Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Past Medical History
2.3. Measurements of 25OHD
2.4. Statistics
3. Results
3.1. Calcium-Phosphate Metabolism Parameters and Inflammatory Markers Status in IBD Pediatric Patients
3.2. Clinical Course of the Disease and Vitamin D Level
3.3. Vitamin D Level and Nutritional Status of ID Children
3.4. Sun Exposure
3.5. Physical Activity
3.6. Vitamin D and Diet
3.7. Medications and Vitamin D Level
4. Discussion
4.1. Laboratory Test Results
4.2. Clinical Course of the Disease and Vitamin D Level
4.3. Sun Exposure
4.4. Vitamin D and Diet
4.5. Physical Activity
4.6. Medications and Vitamin D Level
5. Conclusions
- Both children with IBD and the control group reported a vitamin D deficiency which suggests the need for vitamin D supplementation in children with inflammatory bowel disease as well as their healthy peers, especially in adolescents. Although IBD patients are usually under constant medical supervision, more attention should be drawn to maintain an adequate vitamin D level in this group of patients. There are many risk factors of vitamin D deficiency associated with IBD, regardless of disease activity or location, that may lead to serious complications. On the other hand, healthy adolescents are also at risk of developing a vitamin D deficiency and its adverse effects, probably due to the increased metabolic demand at the time of a growth spurt, inappropriate dietary habits or infrequent sun exposure;
- Daily exposure to sunlight has a beneficial effect on serum vitamin D levels in both study groups. It is, therefore, advisable to promote the presence of children outdoors;
- Nutritional status, diet and physical activity does not seem to modify the level of vitamin D. However, it has been confirmed that amount of vitamin D consumed from the regular diet in both study groups is too low to cover daily requirements. Moreover, IBD patients have a lower physical activity frequency compared to controls, even with a milder clinical course of the disease. Healthy lifestyle habits should be promoted in all children. All of the above observations require verification in larger study groups.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.; Piotrowska, A.; Żmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020, 1268, 19–36. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D insufficiency/deficiency in gastrointestinal disorders. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V50–V54. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Wellington, V.N.A.; Sundaram, V.L.; Singh, S.; Sundaram, U. Dietary Supplementation with Vitamin D, Fish Oil or Resveratrol Modulates the Gut Microbiome in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 23, 206. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C.; Cominelli, F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int. J. Mol. Sci. 2017, 18, 2360. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, V.; White, J.H. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell. Endocrinol. 2017, 453, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020, 10, 3141. [Google Scholar] [CrossRef]
