Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Milk Collection
2.2. Isolation of HMOs
2.3. Composition Analysis of HMOs
2.4. Bacterial Culture
2.5. Cell Culture and Measures of Epithelial Barrier Integrity
2.6. Transepithelial Electrical Resistance (TER)
2.7. FITC-Dextran Permeability Assay
2.8. Immunofluorescence Microscopy
2.9. qPCR for mRNA Expression
- GAPDH, ACCCACTCCTCCACCTTTGAC (forward), CCACCACCCTGTTGCTGTAG (reverse);
- Β-actin, CTGGAACGGTGAAGGTGACA (forward), AAGGGACTTCCTGTAACAATGCA (reverse).
- ZO-1, GAATGATGGTTGGTATGGTGCG (forward), TCAGAAGTGTGTCTACTGTCCG (reverse);
- Claudin-1, AGCTGGCTGAGACACTGAAGA (forward), GAGAGGAAGGCACTGAACCA (reverse);
- Muc1, CCTCACAGTGCTTACAGTTGTT (forward), AGTAGTCGGTGCTGGGATCT (reverse);
- Muc2, TGTAGGCATCGCTCTTCTCA (forward), GACACCATCTACCTCACCCG (reverse);
- Tgfβ, CGGAGTTGTGCGGCAGTGGT (forward), GGCCGGTAGTGAACCCGTTGATG (reverse);
- Il-10, AGGAGGTGATGCCCCAAGCTGA (forward), ATCGATGACAGCGCCGTAGCCTC (reverse);
- CXCL-8, ACTGAGAGTGATTGAGAGTGGAC (forward), AACCCTCTGCACCCAGTTTTC (reverse);
- IL-18, TGCCCTCCTGGCTGCCAACT (forward), TCAGCAGCCATCTTTATTCCTGCG (reverse).
2.10. Immunoblotting
2.11. Statistical Analyses
3. Results
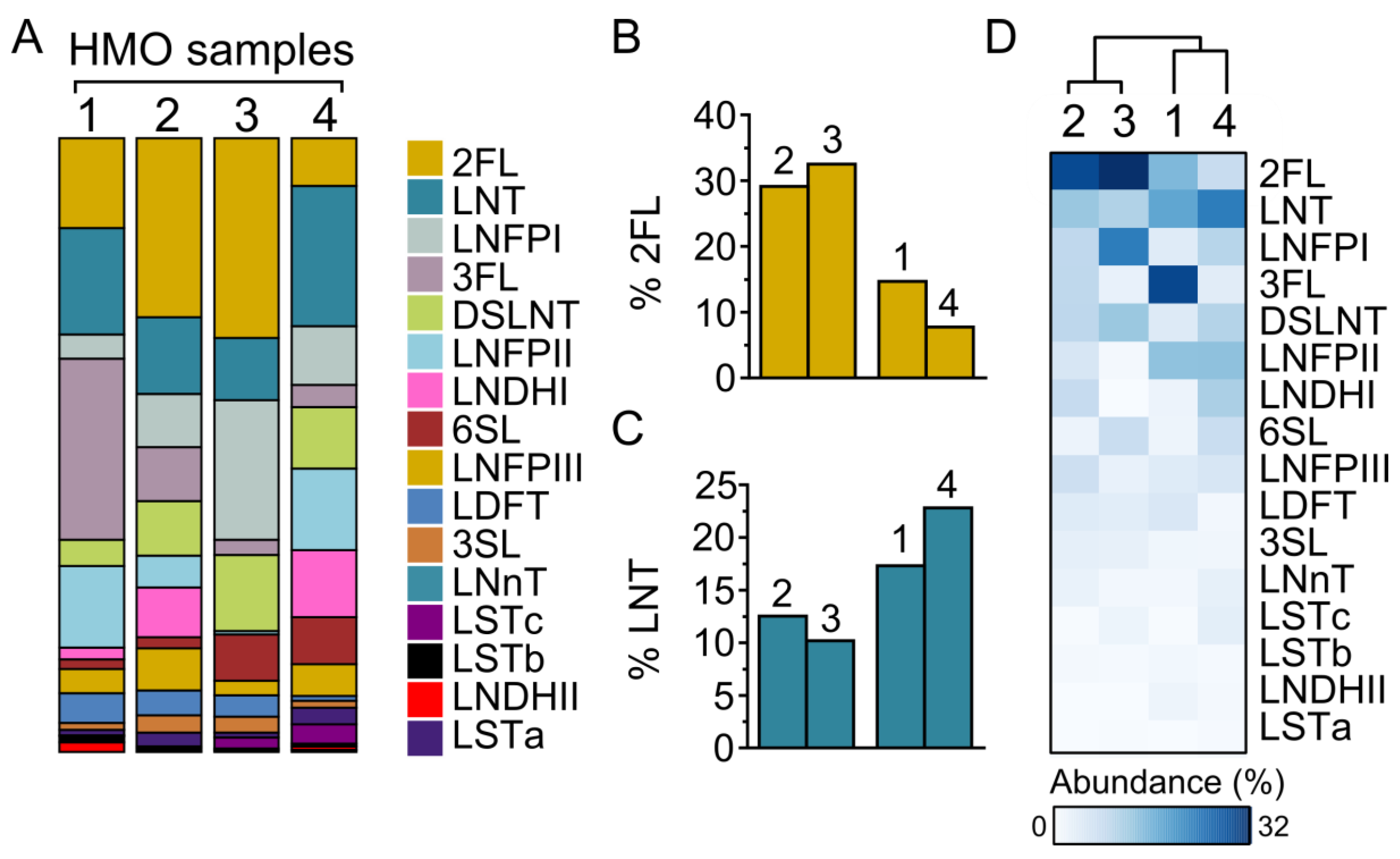
3.1. HMO Composition in Individual Mothers
3.2. HMOs Promote Intestinal Epithelial Barrier Integrity in an Individual-Dependent Manner
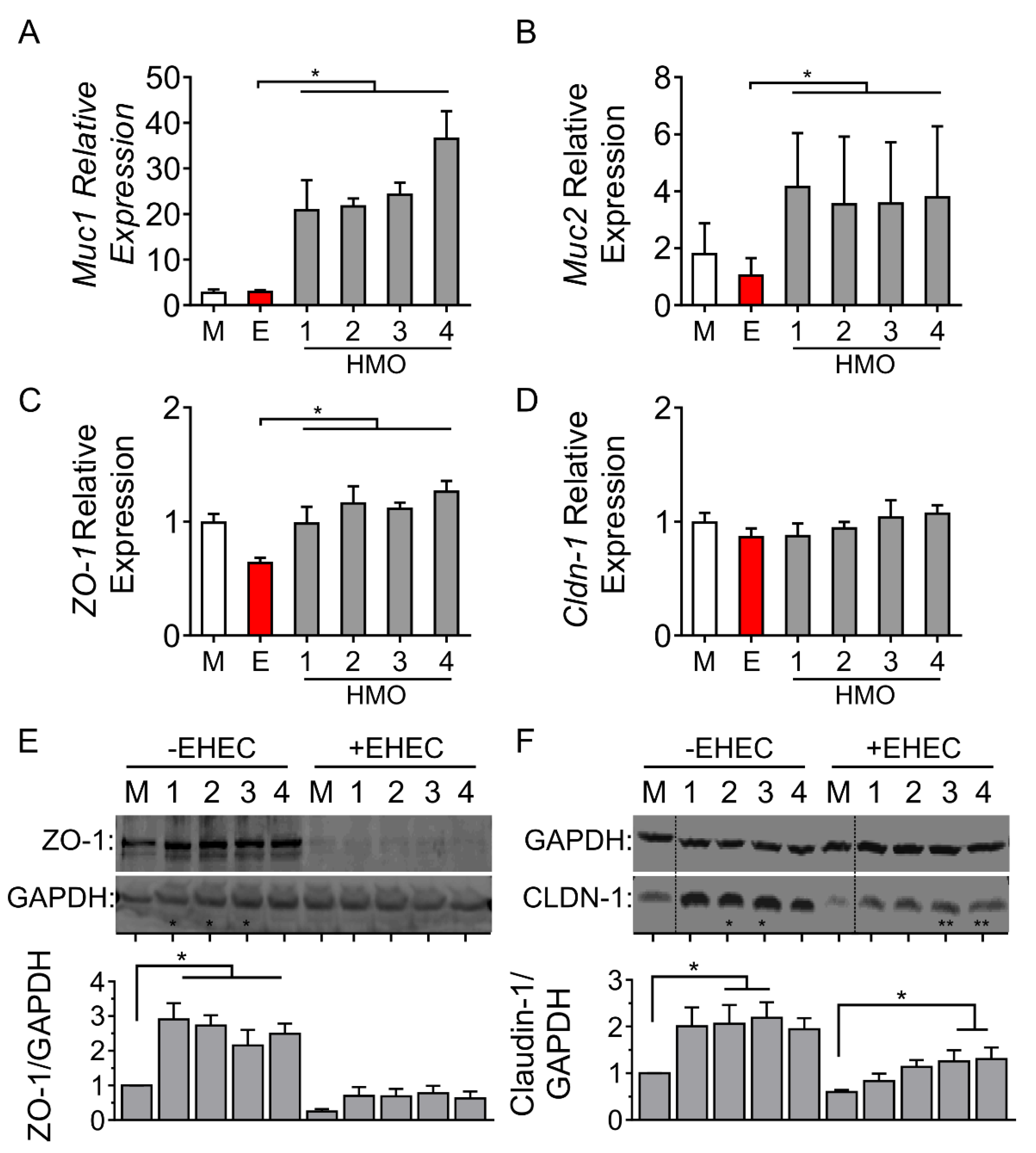
3.3. Individual HMO Blends Alter Barrier-Related Gene Expression
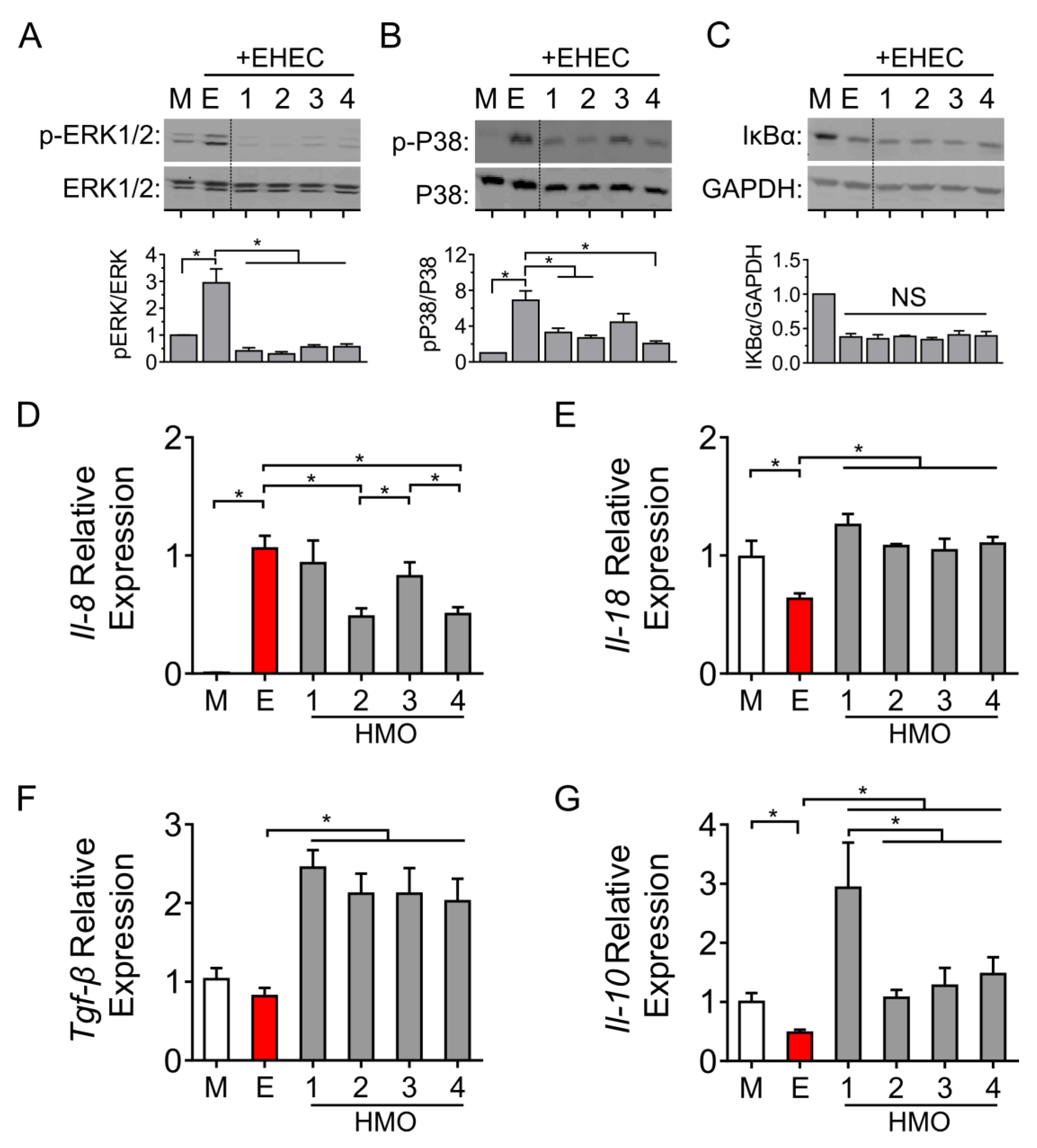
3.4. Individual HMO Blends Vary in Their Ability to Alter Host Inflammatory Responses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HMOs | Human milk oligosaccharides |
| TER | Transepithelial electrical resistance |
| EHEC | Enterohemorrhagic E. coli O157:H7 |
References
- Bode, L.; Raman, A.S.; Murch, S.H.; Rollins, N.C.; Gordon, J.I. Understanding the Mother-Breastmilk-Infant “Triad". Science 2020, 367, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In Vitro Fermentability of Human Milk Oligosaccharides by Several Strains of Bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter Jejuni–Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Sherman, P.M. Multifaceted Effects of Human Milk Oligosaccharides. J. Infect. Dis. 2014, 209, 323–324. