Eating Disorder Day Programs: Is There a Best Format?
Abstract
:1. Introduction
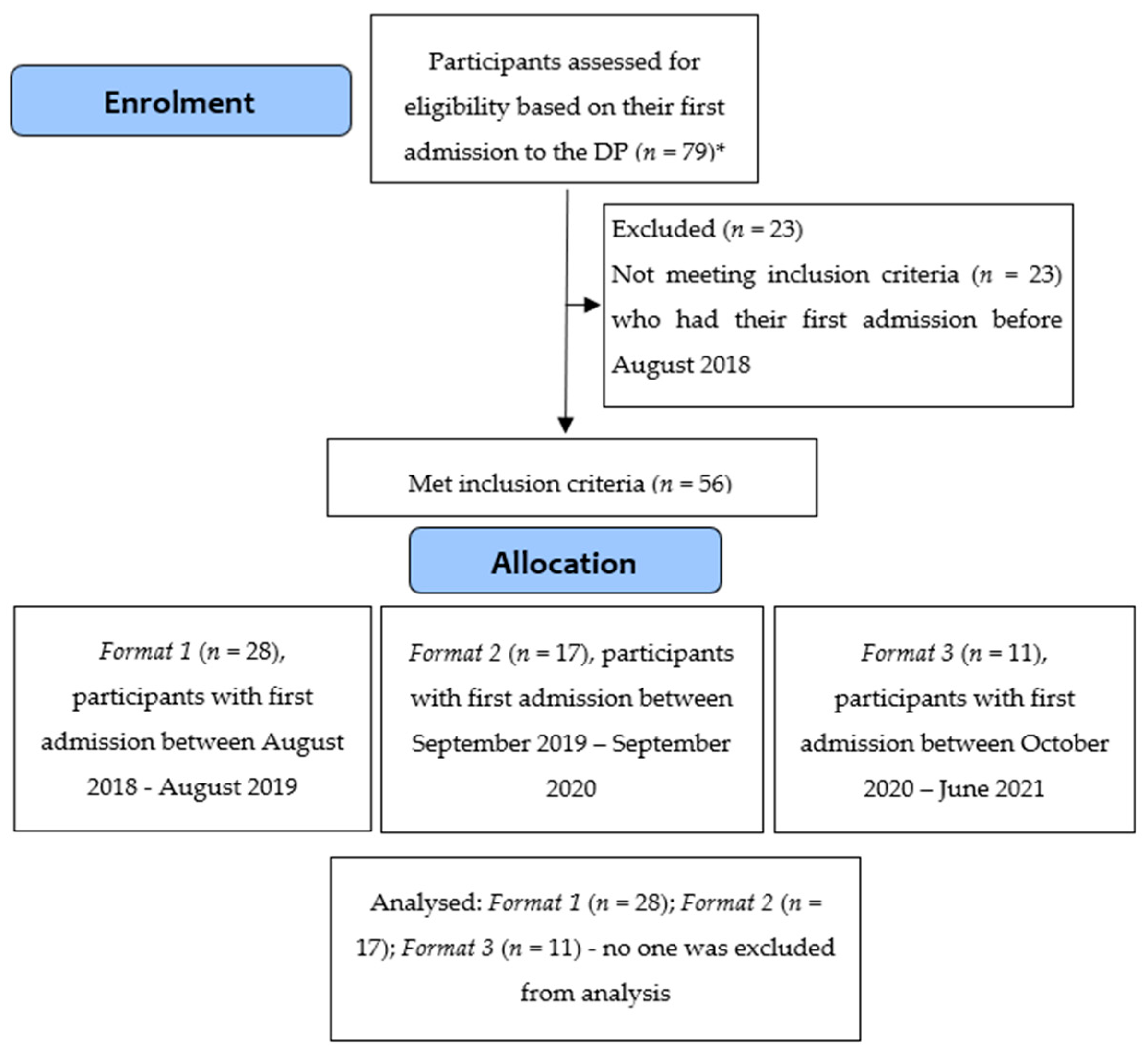
2. Materials and Methods
2.1. Design
2.2. Interventions
2.3. Participants
2.4. Repeated Measures
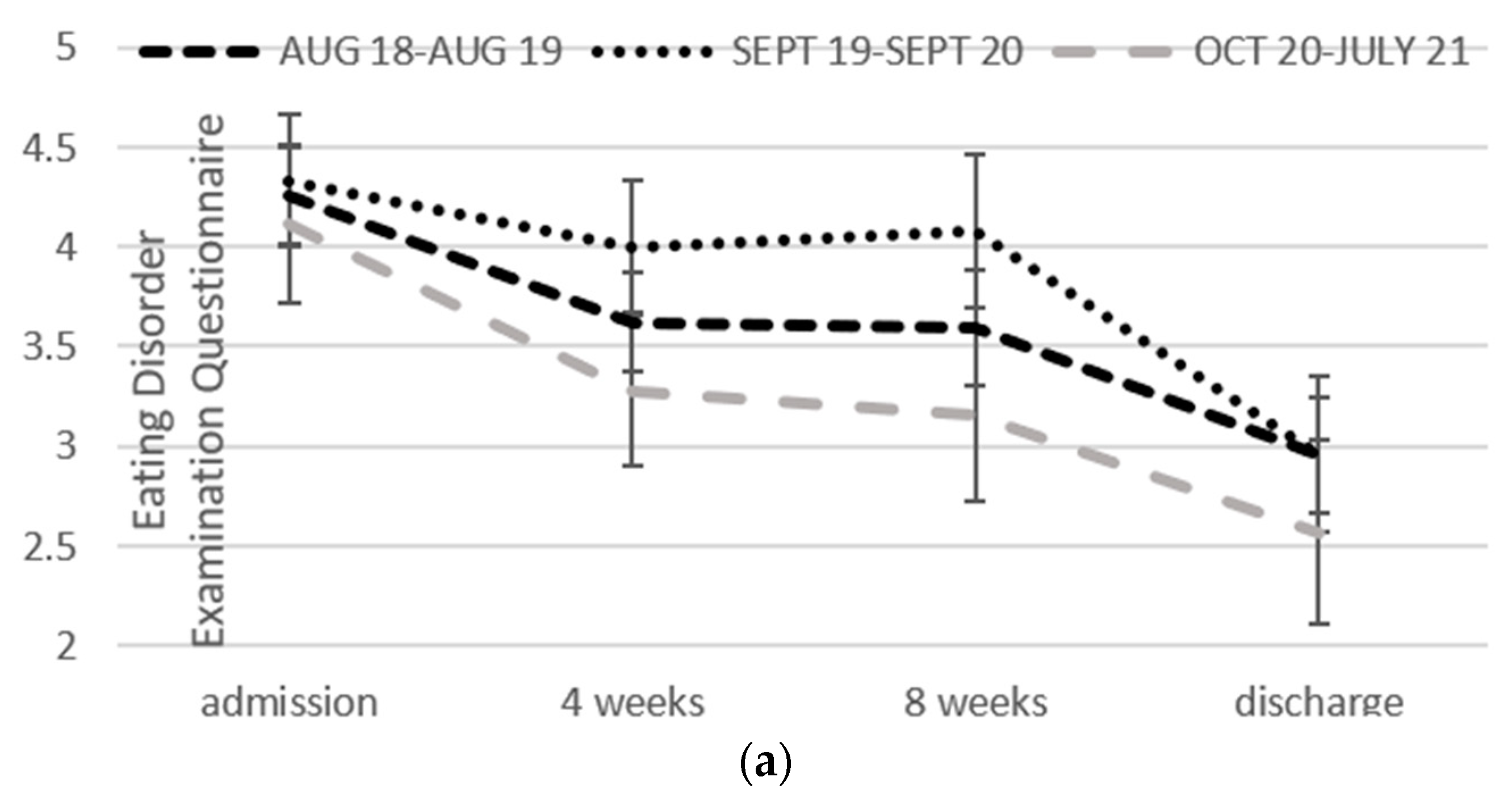
2.4.1. Eating Disorder Examination Questionnaire
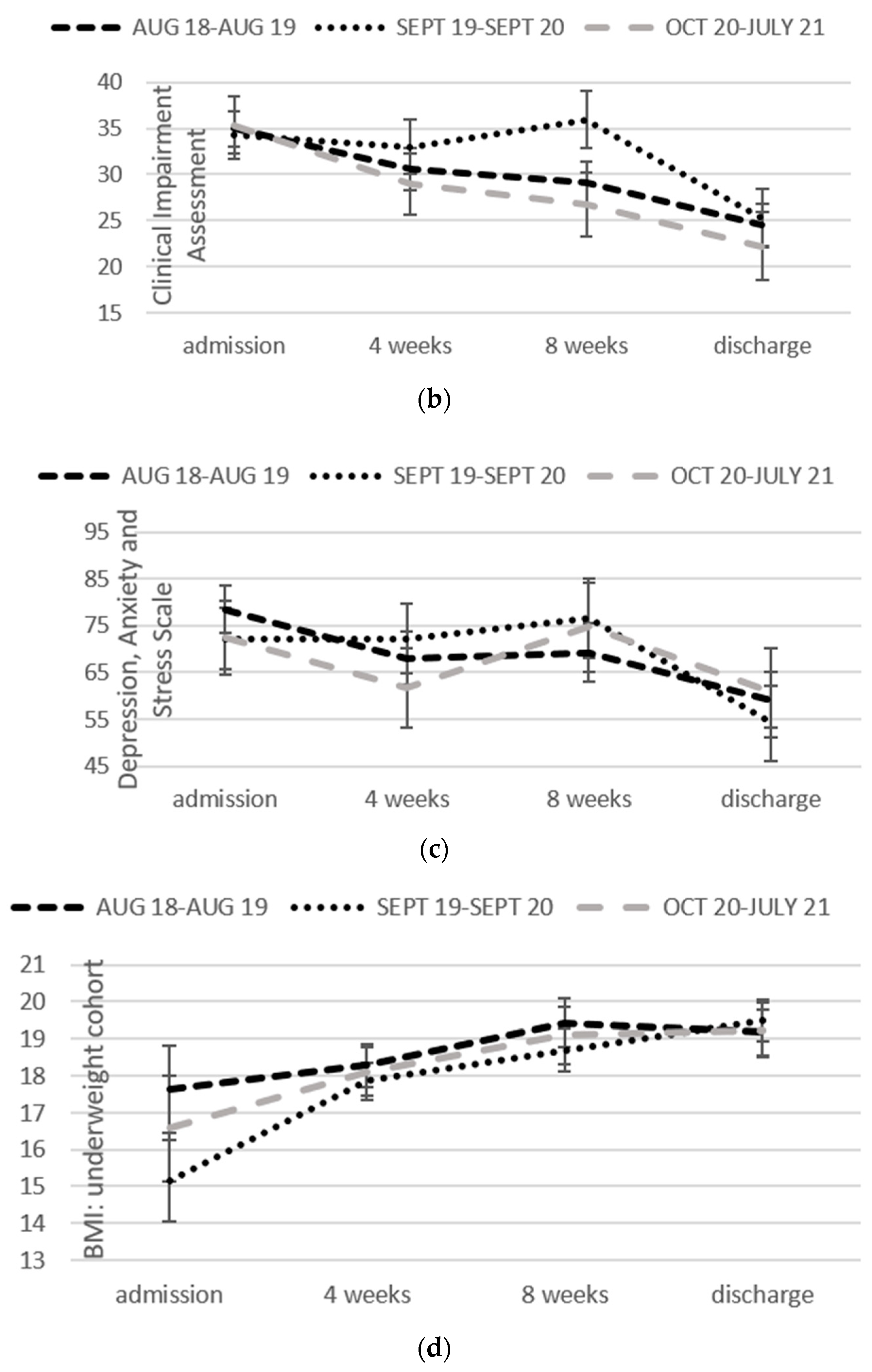
2.4.2. Clinical Impairment Assessment
2.4.3. Depression Anxiety and Stress Scales
2.4.4. Body Mass Index
2.5. Baseline Measures
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Change over Time in Each Format
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, K.; Ramirez, A.L.; Murray, S.B.; Anderson, L.K.; Cusack, A.; Boutelle, K.N.; Kaye, W.H. A narrative review of outcome studies for residential and partial hospital-based treatment for eating disorders. Eur. Eat. Disord. Rev. 2016, 24, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, T.D.; Keski-Rahkonen, A.; Hudson, J. Epidemiology of eating disorders. In Textbook in Psychiatric Epidemiology; Tsuang, M., Tohen, M., Eds.; Wiley: New York, NY, USA, 2011; pp. 343–360. [Google Scholar]