- Holmes, E.A.; Rodney Harris, R.M.; Lucas, R.M. Low Sun Exposure and Vitamin D Deficiency as Risk Factors for Inflammatory Bowel Disease, With a Focus on Childhood Onset. Photochem. Photobiol. 2019, 95, 105–118. [Google Scholar] [CrossRef]
- Veit, L.E.; Maranda, L.; Nwosu, B.U. The nondietary determinants of vitamin D status in pediatric inflammatory bowel disease. Nutrition 2015, 31, 994–999. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: Recommendations for diagnosis—The Porto criteria. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 1–7. [Google Scholar] [CrossRef]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Griffiths, A.M. Acute severe ulcerative colitis in children: A systematic review. Inflamm. Bowel Dis. 2011, 17, 440–449. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Kowalik, T.; Żmijewski, M.; Kowalski, K.; Rola, R.; Bieńkowski, T.; Antosiewicz, J. Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial. Nutrients 2020, 12, 3629. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Yu, W.; Li, D.; Xiang, Z.; Lin, Y.; Yu, C. Association between 25(OH)D Level, Ultraviolet Exposure, Geographical Location, and Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0132036. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Prosnitz, A.R.; Leonard, M.B.; Shults, J.; Zemel, B.S.; Hollis, B.W.; Denson, L.A.; Baldassano, R.N.; Cohen, A.B.; Thayu, M. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 45–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Matary, W.; Sikora, S.; Spady, D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Williams, E.A.; Corfe, B.M. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: What do we know and what do we need to know? Eur. J. Clin. Nutr. 2018, 72, 1358–1363. [Google Scholar] [CrossRef]
- Middleton, J.P.; Bhagavathula, A.P.; Gaye, B.; Alvarez, J.A.; Huang, C.S.; Sauer, C.G.; Tenjarla, G.; Schoen, B.T.; Kumar, A.; Prasad, M.; et al. Vitamin D status and bone mineral density in African American children with Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 587–593. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Cenni, S.; Giugliano, F.P.; Miele, E.; Cirillo, G.; Martinelli, M.; Vitale, A.; Tolone, C.; Staiano, A.; Miraglia Del Giudice, E.; et al. The Role of Inflammation on Vitamin D Levels in a Cohort of Pediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 501–506. [Google Scholar] [CrossRef]
