Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil
Abstract
1. Introduction
2. Methodology
2.1. Study Population
2.2. Anthropometric Measures
2.3. Biochemical Measures
2.4. Assessment of Sun Exposure and Dietary Intake
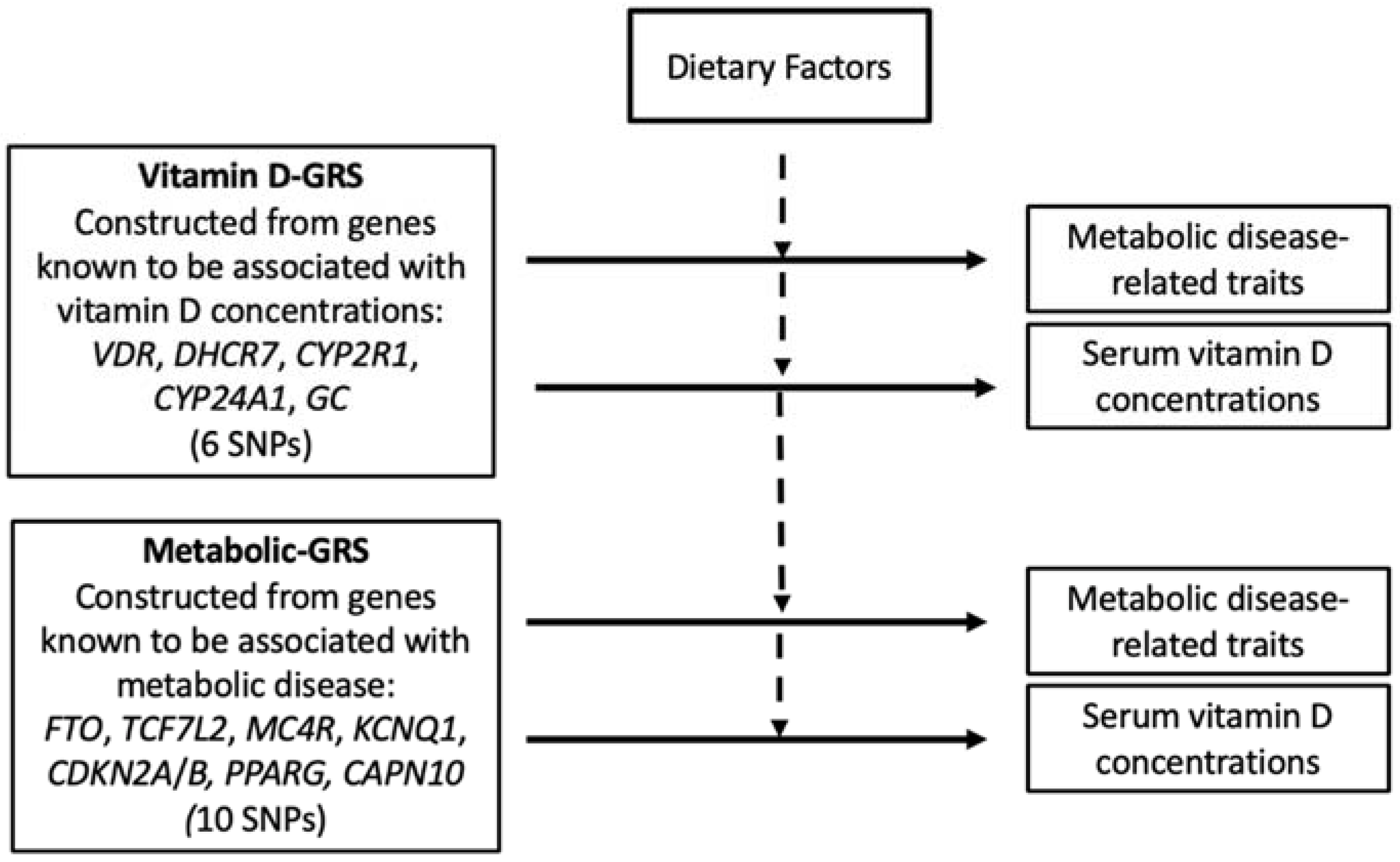
2.5. SNP Selection and Genotyping
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
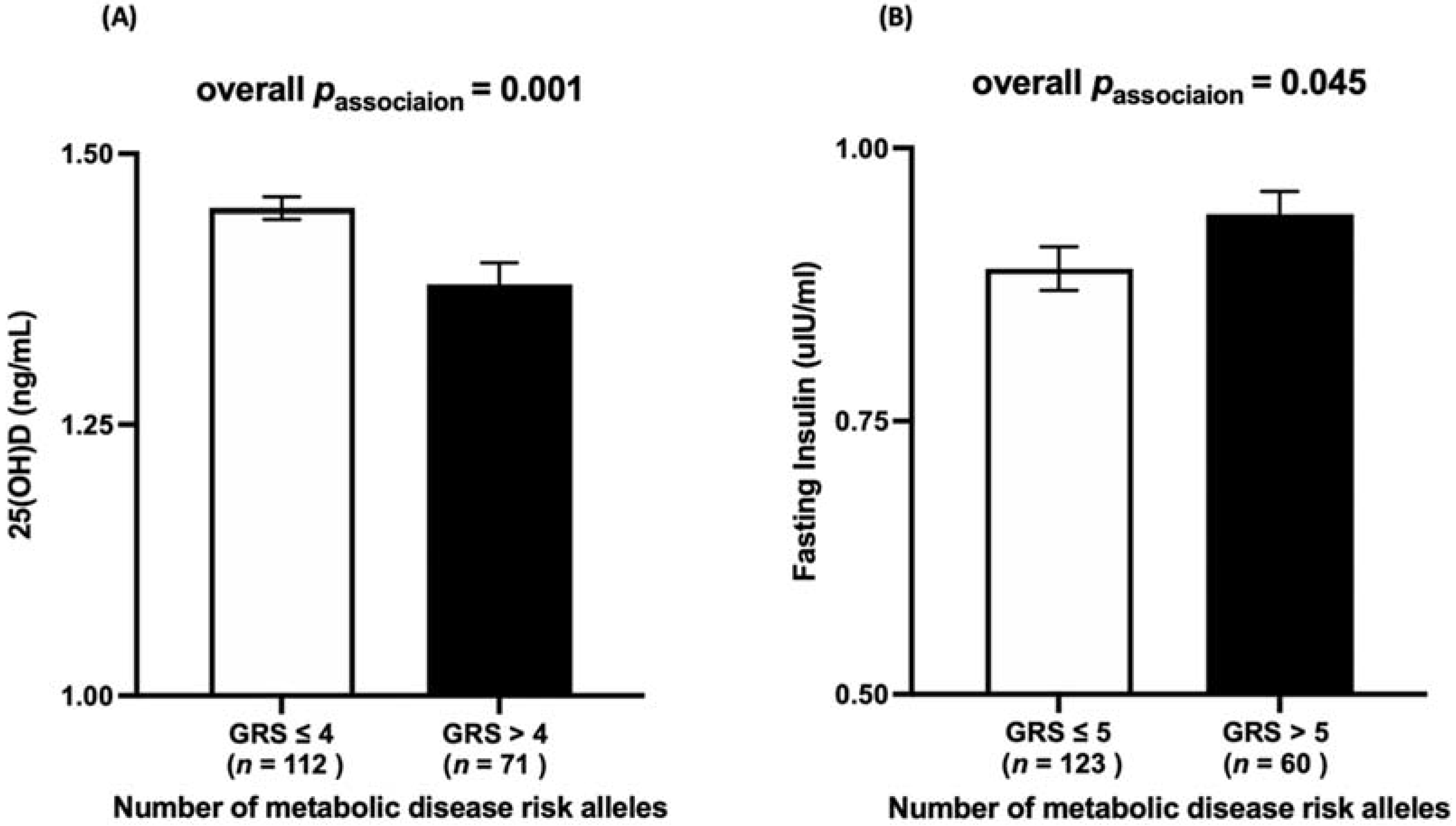
3.2. Association between Vitamin D-GRS and Anthropometric and Biochemical Measurements
3.3. Association between Metabolic-GRS and Anthropometric and Biochemical Measurements
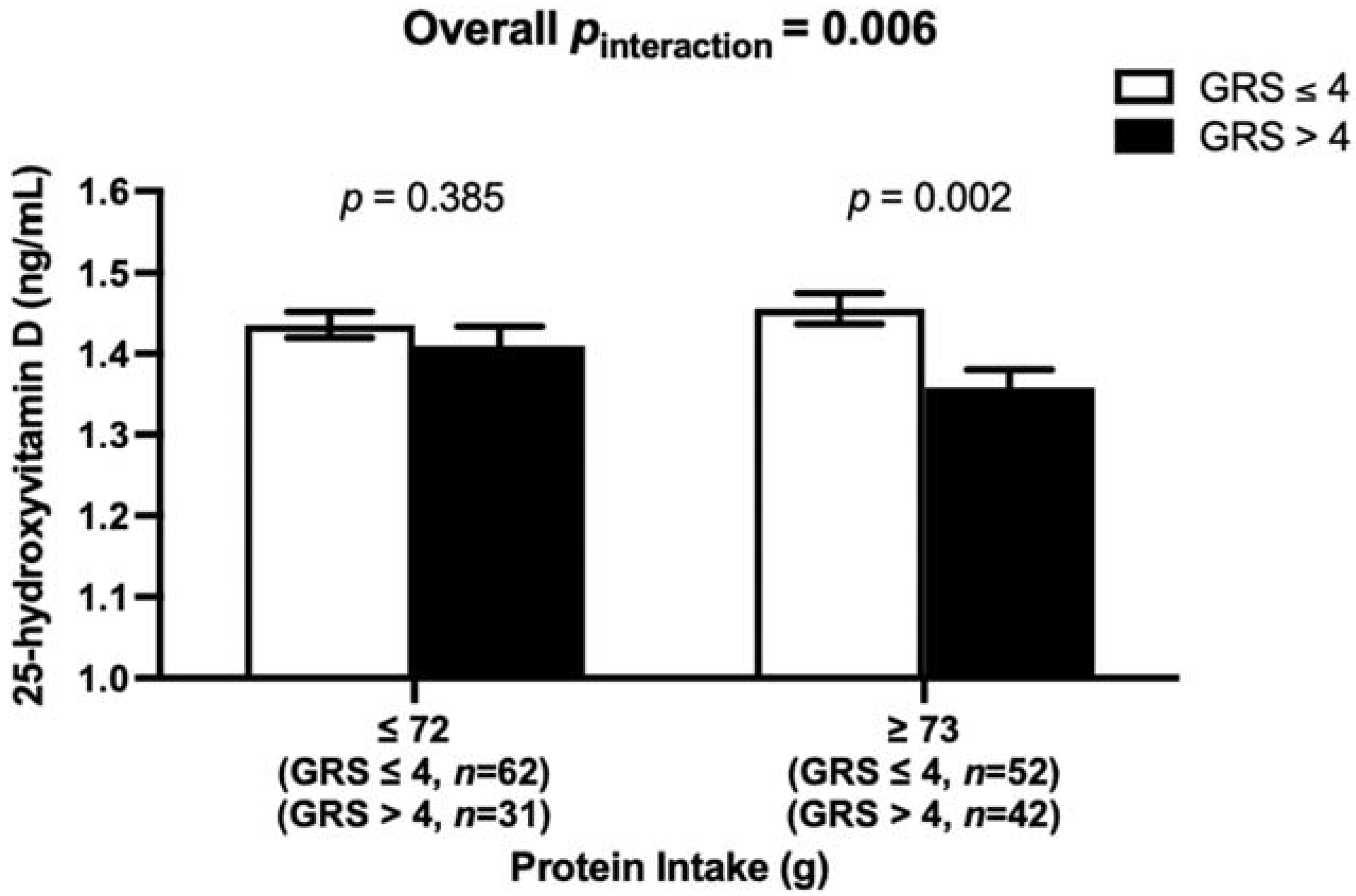
3.4. Interaction between the Vitamin D-GRS and Dietary Factors on Biochemical and Anthropometric Measurements
3.5. Interaction between the Metabolic-GRS and Dietary Factors on Clinical and Biochemical Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin d: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Benhamou, J.; Schindler, C.; Rutishauser, J. Prevalence of vitamin D deficiency in an inpatient population in the Swiss Canton of Basel-country. Swiss. Med. Wkly. 2021, 151, w20470. [Google Scholar] [CrossRef]