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Wu, R.Y.; Horne, R.G.; Ahmed, A.; Lee, D.; Robinson, S.C.; Zhu, H.; Lee, C.; Cadete, M.; Johnson-Henry, K.C.; et al. Human Milk Oligosaccharides Protect against Necrotizing Enterocolitis by Activating Intestinal Cell Differentiation. Mol. Nutr. Food Res. 2020, 64, 2000519. [Google Scholar] [CrossRef]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional Benefits of Prevalent HMOs: Implications for Infant Health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef]
- Bode, L.; Jantscher-Krenn, E. Structure-Function Relationships of Human Milk Oligosaccharides. Adv. Nutr. Int. Rev. J. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive Profiles of Human Milk Oligosaccharides Yield Highly Sensitive and Specific Markers for Determining Secretor Status in Lactating Mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s Normal? Oligosaccharide Concentrations and Profiles in Milk Produced by Healthy Women Vary Geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human Milk Oligosaccharides Inhibit Rotavirus Infectivity in Vitro and in Acutely Infected Piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The Human Milk Oligosaccharide Disialyllacto-N-Tetraose Prevents Necrotising Enterocolitis in Neonatal Rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human Milk Oligosaccharide Composition Predicts Risk of Necrotising Enterocolitis in Preterm Infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Wu, R.Y.; Li, B.; Horne, R.G.; Ahmed, A.; Lee, D.; Robinson, S.C.; Zhu, H.; Cadete, M.; Alganabi, M.; Filler, R.; et al. Structure-function Relationships of Human Milk Oligosaccharides on the Intestinal Epithelial Transcriptome in Caco-2 Cells and a Murine Model of Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2021, 66, 2100893. [Google Scholar] [CrossRef]