- Rigterink, T.; Appleton, L.; Day, A.S. Vitamin D therapy in children with inflammatory bowel disease: A systematic review. World J. Clin. Pediatr. 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef]
- Levin, A.D.; Wadhera, V.; Leach, S.T.; Woodhead, H.J.; Lemberg, D.A.; Mendoza-Cruz, A.C.; Day, A.S. Vitamin D deficiency in children with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Pappa, H.M.; Langereis, E.J.; Grand, R.J.; Gordon, C.M. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, 2583. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.A.; Avan, A.; Bahrami, A.; Khodashenas, E.; Esmaeili, H.; Ferns, G.A.; Abdizadeh, M.F.; Ghayour-Mobarhan, M. High Dose Supplementation of Vitamin D Affects Measures of Systemic Inflammation: Reductions in High Sensitivity C-Reactive Protein Level and Neutrophil to Lymphocyte Ratio (NLR) Distribution. J. Cell. Biochem. 2017, 118, 4317–4322. [Google Scholar] [CrossRef]
- Shepherd, D.; Day, A.S.; Leach, S.T.; Lopez, R.; Messenger, R.; Woodhead, H.J.; Ledder, O.; Lemberg, D.A. Single High-Dose Oral Vitamin D3 Therapy (Stoss): A Solution to Vitamin D Deficiency in Children With Inflammatory Bowel Disease? J. Pediatr. Gastroenterol. Nutr. 2015, 61, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Wingate, K.E.; Jacobson, K.; Issenman, R.; Carroll, M.; Barker, C.; Israel, D.; Brill, H.; Weiler, H.; Barr, S.I.; Li, W.; et al. 25-Hydroxyvitamin D concentrations in children with Crohn’s disease supplemented with either 2000 or 400 IU daily for 6 months: A randomized controlled study. J. Pediatr. 2014, 164, 860–865. [Google Scholar] [CrossRef]
- Joseph, A.J.; George, B.; Pulimood, A.B.; Seshadri, M.S.; Chacko, A. 25 (OH) vitamin D level in Crohn’s disease: Association with sun exposure & disease activity. Indian J. Med. Res. 2009, 130, 133–137. [Google Scholar]
- Sentongo, T.A.; Semaeo, E.J.; Stettler, N.; Piccoli, D.A.; Stallings, V.A.; Zemel, B.S. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am. J. Clin. Nutr. 2002, 76, 1077–1081. [Google Scholar] [CrossRef]
- Jasielska, M.; Grzybowska-Chlebowczyk, U. Hypocalcemia and Vitamin D Deficiency in Children with Inflammatory Bowel Diseases and Lactose Intolerance. Nutrients 2021, 13, 2583. [Google Scholar] [CrossRef]
- Driscoll, R.H.; Meredith, S.C.; Sitrin, M.; Rosenberg, I.H. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology 1982, 83, 1252–1258. [Google Scholar] [CrossRef]