- Brito, A.; Cori, H.; Olivares, M.; Fernanda Mujica, M.; Cediel, G.; López de Romaña, D. Less than adequate vitamin D status and intake in Latin America and the Caribbean:a problem of unknown magnitude. Food Nutr. Bull. 2013, 34, 52–64. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Santos, J.; Carvalho, G.Q.; Santos, D.B.D.; Oliveira, A.M. Epidemiology of vitamin D insufficiency and deficiency in a population in a sunny country: Geospatial meta-analysis in Brazil. Crit. Rev. Food. Sci. Nutr. 2019, 59, 2102–2109. [Google Scholar] [CrossRef]
- Bandeira, F.; Griz, L.; Dreyer, P.; Eufrazino, C.; Bandeira, C.; Freese, E. Vitamin D deficiency: A global perspective. Arq Bras Endocrinol. Metabol. 2006, 50, 640–646. [Google Scholar] [CrossRef]
- Eloi, M.; Horvath, D.V.; Szejnfeld, V.L.; Ortega, J.C.; Rocha, D.A.; Szejnfeld, J.; Castro, C.H. Vitamin D deficiency and seasonal variation over the years in São Paulo, Brazil. Osteoporos. Int. 2016, 27, 3449–3456. [Google Scholar] [CrossRef]
- De Arêa Leão Borges Vera, C.; Callejas Ivan Julio, A.; Durante, L.C. Thermal sensation in outdoor urban spaces: A study in a Tropical Savannah climate, Brazil. Int. J. Biometeorol. 2020, 64, 533–545. [Google Scholar] [CrossRef]
- Peters, B.S.E.; Dos Santos, L.C.; Fisberg, M.; Wood, R.J.; Martini, L.A. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann. Nutr. Metab. 2009, 54, 15–21. [Google Scholar] [CrossRef]
- Triaca, L.M.; dos Santos, A.M.A.; Tejada, C.A.O. Socioeconomic inequalities in obesity in Brazil. Econ. Hum. Biol. 2020, 39, 100906. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Vigitel Brasil 2020—Vigilância de Fatores de Risco e Proteção Para Doenças Crônicas por Inquérito Telefônico. Brasília: Ministério da Saúde. 2021. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/publicacoes-svs/vigitel/relatorio-vigitel-2020-original.pdf/view (accessed on 15 November 2021).