- Peila, C.; Moro, G.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2018, 63, 1800658. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein Kinase C δ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of Human Milk Oligosaccharides in Relation to Milk Groups and Lactational Periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Määttänen, P.; Napper, S.; Scruten, E.; Li, B.; Koike, Y.; Johnson-Henry, K.C.; Pierro, A.; Rossi, L.; Botts, S.R.; et al. Non-Digestible Oligosaccharides Directly Regulate Host Kinome to Modulate Host Inflammatory Responses without Alterations in the Gut Microbiota. Microbiome 2017, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The Genome Sequence of Bifidobacterium Longum Subsp. Infantis Reveals Adaptations for Milk Utilization within the Infant Microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-Milk Glycans That Inhibit Pathogen Binding Protect Breast-Feeding Infants against Infectious Diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, C.; Walvoort, M.T.C.; Faas, M.M.; de Vos, P. Human Milk Oligosaccharides Differently Modulate Goblet Cells Under Homeostatic, Proinflammatory Conditions and ER Stress. Mol. Nutr. Food Res. 2020, 64, e1900976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kong, C.; Wang, W.; Groeneveld, A.; Nauta, A.; Groves, M.R.; Kiewiet, M.B.G.; de Vos, P. The Human Milk Oligosaccharides 3-FL, Lacto-N-Neotetraose, and LDFT Attenuate Tumor Necrosis Factor-α Induced Inflammation in Fetal Intestinal Epithelial Cells In Vitro through Shedding or Interacting with Tumor Necrosis Factor Receptor 1. Mol. Nutr. Food Res. 2021, 65, 2000425. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Totten, S.M.; Garrido, D.A.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Variation in Consumption of Human Milk Oligosaccharides by Infant Gut-Associated Strains of Bifidobacterium Breve. Appl. Environ. Microbiol. 2013, 79, 6040–6049. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.-S.; German, J.B.; et al. Maternal Fucosyltransferase 2 Status Affects the Gut Bifidobacterial Communities of Breastfed Infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter Jejuni Binds Intestinal H(O) Antigen (Fucα1, 2Galβ1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed]