- Pappa, H.M.; Gordon, C.M.; Saslowsky, T.M.; Zholudev, A.; Horr, B.; Shih, M.C.; Grand, R.J. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 2006, 118, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Catto-Smith, A.G.; Zacharin, M. Pathological fractures in paediatric patients with inflammatory bowel disease. Eur. J. Pediatr. 2014, 173, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Laakso, S.; Valta, H.; Verkasalo, M.; Toiviainen-Salo, S.; Viljakainen, H.; Mäkitie, O. Impaired bone health in inflammatory bowel disease: A case-control study in 80 pediatric patients. Calcif. Tissue Int. 2012, 91, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kmieć, P.; Żmijewski, M.; Lizakowska-Kmieć, M.; Sworczak, K. Widespread vitamin D deficiency among adults from northern Poland (54° N) after months of low and high natural UVB radiation. Endokrynol. Pol. 2015, 66, 30–38. [Google Scholar] [CrossRef]
- Alkhouri, R.H.; Hashmi, H.; Baker, R.D.; Gelfond, D.; Baker, S.S. Vitamin and mineral status in patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 89–92. [Google Scholar] [CrossRef]
- Karagüzel, G.; Dilber, B.; Çan, G.; Ökten, A.; Değer, O.; Holick, M.F. Seasonal vitamin D status of healthy schoolchildren and predictors of low vitamin D status. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 654–660. [Google Scholar] [CrossRef]
- Brandvayman, Y.; Rinawi, F.; Shamir, R.; Assa, A. Associations of seasonal patterns and vitamin D levels with onset and flares of pediatric inflammatory bowel disease. Minerva Pediatr. 2021, 73, 42–49. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Bayless, T.M.; Brant, S.R.; Hutfless, S.M. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Aliment. Pharmacol. Ther. 2014, 40, 508–517. [Google Scholar] [CrossRef]
- Rufo, P.A.; Denson, L.A.; Sylvester, F.A.; Szigethy, E.; Sathya, P.; Lu, Y.; Wahbeh, G.T.; Sena, L.M.; Faubion, W.A. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 93–108. [Google Scholar] [CrossRef]
- Kikut, J.; Skonieczna-Żydecka, K.; Sochaczewska, D.; Kordek, A.; Szczuko, M. Differences in Dietary Patterns of Adolescent Patients with IBD. Nutrients 2021, 13, 3119. [Google Scholar] [CrossRef]
- Richman, E.; Rhodes, J.M. Review article: Evidence-based dietary advice for patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Salen, G.; Shefer, S.; Batta, A.K.; Honda, M.; Xu, G.; Tint, G.S.; Matsuzaki, Y.; Shoda, J.; Tanaka, N. Bile acid synthesis in the Smith-Lemli-Opitz syndrome: Effects of dehydrocholesterols on cholesterol 7alpha-hydroxylase and 27-hydroxylase activities in rat liver. J. Lipid Res. 1999, 40, 1520–1528. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Ullrich, J.; Schatz, S.B.; Prell, C.; Koletzko, B.; Koletzko, S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J. Crohns Colitis 2012, 6, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Trivić, I.; Sila, S.; Batoš, A.T.; Mišak, Z.; Kolaček, S.; Hojsak, I. Moderate-to-Vigorous Physical Activity Is Associated with Higher Bone Mineral Density in Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 54–59. [Google Scholar] [CrossRef]
