 6.8 ± 0.5 | −0.7 ± 0.6 | 0.45 |
| IWR error (number) | 0.2 ± 0.2 | 0.0 | 0.4 ± 0.2 | 0.1 ± 0.2 | 0.6 ± 0.2 | 0.01 ± 0.1 | 0.3 ± 0.2 | −0.1 ± 0.1 | 0.83 |
| NWM (% accuracy) | 98.0 ± 0.3 | −0.23 ± 1.5 | 93.8 ± 1.7 | 1.2 ± 0.7 | 96.5 ± 1.4 | −1.3 ± 1.6 | 91.1 ± 3.4 | 1.3 ± 1.4 | 0.61 |
| NWM-RT (ms) | 1126 ± 81 | 3 ± 37 | 1288 ± 179 | −185 ± 143 | 1087 ± 77 | 63 ± 61 | 1091 ± 48 | −78 ± 46 | 0.76 |
| CRT (% accuracy) | 98.4 ± 0.5 | −0.6 ± 0.6 | 98.0 ± 0.5 | 0.8 ± 0.6 | 98.7 ± 0.5 | −0.1 ± 0.4 | 98.8 ± 0.5 | −0.6 ± 0.5 | 0.49 |
| CRT RT (ms) | 570 ± 37 | −18 ± 21 | 622 ± 30 | −31 ± 27 | 641 ± 46 | −4 ± 0.5 | 603 ± 27 | −6 ± 21 | 0.21 |
| Stroop % accuracy * | 99.3 ± 0.4 | 0.29 ± 0.3 | 98.8 ± 0.8 | 6.5 ± 7.1 | 99.9 ± 0.1 | −0.2 ± 0.2 | 99.8 ± 0.1 | −1.9 ± 10 | 0.79 |
| Stroop RT (ms) * | 1304 ± 28 | −45 ± 48 | 1434 ± 70 | 128 ± 195 | 1523 ± 174 | −40 ± 74 | 1261 ± 38 | 51 ± 159 | 0.78 |
| DV (% accuracy) | 96.5 ± 1.4 | −2.5 ± 1.7 | 90.4 ± 2.2 | −7.2 ± 7.9 | 86.1 ± 3.7 | 3.8 ± 2.3 | 84.8 ± 4.6 | −0.89 ± 2.0 | 0.49 |
| DV RT (ms) | 483 ± 11 | −2.8 ± 7.7 | 469 ± 10 | −41.5 ± 38 | 512 ± 11 | 33 ± 40 | 498 ± 8 | −0.03 ± 4 | 0.36 |
| DV false alarms (number) | 1.3 ± 0.3 | 0.7 ± 1.0 | 4.2 ± 0.7 | −0.3 ± 1.0 | 5.7 ± 1.1 | 0.2 ± 0.8 | 5.6 ± 1.2 | −0.4 ± 0.8 | 0.79 |
| Corsi blocks span | 5.6 ± 0.1 | 0.1 ± 0.1 | 5.4 ± 0.2 | 0.1 ± 0.2 | 5.2 ± 0.4 | 0.3 ± 0.3 | 5.4 ± 0.3 | −0.3 ± 0.4 | 0.29 |
| P&B thinking time | 4106 ± 582 | −367 ± 349 | 5221 ± 429 | −1066 ± 382 | 4600 ± 317 | −695 ± 263 | 5092 ± 541 | −653 ± 345 | 0.46 |
| P&B working time (ms) | 11,640 ± 747 | −974 ± 460 | 14,311 ± 980 | −2325 ± 893 | 14,186 ± 1675 | −1882 ± 144 | 12,624 ± 729 | −963 ± 641 | 0.59 |
| P&B errors | 3.7 ± 2.5 | 0.8 ± 1.5 | 2.2 ± 1.9 | −0.2 ± 0.8 | 4.2 ± 4.6 | −1.3 ± 1.6 | 3.3 ± 3.3 | −2.3 ± 1.1 | 0.48 |
| DWR correct (number) | 3.8 ± 0.5 | 0.3 ± 0.5 | 5.3 ± 0.4 | −0.8 ± 0.3 | 3.5 ± 0.5 | 0.8 ± 0.3 | 5.0 ± 0.5 | −0.1 ± 0.6 | 0.03 |
| DWR error (number) | 0.6 ± 0.2 | 0.4 ± 0.2 | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.1 ± 0.1 | 0.6 ± 0.2 | 0.1 ± 0.3 | 0.65 |
| WR (%accuracy) | 77.0 ± 7.9 | −1.3 ± 4.3 | 79.4 ± 8.1 | 0.7 ± 2.2 | 78.6 ± 12.7 | −0.7 ± 3.6 | 82.4 ± 7.8 | −2.2 ± 2.7 | 0.77 |
| WR RT (ms) | 1277 ± 187 | −14 ± 103 | 1085 ± 191 | 17 ± 39 | 1226 ± 316 | 6.1 ± 52 | 1287 ± 480 | 65 ± 48 | 0.39 |
| TMT-a (s) | 24.1 ± 1.7 | −2.4 ± 1.5 | 28.1 ± 1.8 | −2.5 ± 2.2 | 28.5 ± 2.7 | −0.1 ± 1.8 | 24.6 ± 1.5 | −1.2 ± 1.3 | 0.75 |