- Duska-McEwen, G.; Senft, A.P.; Ruetschilling, T.L.; Barrett, E.G.; Buck, R.H. Human Milk Oligosaccharides Enhance Innate Immunity to Respiratory Syncytial Virus and Influenza in vitro. Food Nutr. Sci. 2014, 5, 1387–1398. [Google Scholar] [CrossRef]
- Hu, L.; Sankaran, B.; Laucirica, D.R.; Patil, K.; Salmen, W.; Ferreon, A.C.M.; Tsoi, P.S.; Lasanajak, Y.; Smith, D.F.; Ramani, S.; et al. Glycan Recognition in Globally Dominant Human Rotaviruses. Nat. Commun. 2018, 9, 2631. [Google Scholar] [CrossRef]
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J.; Robertson, B.; Autran, C.A.; Shinge, D.; Rani, S.; Anandan, S.; Hu, L.; et al. Human Milk Oligosaccharides, Milk Microbiome and Infant Gut Microbiome Modulate Neonatal Rotavirus Infection. Nat. Commun. 2018, 9, 5010. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.M.; Goode, P.L.; Mobasseri, A.; Zopf, D. Inhibition of Helicobacter Pylori Binding to Gastrointestinal Epithelial Cells by Sialic Acid-Containing Oligosaccharides. Infect. Immun. 1997, 65, 750–757. [Google Scholar] [CrossRef]
- Holscher, H.D.; Davis, S.R.; Tappenden, K.A. Human Milk Oligosaccharides Influence Maturation of Human Intestinal Caco-2Bbe and HT-29 Cell Lines. J. Nutr. 2014, 144, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bode, L.; Tappenden, K.A. Human Milk Oligosaccharides Influence Intestinal Epithelial Cell Maturation In Vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Modulates CD14 Expression in Human Enterocytes, Thereby Attenuating LPS-Induced Inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef]
- Natividad, J.M.; Rytz, A.; Keddani, S.; Bergonzelli, G.; Garcia-Rodenas, C.L. Blends of Human Milk Oligosaccharides Confer Intestinal Epithelial Barrier Protection In Vitro. Nutrients 2020, 12, 3047. [Google Scholar] [CrossRef]
- Zhao, G.; Williams, J.; Washington, M.K.; Yang, Y.; Long, J.; Townsend, S.D.; Yan, F. 2′-Fucosyllactose Ameliorates Chemotherapy-Induced Intestinal Mucositis by Protecting Intestinal Epithelial Cells Against Apoptosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 13, 441–457. [Google Scholar] [CrossRef]