- Raman, M.; Rajagopalan, V.; Kaur, S.; Reimer, R.A.; Ma, C.; Ghosh, S.; Vallance, J. Physical Activity in Patients with Inflammatory Bowel Disease: A Narrative Review. Inflamm. Bowel Dis. 2021, 4, izab218. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Society, E. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis—An Evidence-based Consensus Guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-based Guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J. Gastroenterol. 2010, 45, 399–405. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; Garcia-Maurino, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Thayu, M.; Leonard, M.B.; Hyams, J.S.; Crandall, W.V.; Kugathasan, S.; Otley, A.R.; Olson, A.; Johanns, J.; Marano, C.W.; Heuschkel, R.B.; et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: Results of the REACH study. Clin. Gastroenterol. Hepatol. 2008, 6, 1378–1384. [Google Scholar] [CrossRef]

| Characteristic | Number of Patients (%) | |||
|---|---|---|---|---|
| IBD (N = 62) | CD (N = 34) | UC (N = 28) | C (N = 47) | |
| Gender: F M | 23 (37) 39 (63) | 10 (30) 24 (70) | 13 (46) 15 (54) | 23 (49) 24 (51) |
| Season of the 25OHD analysis: summer winter | 16 (26) 46 (74) | 10 (30) 24 (70) | 6 (21) 22 (79) | 0 (0) 47 (100) |
| Clinical course | ||||
| PCDAI/PUCAI score: 1/2/3/4 | 10/16/4/4 | 13/13/3/0 | ||
| Endoscopic location CD: L1/L2/L3/L4 UC: E1/E2/E3/E4 | 2/21/11/0 | 5/6/12/5 | ||
| Surgical abdominal interventions | 5 (8) | 4 | 1 | 0 (0) |
| Bone fractures | 11 (18) | 7 | 4 | 3 (6) |
| Medications: | ||||
| Dose of vitamin D supplementation: <600 IU 601–1000 IU >1001 IU | 33 (53) 16 (26) 10 (16) 7 (11) | 15 8 4 4 | 18 8 6 3 | 7 (15) 5 (11) 2 (4) 0 (0) |
| Steroids | 29 (47) | 19 | 10 | 0 (0) |
| Steroids + Vitamin D | 17 (27) | 8 | 9 | 0 (0) |
| Azathioprine | 18 (29) | 13 | 5 | 0 (0) |
| Biological treatment | 12 (19) | 9 | 3 | 0 (0) |
| Nutrition: enteral/parenteral | 12 (62) 11/1 | 10/1 | 0/1 | 0 (0) |
| IBD | CD | UC | C | p | |
|---|---|---|---|---|---|
| Weight | 48.5 (37.9–58.0) | 48.4 (41.0–53.0) | 50.5 (33.5–60.5) | 50.0 (42.0–56.77) | NS |
| Weight z-score | −0.88 ± 1.04 | −0.99 ± 1.07 | 0.74 ± 1.02 | −0.014 ± 0.73 | p < 0.05 |
| Height | 1.625 (1.51–1.73) | 1.63 (1.57–1.7) | 1.62 (1.48–1.75) | 1.62 (1.55–1.7) | NS |
| Height z-score | −0.51 (−1.26–0.29) | −0.51 (−1.03–0.15) | −0.31 (−1.74–0.78) | −0.18 (−0.63–0.29) | NS |
| BMI | 18.16 (16.44–19.53) | 17.8 (16.44–19.03) | 18.53 (16.43–20.27) | 18.52 (17.67–19.72) | NS |
| BMI z-score | −0.69 ± 1.01 | −0.85 ± 0.98 | −0.49 ± 1.02 | −0.19 ± 0.73 | p < 0.05 |
| Season/Supplementation | 25OHD Level [ng/mL] |
|---|---|
| IBD summer | 14.0 (11.65–26.65) |
| IBD summer supp + | 14.5 (12.38–42.53) |