- Dos Passos, C.M.; Maia, E.G.; Levy, R.B.; Martins, A.P.B.; Claro, R.M. Association between the price of ultra-processed foods and obesity in Brazil. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 589–598. [Google Scholar] [CrossRef]
- Correr, C.J.; Coura-Vital, W.; Frade, J.C.Q.P.; Nascimento, R.C.R.M.; Nascimento, L.G.; Pinheiro, E.B.; Ferreira, W.M.; Reis, J.S.; Melo, K.F.S.; Pontarolo, R.; et al. Prevalence of people at risk of developing type 2 diabetes mellitus and the involvement of community pharmacies in a national screening campaign: A pioneer action in Brazil. Diabetol. Metab. Syndr. 2020, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, W.F.; Silva Júnior, W.S. Diabetes care in Brazil. Ann. Glob. Health 2015, 81, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Salomone, E.; Pontecorvi, A.; Giaccari, A. Vitamin D Deficiency: A new risk factor for type 2 diabetes. Ann. Nutr. Metab. 2012, 61, 337–348. [Google Scholar] [CrossRef]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef]
- Scragg, R. Vitamin D and type 2 diabetes. Are We Ready Prev. Trial? 2008, 57, 2565–2566. [Google Scholar] [CrossRef][Green Version]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D deficiency: Consequence or cause of obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Alathari, B.E.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Tasrif, N.; Yani, F.F.; Sudji, I.R.; Lovegrove, J.A.; Lipoeto, N.I.; Vimaleswaran, K.S. Interaction between vitamin D-related genetic risk score and carbohydrate intake on body fat composition: A study in southeast asian minangkabau women. Nutrients 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Bodhini, D.; Jayashri, R.; Lakshmipriya, N.; Shanthi Rani, C.S.; Sudha, V.; Lovegrove, J.A.; Anjana, R.M.; Mohan, V.; Radha, V.; et al. A Nutrigenetic approach to investigate the relationship between metabolic traits and vitamin d status in an Asian Indian population. Nutrients 2020, 12, 1357. [Google Scholar] [CrossRef]
- Fenech, M.; El-Sohemy, A.; Cahill, L.; Ferguson, L.R.; French, T.-A.C.; Tai, E.S.; Milner, J.; Koh, W.-P.; Xie, L.; Zucker, M.; et al. Nutrigenetics and nutrigenomics: Viewpoints on the current status and applications in nutrition research and practice. J. Nutr. Nutr. 2011, 4, 69–89. [Google Scholar] [CrossRef]
- Hüls, A.; Krämer, U.; Carlsten, C.; Schikowski, T.; Ickstadt, K.; Schwender, H. Comparison of weighting approaches for genetic risk scores in gene-environment interaction studies. BMC Genet. 2017, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Udler, M.S.; McCarthy, M.I.; Florez, J.C.; Mahajan, A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr. Rev. 2019, 40, 1500–1520. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. Gene–nutrient interactions on metabolic diseases: Findings from the GeNuIne collaboration. Nutr. Bull. 2017, 42, 80–86. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. A nutrigenetics approach to study the impact of genetic and lifestyle factors on cardiometabolic traits in various ethnic groups: Findings from the GeNuIne Collaboration. Proc. Nutr. Soc. 2020, 79, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. GeNuIne (gene–nutrient interactions) Collaboration: Towards implementing multi-ethnic population-based nutrigenetic studies of vitamin B12 and D deficiencies and metabolic diseases. Proc. Nutr. Soc. 2021, 80, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Isgin-Atici, K.; Alathari, B.E.; Turan-Demirci, B.; Sendur, S.N.; Lay, I.; Ellahi, B.; Alikasifoglu, M.; Erbas, T.; Buyuktuncer, Z.; Santhanakrishnan, V.K. Interaction between dietary fat intake and metabolic genetic risk score on 25-hydroxyvitamin D concentrations in a Turkish adult population. Nutrients 2022, 14, 382. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Lohman, T.; Roche, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- De Lorenzo, A.; Martinoli, R.; Vaia, F.; Di Renzo, L. Normal weight obese (NWO) women: An evaluation of a candidate new syndrome. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 513–523. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Kwon, H.S.; Song, K.H.; Yim, H.W.; Lee, W.C.; Park, Y.M. Normal weight obesity in Korean adults. Clin. Endocrinol. 2014, 80, 214–220. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Wagner, D.; Hanwell, H.E.; Vieth, R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin. Biochem. 2009, 42, 1549–1556. [Google Scholar] [CrossRef]
- Climate-Data.org. Goiás Climate. 2021. Available online: https://en.climate-data.org/south-america/brazil/goias/goias-879942/ (accessed on 12 August 2021).