- Wejryd, E.; Martí, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T. Low Diversity of Human Milk Oligosaccharides Is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients 2018, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human Milk Oligosaccharide DSLNT and Gut Microbiome in Preterm Infants Predicts Necrotising Enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Pell, L.G.; Ohuma, E.O.; Yonemitsu, C.; Loutet, M.G.; Ahmed, T.; Mahmud, A.A.; Azad, M.B.; Bode, L.; Roth, D.E. The Human-Milk Oligosaccharide Profile of Lactating Women in Dhaka, Bangladesh. Curr. Dev. Nutr. 2021, 5, nzab137. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Kunz, C.; Rudloff, S.; García-Mantrana, I.; Crehuá-Gaudiza, E.; Martínez-Costa, C.; Collado, M.C. Association of Maternal Secretor Status and Human Milk Oligosaccharides With Milk Microbiota: An Observational Pilot Study. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming Growth Factor-Beta Regulation of Epithelial Tight Junction Proteins Enhances Barrier Function and Blocks Enterohemorrhagic Escherichia Coli O157:H7-Induced Increased Permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Kong, C.; Elderman, M.; Cheng, L.; de Haan, B.J.; Nauta, A.; de Vos, P. Modulation of Intestinal Epithelial Glycocalyx Development by Human Milk Oligosaccharides and Non-Digestible Carbohydrates. Mol. Nutr. Food Res. 2019, 63, e1900303. [Google Scholar] [CrossRef]
- Benkoe, T.; Reck, C.; Pones, M.; Weninger, M.; Gleiss, A.; Stift, A.; Rebhandl, W. Interleukin-8 Predicts 60-Day Mortality in Premature Infants with Necrotizing Enterocolitis. J. Pediatr. Surg. 2014, 49, 385–389. [Google Scholar] [CrossRef]
- Kong, C.; Cheng, L.; Krenning, G.; Fledderus, J.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P. Human Milk Oligosaccharides Mediate the Crosstalk Between Intestinal Epithelial Caco-2 Cells and Lactobacillus PlantarumWCFS1in an In Vitro Model with Intestinal Peristaltic Shear Force. J. Nutr. 2020, 150, 2077–2088. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human Colostrum Oligosaccharides Modulate Major Immunologic Pathways of Immature Human Intestine. Mucosal. Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Kurakevich, E.; Hennet, T.; Hausmann, M.; Rogler, G.; Borsig, L. Milk Oligosaccharide Sialyl(2,3)Lactose Activates Intestinal CD11c+ Cells through TLR4. Proc. Natl. Acad. Sci. USA 2013, 110, 17444–17449. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3′Sialyllactose-Poor Milk during Lactation Impairs Cognitive Capabilities in Adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The Human Milk Oligosaccharides 2′-Fucosyllactose and 6′-Sialyllactose Protect against the Development of Necrotizing Enterocolitis by Inhibiting Toll-like Receptor 4 Signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Simiantonaki, N.; Kurzik-Dumke, U.; Karyofylli, G.; Jayasinghe, C.; Michel-Schmidt, R.; Kirkpatrick, C.J. Reduced Expression of TLR4 Is Associated with the Metastatic Status of Human Colorectal Cancer. Int. J. Mol. Med. 2007, 20, 21–29. [Google Scholar] [CrossRef]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Berger, B.; Carnielli, V.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-D.; Li, N.; Tian, X.-Q.; Huang, P.-L. Caco-2 and LS174T Cell Lines Provide Different Models for Studying Mucin Expression in Colon Cancer. Tissue Cell 2011, 43, 201–206. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.Y.; Botts, S.R.; Johnson-Henry, K.C.; Landberg, E.; Abrahamsson, T.R.; Sherman, P.M. Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study. Nutrients 2022, 14, 1014. https://doi.org/10.3390/nu14051014
Wu RY, Botts SR, Johnson-Henry KC, Landberg E, Abrahamsson TR, Sherman PM. Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study. Nutrients. 2022; 14(5):1014. https://doi.org/10.3390/nu14051014
Chicago/Turabian StyleWu, Richard Y., Steven R. Botts, Kathene C. Johnson-Henry, Eva Landberg, Thomas R. Abrahamsson, and Philip M. Sherman. 2022. "Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study" Nutrients 14, no. 5: 1014. https://doi.org/10.3390/nu14051014
APA StyleWu, R. Y., Botts, S. R., Johnson-Henry, K. C., Landberg, E., Abrahamsson, T. R., & Sherman, P. M. (2022). Variations in the Composition of Human Milk Oligosaccharides Correlates with Effects on Both the Intestinal Epithelial Barrier and Host Inflammation: A Pilot Study. Nutrients, 14(5), 1014. https://doi.org/10.3390/nu14051014