| IBD summer supp − | 13.4 (11.43–23.48) |
| IBD winter | 19.25 (14.4–23.2) |
| IBD winter supp + | 22.25 (17.85–25.4) |
| IBD winter supp − | 14.45 (13.7–19.2) |
| C winter | 15.5 (11.83–18.7) |
| C winter supp + | 16.2 (12.73–19.38) |
| C winter supp − | 13.55 (9.55–15.85) |
| Mean Value +/− SD or Median (25–75%) | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Reference Value | CD | UC | IBD | C | CD vs. C | UC vs. C | IBD vs. C | CD vs. UC |
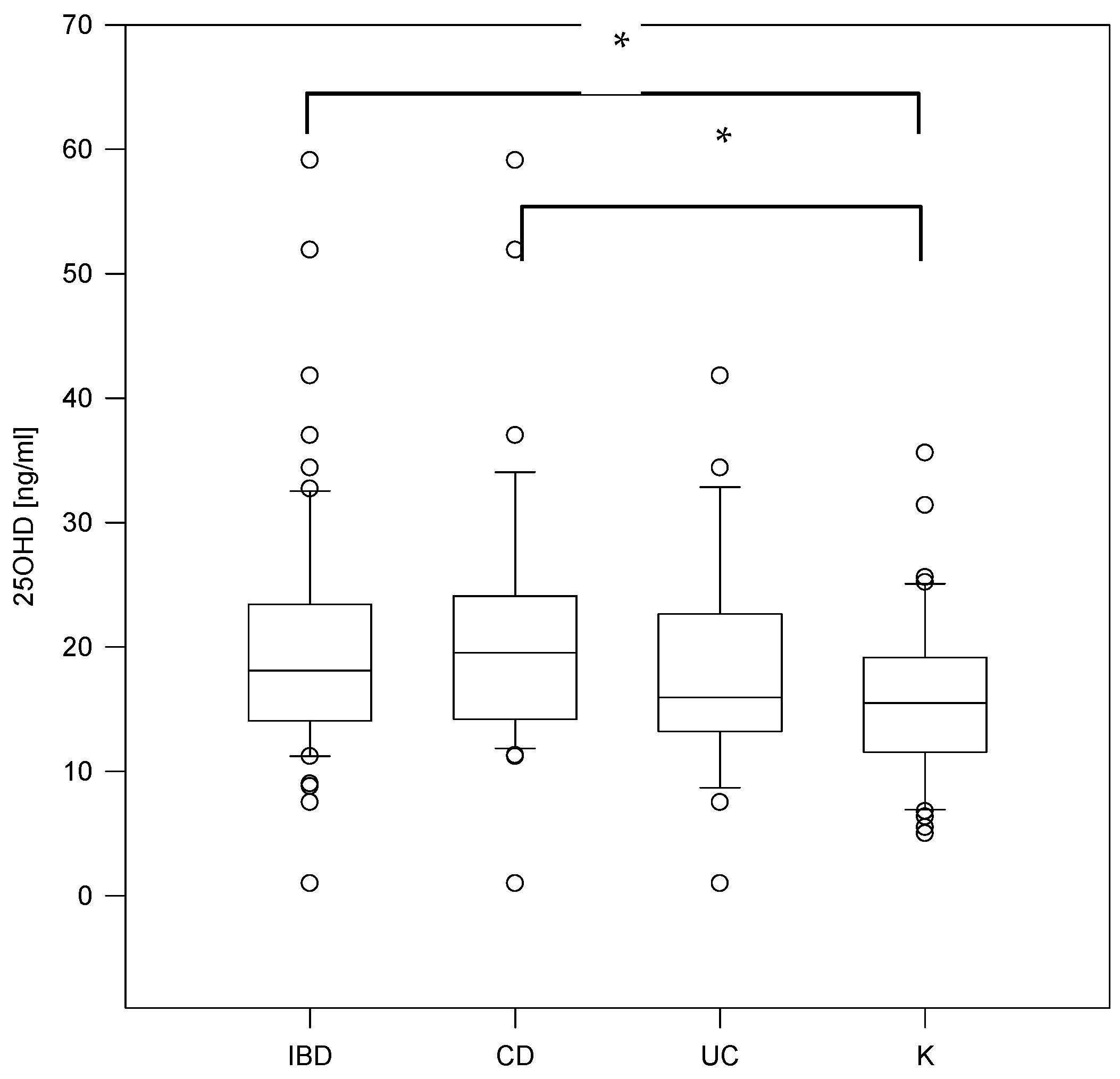
| 25OHD [ng/mL] | (30–50) | 19.55 (14.2–23.8) | 15.95 (13.3–22.65) | 18.1 (14.2–23.3) | 15.5 (11.83–18.7) | 0.047 | NS | 0.03 | NS |
| PTH [pg/mL] | (10–62) | 22.1 (11.7–31.0) | 18.4 (11.25–26.85) | 22.0 (11.5–27.9) | 17.2 (12.5–24.25) | NS | NS | NS | NS |
| Ca [mg/dL] | (8.4–10.2) | 9.78 (9.3–10.07) | 9.78 (9.46–10.06) | 9.70 (9.3–10.07) | 10.0 (9.81–10.3) | 0.002 | 0.002 | <0.001 | NS |
| P [mg/dL] | (2.9–5.1) | 4.3 (4.1–4.8) | 4.4 (4.0–4.99) | 4.35 (4.0–4.9) | 4.4 (4.1–5.0) | NS | NS | NS | NS |
| ALP [U/L] | (<390) | 117.5 (95.0–171.0) | 124.0 (98.5–200.0) | 117.5 (97.0–194.0) | 177.0 (90.5–229.5) | NS | NS | NS | NS |
| Urine Ca/cr ratio [mg/mg] | (0.01–0.25) | 0.125 (0.07–0.2) | 0.16 (0.09–0.21) | 0.13 (0.07–0.2) | NS | ||||
| ESR [mm/h] | 24.5 (13.0–40.0) | 25.0 (15.0–35.0) | 21.5 (10.5–52.5) | ||||||
| CRP [mg/L] | (1–5) | 2.25 (1.0–15.0) | 3.05 (1.0–16.5) | 2.75 (1.0–15.0) | 1.0 (1.0–1.0) | <0.001 | <0.0001 | <0.001 | NS |
| WBC [G/L] | (3.5–10) | 7.52 (5.4–8.8) | 9.18 (7.76–12.05) | 8.155 (6.15–10.59) | 6.73 (5.73–8.23) | NS | <0.001 | 0.009 | <0.001 |
| Neu [G/L] | (1.8–7.7) | 4.235 (2.59–6.5) | 5.79 (3.82–8.65) | 4.75 (3.02–6.6) | 3.86 (3.03–4.75) | NS | <0.001 | 0.058 | NS |
| Hb [g/dL] | (K:12–15; M:12.5–16.1) | 12.14 ± 1.16 | 12.11 ± 1.77 | 12.13 ± 1.45 | 13.62 ± 1.24 | <0.001 | <0.001 | <0.001 | NS |
| PLT [G/L] | (125–400) | 351.0 (299.0–420.0) | 343.5 (286.5–420.5) | 351.0 (290.0–420.0) | 268.0 (236.0–324.5) | <0.001 | <0.001 | <0.001 | NS |
| Season | Subgroup | Number of Patients in % | p | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 + 2 | |||
| Spring | IBD | 38.71 | 37.1 | 17.74 | 6.45 | 75.81 | 0.038 |
| CD | 41.18 | 35.29 | 14.71 | 8.82 | 76.47 | ||
| UC | 35.71 | 39.29 | 21.43 | 35.7 | 75.00 | ||
| C | 31.91 | 63.83 | 4.26 | 0.00 | 95.74 | ||
| Autumn | IBD | 29.03 | 45.16 | 20.97 | 4.84 | 74.19 | 0.002 |
| CD | 26.47 | 50.0 | 17.65 | 5.88 | 76.47 | ||
| UC | 32.14 | 39.29 | 25.0 | 3.57 | 71.43 | ||
| C | 12.77 | 85.11 | 2.13 | 0.00 | 97.88 | ||
| Summer | IBD | 59.68 | 27.42 | 8.06 | 4.84 | 87.1 | 0.144 |
| CD | 55.88 | 29.41 | 8.82 | 5.88 | 85.29 | ||
| UC | 64.29 | 25.0 | 7.14 | 3.57 | 89.29 | ||
| C | 40.43 | 53.19 | 6.38 | 0.00 | 93.62 | ||
| Winter | IBD | 22.58 | 41.93 | 29.03 | 6.45 | 64.51 | 0.0015 |