- Spark, W. Average Weather in Goiás. 2021. Available online: https://weatherspark.com/y/29864/Average-Weather-in-Goi%C3%A1s-Brazil-Year-Round (accessed on 12 August 2021).
- Køster, B.; Søndergaard, J.; Nielsen, J.B.; Allen, M.; Olsen, A.; Bentzen, J. The validated sun exposure questionnaire: Association of objective and subjective measures of sun exposure in a Danish population-based sample. Br. J. Dermatol. 2017, 176, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nakhl, S.; Sleilaty, G.; Chouery, E.; Salem, N.; Chahine, R.; Farès, N. FokI vitamin D receptor gene polymorphism and serum 25-hydroxyvitamin D in patients with cardiovascular risk. Arch. Med. Sci. Atheroscler Dis. 2019, 4, e298–e303. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, G.; Temel, S.G.; Ergoren, M.C. Strong association between VDR FokI (rs2228570) gene variant and serum vitamin D levels in Turkish cypriots. Mol. Biol. Rep. 2019, 46, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Foucan, L.; Velayoudom-Cephise, F.L.; Larifla, L.; Armand, C.; Deloumeaux, J.; Fagour, C.; Plumasseau, J.; Portlis, M.L.; Liu, L.; Bonnet, F.; et al. Polymorphisms in GC and NADSYN1 Genes are associated with vitamin D status and metabolic profile in Non-diabetic adults. BMC Endocr. Disord. 2013, 13, 36–43. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Xu, X.; Mao, J.; Zhang, M.; Liu, H.; Li, H.; Lei, H.; Han, L.; Gao, M. Vitamin D Deficiency in Uygurs and Kazaks is associated with polymorphisms in CYP2R1 and DHCR7/NADSYN1 Genes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1960–1968. [Google Scholar]
- Zhang, Y.; Wang, X.; Liu, Y.; Qu, H.; Qu, S.; Wang, W.; Ren, L. The GC, CYP2R1 and DHCR7 genes are associated with vitamin D levels in northeastern Han Chinese children. Swiss. Med. Wkly. 2012, 142, w13636. [Google Scholar] [CrossRef]
- Elkum, N.; Alkayal, F.; Noronha, F.; Ali, M.M.; Melhem, M.; Al-Arouj, M.; Bennakhi, A.; Behbehani, K.; Alsmadi, O.; Abubaker, J. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS ONE 2014, 9, e113102. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Yongjoo Park, C.; Jo, G.; Yoen Kim, O.; Shin, M.J. Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: Results from the Korea National Health and Nutrition Examination Survey 2011–2012. Endocr. J. 2018, 65, 881–891. [Google Scholar] [CrossRef]
- Bego, T.; Čaušević, A.; Dujić, T.; Malenica, M.; Velija-Asimi, Z.; Prnjavorac, B.; Marc, J.; Nekvindová, J.; Palička, V.; Semiz, S. Association of FTO gene variant (rs8050136) with type 2 diabetes and markers of obesity, glycaemic control and inflammation. J. Med. Biochem. 2019, 38, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chris, A.; Narila, N.; Flori, S.; Risfy, P.; Intan, A.; Zeti, H.; Witri, A.; Hari, H.; Nouval, S.; Endah, W.; et al. Preliminary study: Identification of DNA variation with SNP numbers RS1137101 and RS8050136 in patient’s type 2 diabetes mellitus at salsabila clinic bogor—Indonesia. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019, 21, 931–934. [Google Scholar]
- Li, H.; Kilpelainen, T.O.; Liu, C.; Zhu, J.; Liu, Y.; Hu, C.; Yang, Z.; Zhang, W.; Bao, W.; Cha, S.; et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 2012, 55, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.K.; Karpe, F.; Gu, H.F.; Brismar, K.; Fall, C.H.; Ingelsson, E.; Fall, T. FTO genetic variants and risk of obesity and type 2 diabetes: A meta-analysis of 28,394 Indians. Obesity 2014, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Angquist, L.; Hansen, R.D.; Daphne, L.v.d.A.; Bouatia-Naji, N.; Holst, C.; Tjonneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; et al. Association between FTO variant and change in body weight and its interaction with dietary factors: The DiOGenes study. Obesity 2012, 20, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Bodhini, D.; Lakshmipriya, N.; Ramya, K.; Anjana, R.M.; Sudha, V.; Lovegrove, J.A.; Kinra, S.; Mohan, V.; Radha, V. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Bodhini, D.; Gaal, S.; Shatwan, I.; Ramya, K.; Ellahi, B.; Surendran, S.; Sudha, V.; Anjana, M.R.; Mohan, V.; Lovegrove, J.A.; et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE 2017, 12, e0188382. [Google Scholar] [CrossRef]