| TMT-b (s) | 52.1 ± 3.6 | −8.1 ± 3.0 | 52.2 ± 4.2 | 2.2 ± 4.9 | 56.3 ± 5.5 | −7.1 ± 2.4 | 55.0 ± 4.5 | −9.7 ± 3.9 | 0.11 |
| Measure | High NO3− (n = 10) | Medium NO3− (n = 13) | Low NO3− (n = 14) | Placebo (n = 13) | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 13-Weeks (Change) | Baseline | 13-Weeks (Change) | Baseline | 13-Weeks (Change) | Baseline | 13-Weeks (Change) | ||
| Accuracy of attention (%) | 96.6 ± 0.8 | −1.8 ± 0.8 | 93.2 ± 1.3 | −2.2 ± 2.3 | 94.2 ± 1.4 | −0.8 ± 1.0 | 91.7 ± 2.2 | 7.2 ± 7.2 | 0.88 |
| Speed of attention (msec) | 735 ± 36 | −6.1 ± 13.5 | 788 ± 54 | −95.7 ± 52.3 | 763 ± 37 | 17.9 ± 21.3 | 742 ± 21 | 32 ± 62.8 | 0.16 |
| Accuracy of working memory (%) | 96.3 ± 1.7 | −0.2 ± 1.5 | 91.6 ± 2.8 | 1.2 ± 0.7 | 96.4 ± 1.2 | −1.3 ± 1.7 | 91.3 ± 3.2 | 8.0 ± 6.8 | 0.49 |
| Accuracy of episodic memory (%) | 45.2 ± 1.9 | 0.7 ± 3.1 | 52.0 ± 2.1 | −1.2 ± 1.8 | 46.3 ± 2.5 | 1.4 ± 1.3 | 54.8 ± 2.9 | 2.5 ± 6.1 | 0.42 |
| Speed of memory (msec) | 1205 ± 51 | −5.6 ± 44.3 | 1186 ± 72 | −83.7 ± 67.2 | 1155 ± 66 | 34.7 ± 32.1 | 1214 ± 76 | 88.2 ± 97.5 | 0.37 |
| Overall speed (msec) | 947 ± 25 | −15.8 ± 15.2 | 969 ± 37 | −42.3 ± 40.6 | 997 ± 57 | 2.5 ± 25.1 | 954 ± 35 | 76.3 ± 73.1 | 0.77 |
| Overall accuracy (%) | 74.6 ± 1.2 | 0.7 ± 1.6 | 75.7 ± 1.3 | −1.1 ± 1.6 | 73.9 ± 1.4 | 0.2 ± 0.6 | 76.9 ± 1.5 | 4.8 ± 6.6 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babateen, A.M.; Shannon, O.M.; O’Brien, G.M.; Okello, E.; Smith, E.; Olgacer, D.; Koehl, C.; Fostier, W.; Wightman, E.; Kennedy, D.; et al. Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial. Nutrients 2022, 14, 1052. https://doi.org/10.3390/nu14051052
Babateen AM, Shannon OM, O’Brien GM, Okello E, Smith E, Olgacer D, Koehl C, Fostier W, Wightman E, Kennedy D, et al. Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial. Nutrients. 2022; 14(5):1052. https://doi.org/10.3390/nu14051052
Chicago/Turabian StyleBabateen, Abrar M., Oliver M. Shannon, Gerard M. O’Brien, Edward Okello, Ellen Smith, Dilara Olgacer, Christina Koehl, William Fostier, Emma Wightman, David Kennedy, and et al. 2022. "Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial" Nutrients 14, no. 5: 1052. https://doi.org/10.3390/nu14051052
APA StyleBabateen, A. M., Shannon, O. M., O’Brien, G. M., Okello, E., Smith, E., Olgacer, D., Koehl, C., Fostier, W., Wightman, E., Kennedy, D., Mathers, J. C., & Siervo, M. (2022). Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial. Nutrients, 14(5), 1052. https://doi.org/10.3390/nu14051052