| CD | 20.59 | 47.06 | 23.53 | 8.82 | 67.65 | ||
| UC | 25.00 | 35.71 | 35.71 | 3.57 | 60.71 | ||
| C | 4.26 | 85.11 | 10.64 | 0.00 | 89.37 | ||
| Points | Vitamin D Level (ng/mL) |
|---|---|
| 0 | 12.1 (11.43–20.43) |
| 4 | 14.5 (13.8–19.3) |
| 5 | 15.75 (13.85–17.9) |
| 6 | 14.9 (14.2–22.13) |
| 7 | 8.5 (7.03–20.43) |
| 8 | 13.4 (11.1–17.1) |
| 9 | 17.2 (14.0–22.83) |
| 10 | 20.0 (16.95–28.2) |
| 11 | 19.6 (17.3–22.53) |
| 12 | 24.55 (17.6–32.7) |
| Points | Vitamin D Level (ng/mL) |
|---|---|
| 0 | 12.1 (11.43–20.43) |
| 1 | 13.95 (12.6–15.35) |
| 2 | 15.5 (11.6–18.7) |
| 3 | 19.45 (14.0–24.2) |
| Points | IBD | C |
|---|---|---|
| 0–3 | 12.1 (11.42–20.42) | |
| 4–6 | 14.5 (14.02–19.6) | 14.35 (13.8–14.9) |
| 7–9 | 15.2 (11.57–22.67) | 13.4 (10.85–17.87) |
| 10–12 | 15.2 (11.57–22.67) | 18.75 (16.95–23.5) |
| Points | IBS Sup | IBD No Sup | p | C Sup | C No Sup | p |
|---|---|---|---|---|---|---|
| 0–3 | 23.2 (23.2–23.2) | 11.65 (11.2–12.1) | * | * | * | * |
| 4–6 | 18.9 (14.35–21.25) | 14.5 (12.95–15.2) | 0.05 | 13.8 (13.8–13.8) | 14.9 (14.9–14.9) | NS |
| 7–9 | 22.6 (13.65–27.95) | 14.2 (11.3–18.1) | 0.06 | 10.7 (7.55–13.42) | 13.65 (11.3–18.4) | 0.07 |
| 10–12 | 23.8 (18.53–33.98) | 19.7 (14.3–24.25) | 0.16 | 26.75 (17.9–35.6) | 18.7 (16.2–22.1) | 0.19 |
| Points | Total Sup | Total No Sup | p |
|---|---|---|---|
| 0–3 | 23.2 | 11.65 (11.2–12.1) | - |
| 4–6 | 18.5 (14.1–20.87) | 14.5 (13.3–14.9) | 0.17 |
| 7–9 | 17.14 ± 9.61 | 14.69 ± 6.07 | 0.26 |
| 10–12 | 23.8 (18.05–34.7) | 19.4 (15.5–22.1) | 0.05 |
| 1 | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| Milk | IBD | 22.58 | 29.03 | 14.51 | 33.87 | 0.0009 |
| C | 34.04 | 53.19 | 12.77 | 0 | ||
| Yogurt | IBD | 29.03 | 38.71 | 16.13 | 16.13 | 0.049 |
| C | 34.04 | 51.06 | 14.89 | 0 | ||
| Yellow cheese | IBD | 38.71 | 43.55 | 11.29 | 6.45 | 0.02 |
| C | 44.68 | 55.32 | 0 | 0 | ||
| Eggs | IBD | 3.22 | 80.64 | 12.91 | 3.22 | 0.00085 |
| C | 0 | 48.94 | 51.06 | 0 | ||
| Fat fish | IBD | 0 | 8.06 | 40.32 | 51.61 | 0.00004 |
| C | 0 | 0 | 44.68 | 55.32 |
| IBD | C | p | |
|---|---|---|---|
| Kcal | 1650.95 (1483.37–1939.07) | 1787.75 (1512.22–2073.3) | NS |
| %EAR | 72.45 ± 14.13 | 83.4 ± 17.44 | <0.001 |
| Protein (g) | 69.64 ± 16.07 | 75.98 ± 16.25 | 0.044 |
| Protein (g/kg) | 1.46 (1.22–1.82) | 1.47 (1.27–1.89) | NS |
| Protein (%) | 16.81 (14.31–18.49) | 16.02 (14.35–19.17) | NS |
| Fat (g) | 53.85 (45.84–70.02) | 56.21 (45.78–83.19) | NS |
| Fat (%) | 30.43 ± 5.76 | 29.34 ± 47.22 | NS |
| Carbs (g) | 232.21 (197.58–273.75) | 245.12 (207.99–291.56) | NS |
| Carbs (%) | 52.93 ± 96.14 | 53.27 ± 7.17 | NS |
| * | 15.3:20.5:24.3:17.3:20.8 | 13.9:20.6:23.6:17.9:20.2 | NS |
| Vit D (IU) | 79.63 (56.53–107.56) | 85.14 (62.27–114.39 | NS |