- Chauhan, G.; Spurgeon, C.J.; Tabassum, R.; Bhaskar, S.; Kulkarni, S.R.; Mahajan, A.; Chavali, S.; Kumar, M.V.K.; Prakash, S.; Dwivedi, O.P.; et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5164 Indians. Diabetes 2010, 59, 2068–2074. [Google Scholar] [CrossRef]
- Chidambaram, M.; Liju, S.; Saboo, B.; Sathyavani, K.; Viswanathan, V.; Pankratz, N.; Gross, M.; Mohan, V.; Radha, V. Replication of genom—Wide association signals in Asian Indians with early-onset type 2 diabetes. Acta Diabetol 2016, 53, 915–923. [Google Scholar] [CrossRef]
- Gupta, V.; Khadgawat, R.; Ng, H.K.T.; Walia, G.K.; Kalla, L.; Rao, V.R.; Sachdeva, M.P. Association of TCF7L2 and ADIPOQ with body mass index, waist-hip ratio, and systolic blood pressure in an endogamous ethnic group of India. Genet. Test. Mol. Biomark. 2012, 16, 948–951. [Google Scholar] [CrossRef]
- Khan, I.A.; Poornima, S.; Jahan, P.; Rao, P.; Hasan, Q. Type 2 Diabetes mellitus and the association of candidate genes in Asian Indian population from Hyderabad, India. J. Clin. Diagn. Res. 2015, 9, Gc01–Gc05. [Google Scholar] [CrossRef] [PubMed]
- Phani, N.M.; Adhikari, P.; Nagri, S.K.; D’Souza, S.C.; Satyamoorthy, K.; Rai, P.S. Replication and Relevance of multiple susceptibility loci discovered from genome wide association studies for type 2 diabetes in an indian population. PLoS ONE 2016, 11, e0157364. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mittal, B.; Prakash, J.; Srivastava, P.; Srivastava, N.; Srivastava, N. A multianalytical approach to evaluate the association of 55 SNPs in 28 genes with obesity risk in North Indian adults. Am. J. Hum. Biol. 2017, 29, e22923. [Google Scholar] [CrossRef] [PubMed]
- Apalasamy, Y.D.; Ming, M.F.; Rampal, S.; Bulgiba, A.; Mohamed, Z. Association of melanocortin-4 receptor gene polymorphisms with obesity-related parameters in Malaysian Malays. Ann. Hum. Biol. 2013, 40, 102–106. [Google Scholar] [CrossRef]
- Srivastava, A.; Mittal, B.; Prakash, J.; Narain, V.S.; Natu, S.M.; Srivastava, N. Evaluation of MC4R [rs17782313, rs17700633], AGRP [rs3412352] and POMC [rs1042571] polymorphisms with obesity in Northern India. Oman. Med. J. 2014, 29, 114–118. [Google Scholar] [CrossRef]
- Srivastava, A.; Mittal, B.; Prakash, J.; Srivastava, P.; Srivastava, N. Analysis of MC4R rs17782313, POMC rs1042571, APOE-Hha1 and AGRP rs3412352 genetic variants with susceptibility to obesity risk in North Indians. Ann. Hum. Biol. 2016, 43, 285–288. [Google Scholar] [CrossRef]
- Vasan, S.K.; Fall, T.; Neville, M.J.; Antonisamy, B.; Fall, C.H.; Geethanjali, F.S.; Gu, H.F.; Raghupathy, P.; Samuel, P.; Thomas, N.; et al. Associations of variants in FTO and near MC4R with obesity traits in South Asian Indians. Obesity 2012, 20, 2268–2277. [Google Scholar] [CrossRef]
- Been, L.F.; Ralhan, S.; Wander, G.S.; Mehra, N.K.; Singh, J.; Mulvihill, J.J.; Aston, C.E.; Sanghera, D.K. Variants in KCNQ1 increase type II diabetes susceptibility in South Asians: A study of 3310 subjects from India and the US. BMC Med. Genet. 2011, 12, 18. [Google Scholar] [CrossRef]
- Qi, Q.; Li, H.; Loos, R.J.F.; Liu, C.; Wu, Y.; Hu, F.B.; Wu, H.; Lu, L.; Yu, Z.; Lin, X. Common variants in KCNQ1 are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Hum. Mol. Genet. 2009, 18, 3508–3515. [Google Scholar] [CrossRef]
- Binh, T.Q.; Thu, N.T.T.; Phuong, P.T.; Nhung, B.T.; Nhung, T.T.H. CDKN2A-rs10811661 polymorphism, waist-hip ratio, systolic blood pressure, and dyslipidemia are the independent risk factors for prediabetes in a Vietnamese population. BMC Genet. 2015, 16, 1–8. [Google Scholar] [CrossRef][Green Version]