| Ca (mg) | 587.542 (461.59–758.59) | 699.423 (547.32–874.96) | 0.034 |
| P (mg) | 1051.42 (915.239–1146.65) | 1451.02 (1058.46–1305.51) | NS |
| Ca:P | 0.580 (0.49–0.72) | 0.62 (0.51–0.72) | NS |
| Characteristic | 25OHD [ng/mL]; Mean ± SD or Median (25–75%) | ||||
|---|---|---|---|---|---|
| IBD | CD | UC | C | p | |
| Vitamin D supplementation | Y–22.0 (16.33–26.08) N–14.4 (12.33–19.33) | Y–23.25 (20.15–28.45) N–14.45 (13.3–19.2) | Y–20.23 ± 9.28 N–15.52 ± 8.16 | Y–13.55 (9.55–15.85) N–16.2 (12.73–19.38) | IBD 601–1000 vs. IBD vitD– p = 0.002 IBD vitD 601–1000 vs. C vitD- p = 0.003 CD 601–1000 vs. CD vitD– p = 0.002 CD Y vs. N < 0.001 UC – NS |
| Dose of vitamin D supplementation: | |||||
| <600 IU | 19.8 (16.48–22.55) | 20.9 (19.55–36.95) | 16.68 ± 6.48 | 13.8 (9.63–16.88) | |
| 601–1000 IU | 31.1 (21.18–35.92) | 34.05 (25.9–48.05) | 23.27 ± 9.12 | ||
| >1001 IU | 20.5 (15.075–25.2) | 23.25 (19.3–24.4) | 21.19 ± 10.87 | 10.7 | |
| Steroids | Y–18.1 (13.38–23.23) N–18.1 (14.2–24.0) | Y–19.75 (14.35–24.15) N–18.95 (14.2–23.8) | Y–15.74 ± 7.71 N–19.63 ± 9.5 | NS | |
| Steroids+ vitD+ (S+D+) | 20.5 (14.03–23.73) | 23.2 (20.2–28.0) | 16.2 ± 8.11 | IBD D+ S- vs. IBD D- S- p = 0.003 IBD D+ S- vs. IBD D- S+ p = 0.003 CD D+ S- vs. CD D- S- p = 0.004 CD D+ S+ vs. CD D- S- p = 0.004 | |
| Steroids + vitD– (S+D–) | 14.5 (12.85–19.7) | 14.5 (13.33–19.95) | 12.1 ± 0 | ||
| Steroids - vitD+ (S-D+) | 22.6 (18.3–29.0) | 23.8 (20.35–29.77) | 23.81 ± 9.16 | ||
| Steroids – vitD– (S–D–) | 14.2 (11.75–17.9) | 14.2 (11.9–16.43) | 15.86 ± 8.52 | ||
| Azathioprine | Y–18.1 (13.95–23.28) N–18.1 (14.2–23.53) | * | * | NS | |
| Biological treatment | Y-22.6 (14.0–27.3) N–17.45 (14.2–22.6) | * | * | NS | |
| Nutrition (parenteral/enteral): | Y–19.5 (13.95–30.65) N–17.85 (14.2–23.2) | * | * | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śledzińska, K.; Landowski, P.; Żmijewski, M.A.; Kamińska, B.; Kowalski, K.; Liberek, A. Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease. Nutrients 2022, 14, 1029. https://doi.org/10.3390/nu14051029
Śledzińska K, Landowski P, Żmijewski MA, Kamińska B, Kowalski K, Liberek A. Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease. Nutrients. 2022; 14(5):1029. https://doi.org/10.3390/nu14051029
Chicago/Turabian StyleŚledzińska, Karolina, Piotr Landowski, Michał A. Żmijewski, Barbara Kamińska, Konrad Kowalski, and Anna Liberek. 2022. "Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease" Nutrients 14, no. 5: 1029. https://doi.org/10.3390/nu14051029
APA StyleŚledzińska, K., Landowski, P., Żmijewski, M. A., Kamińska, B., Kowalski, K., & Liberek, A. (2022). Diet, Sun, Physical Activity and Vitamin D Status in Children with Inflammatory Bowel Disease. Nutrients, 14(5), 1029. https://doi.org/10.3390/nu14051029