- Plengvidhya, N.; Chanprasert, C.; Chongjaroen, N.; Yenchitsomanus, P.-t.; Homsanit, M.; Tangjittipokin, W. Impact of KCNQ1, CDKN2A/2B, CDKAL1, HHEX, MTNR1B, SLC30A8, TCF7L2, and UBE2E2 on risk of developing type 2 diabetes in Thai population. BMC Med. Genet. 2018, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiao, J.; He, J.; Zhang, X.; Hong, J.; Kong, X.; Mills, K.T.; Weng, J.; Jia, W.; Yang, W. Cross-sectional and longitudinal replication analyses of genome-wide association loci of type 2 diabetes in Han Chinese. PLoS ONE 2014, 9, e91790. [Google Scholar] [CrossRef]
- Ho, J.S.K.; Germer, S.; Tam, C.H.T.; So, W.-Y.; Martin, M.; Ma, R.C.W.; Chan, J.C.N.; Ng, M.C.Y. Association of the PPARG Pro12Ala polymorphism with type 2 diabetes and incident coronary heart disease in a Hong Kong Chinese population. Diabetes Res. Clin. Pract. 2012, 97, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Srivastava, N.; Awasthi, S.; Agarwal, C.; Natu, S.; Rajpal, N.; Mittal, B. Association of PPAR-γ gene polymorphisms with obesity and obesity-associated phenotypes in North Indian population. Am. J. Hum. Biol. 2012, 24, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, D.K.; Ortega, L.; Han, S.; Singh, J.; Ralhan, S.K.; Wander, G.S.; Mehra, N.K.; Mulvihill, J.J.; Ferrell, R.E.; Nath, S.K.; et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTOvariants confer a significant risk. BMC Med. Genet. 2008, 9, 59. [Google Scholar] [CrossRef]
- Cui, J.; Xu, X.; Yin, S.; Chen, F.; Li, P.; Song, C. Meta-analysis of the association between four CAPN10 gene variants and gestational diabetes mellitus. Arch. Gynecol. Obstet. 2016, 294, 447–453. [Google Scholar] [CrossRef]
- Bodhini, D.; Radha, V.; Ghosh, S.; Sanapala, K.R.; Majumder, P.P.; Satyanarayana Rao, M.R.; Mohan, V. Association of calpain 10 gene polymorphisms with type 2 diabetes mellitus in Southern Indians. Metabolism 2011, 60, 681–688. [Google Scholar] [CrossRef]
- Moreira, C.A.; Ferreira, C.; Madeira, M.; Silva, B.C.C.; Maeda, S.S.; Batista, M.C.; Bandeira, F.; Borba, V.Z.C.; Lazaretti-Castro, M. Reference values of 25-hydroxyvitamin D revisited: A position statement from the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian society of clinical pathology/laboratory medicine (SBPC). Arch. Endocrinol. Metab. 2020, 64, 462–478. [Google Scholar] [CrossRef]
- De Souza, R.J.; Bray, G.A.; Carey, V.J.; Hall, K.D.; LeBoff, M.S.; Loria, C.M.; Laranjo, N.M.; Sacks, F.M.; Smith, S.R. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: Results from the POUNDS LOST trial. Am. J. Clin. Nutr. 2012, 95, 614–625. [Google Scholar] [CrossRef]
- Zhou, T.; Sun, D.; Heianza, Y.; Li, X.; Champagne, C.M.; LeBoff, M.S.; Shang, X.; Pei, X.; Bray, G.A.; Sacks, F.M.; et al. Genetically determined vitamin D levels and change in bone density during a weight-loss diet intervention: The preventing overweight using novel dietary strategies (pounds lost) trial. Am. J. Clin. Nutr. 2018, 108, 1129–1134. [Google Scholar] [CrossRef]
- Bonjour, J.-P.; Kraenzlin, M.; Levasseur, R.; Warren, M.; Whiting, S. Dairy in adulthood: From foods to nutrient interactions on bone and skeletal muscle health. J. Am. Coll. Nutr. 2013, 32, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Baig, J.A.; Sheikh, S.A.; Islam, I.; Kumar, M. Vitamin D status among vegetarians and non-vegetarians. J. Ayub. Med. Coll. Abbottabad. 2013, 25, 152–155. [Google Scholar] [PubMed]
- Souza, R.A.; Yokoo, E.M.; Sichieri, R.; Pereira, R.A. Energy and macronutrient intakes in Brazil: Results of the first nationwide individual dietary survey. Public Health Nutr. 2015, 18, 3086–3095. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2007. [Google Scholar]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation, Geneva, 28 January–1 February 2002. 2003. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/42665 (accessed on 9 December 2021).
- World Health Organization. Prevention of cardiovascular disease:Pocket guidelines for assessment and management of cardiovascular risk:(WHO/ISH cardiovascular risk prediction charts for the European Region). World Health Organization. 2007. Available online: https://apps.who.int/iris/andle/10665/43784 (accessed on 9 December 2021).
- Berry, D.J.; Vimaleswaran, K.S.; Whittaker, J.C.; Hingorani, A.D.; Hypponen, E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS ONE 2012, 7, e37465. [Google Scholar] [CrossRef]
- Dudbridge, F. Polygenic epidemiology. Genet. Epidemiol. 2016, 40, 268–272. [Google Scholar] [CrossRef]
- Liu, W.; Icitovic, N.; Shaffer, M.L.; Chase, G.A. The impact of population heterogeneity on risk estimation in genetic counseling. BMC Med. Genet. 2004, 5, 18. [Google Scholar] [CrossRef][Green Version]
- Hellwege, J.N.; Keaton, J.M.; Giri, A.; Gao, X.; Velez Edwards, D.R.; Edwards, T.L. Population Stratification in genetic association studies. Curr. Protoc. Hum. Genet. 2017, 95, 1.22.1–1.22.23. [Google Scholar] [CrossRef]
- Kehdy, F.S.; Gouveia, M.H.; Machado, M.; Magalhães, W.C.; Horimoto, A.R.; Horta, B.L.; Moreira, R.G.; Leal, T.P.; Scliar, M.O.; Soares-Souza, G.B.; et al. Origin and dynamics of admixture in Brazilians and its effect on the pattern of deleterious mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 8696–8701. [Google Scholar] [CrossRef]
- Souza, A.M.; Resende, S.S.; Sousa, T.N.; Brito, C.F.A. A systematic scoping review of the genetic ancestry of the Brazilian population. Genet. Mol. Biol. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. 2021. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 12 August 2021).
| Characteristics of Study Participants | n | Normal Vitamin D Status 25(OH)D ≥ 20 ng/mL | n | Deficient Vitamin D Status 25(OH)D < 20 ng/mL | p Value |
|---|---|---|---|---|---|
| Age (years) | 154 | 21.32 ± 1.71 | 31 | 21.35 ± 1.56 | 0.928 |
| BMI (kg/m2) | 154 | 23.01 ± 3.87 | 31 | 23.76 ± 5.66 | 0.370 |
| WC (cm) | 154 | 74.05 ± 11.89 | 31 | 76.60 ± 14.04 | 0.291 |
| BFP (%) | 154 | 33.76 ± 10.65 | 31 | 34.57 ± 11.05 | 0.702 |
| Glucose (mg/dl) | 156 | 86.74 ± 6.79 | 31 | 88.35 ± 7.29 | 0.235 |
| HbA1c (%) | 156 | 4.73 ± 0.26 | 31 | 4.72 ± 0.22 | 0.911 |
| Fasting Insulin (uIU/mL) | 156 | 8.72 ± 3.69 | 31 | 8.80 ± 4.09 | 0.911 |
| Total Energy Intake (kcal) | 156 | 1793 ± 591 | 31 | 2024.12 ± 676.96 | 0.054 |
| Total Protein (g) | 156 | 75.20 ± 28.17 | 31 | 90.43 ± 33.48 | 0.008 |
| Total Carbohydrate (g) | 156 | 230.67 ± 84.32 | 31 | 258.08 ± 99.59 | 0.111 |
| Total Fat (g) | 156 | 63.34 ± 23.43 | 31 | 70.017 ± 24.88 | 0.153 |
| Dietary Fiber (g) | 156 | 14.45 ± 8.48 | 31 | 16.39 ± 9.68 | 0.258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alathari, B.E.; Cruvinel, N.T.; da Silva, N.R.; Chandrabose, M.; Lovegrove, J.A.; Horst, M.A.; Vimaleswaran, K.S. Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil. Nutrients 2022, 14, 1015. https://doi.org/10.3390/nu14051015
Alathari BE, Cruvinel NT, da Silva NR, Chandrabose M, Lovegrove JA, Horst MA, Vimaleswaran KS. Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil. Nutrients. 2022; 14(5):1015. https://doi.org/10.3390/nu14051015
Chicago/Turabian StyleAlathari, Buthaina E., Nathália Teixeira Cruvinel, Nara Rubia da Silva, Mathurra Chandrabose, Julie A. Lovegrove, Maria A. Horst, and Karani S. Vimaleswaran. 2022. "Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil" Nutrients 14, no. 5: 1015. https://doi.org/10.3390/nu14051015
APA StyleAlathari, B. E., Cruvinel, N. T., da Silva, N. R., Chandrabose, M., Lovegrove, J. A., Horst, M. A., & Vimaleswaran, K. S. (2022). Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil. Nutrients, 14(5), 1015. https://doi.org/10.3390/nu14051015