- Piran, N.; Langdon, L.; Kaplan, A.; Garfinkel, P.E. Evaluation of a day hospital program for eating disorders. Int. J. Eat. Disord. 1989, 8, 523–532. [Google Scholar] [CrossRef]
- McFarlane, T.; MacDonald, D.E.; Trottier, K.; Olmsted, M.P. The effectiveness of an individualized form of day hospital treatment. Eat Disord. 2015, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Thaler, L.; Wilson, S.; Coehlo, J.S.; Mazanek Antunes, J.; Israel, M.; Steiger, H. Mandating weekly weight gain in a day treatment program for eating disorders. Int. J. Eat. Disord. 2014, 47, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Melvin, G.A.; Newman, L.; Jones, M.; Taffe, J.; Gordon, M. Day program for young people with anorexia nervosa. Austral. Psychiatry 2015, 23, 249–253. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B.; Schwarte, R.; Krei, M.; Egberts, K.; Warnke, A.; Wewetzer, C.; Pfeiffer, E.; Fleischhaker, C.; Scherag, A.; Holtkamp, K.; et al. Day-patient treatment after short inpatient care versus continued inpatient treatment in adolescents with anorexia nervosa (ANDI): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2014, 383, 1222–1229. [Google Scholar] [CrossRef]
- Zeeck, A.; Weber, S.; Sandholz, A.; Joos, A.; Hartmaan, A. Stability of long-term outcomes in bulimia nervosa: A 3-year follow-up. J. Clin. Psychol. 2011, 4, 318–327. [Google Scholar] [CrossRef]
- Kong, S. Day treatment programme for patients with eating disorders: Randomized controlled trial. J. Adv. Nurs. 2005, 51, 5–14. [Google Scholar] [CrossRef]
- Ben-Porath, D.D.; Wisniewski, L.; Warren, M. Outcomes of a day treatment program for eating disorders using clinical and statistical significance. J. Contemp. Psychother. 2010, 40, 115–123. [Google Scholar] [CrossRef]
- Högdahl, L.; Birgegård, A.; Björck, C. How effective is bibliotherapy-based self-help cognitive behavioural therapy with Internet support in clinical settings? Results from a pilot study. Eat Weight Disord. 2013, 18, 37–44. [Google Scholar] [CrossRef]
- van den Berg, E.; Schlochtermeier, D.; Koenders, J.; de Mooij, L.; Gouiaan, A.; Blankers, M.; Peen, J.; Dekker, J. Implementing cognitive behavioral therapy-enhanced in a routine inpatient and outpatient setting: Comparing effectiveness and treatment costs in two consecutive cohorts. Int. J. Eat. Disord. 2020, 53, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Bodnar, E.; Gamberg, S.; Bartel, S.J.; Waller, G.; Nunes, A.; Dixon, L.; Keshen, A. The costs and benefits of intensive day treatment programs and outpatient treatments for eating disorders: An idea worth researching. Int. J. Eat. Disord. 2021, 54, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- McKnight, R.; Boughton, N. A patient’s journey. Anorexia nervosa. Br. Med. J. 2009, 339, b3800. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Treasure, J. Anorexia nervosa: Valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br. J. Clin. Psychol. 2006, 45, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Wade, T.D.; Eshkevari, E.; Guerin, C.; Smith, J.; Hoskin, D. Examination of a day program for eating disorders: Impact on 3-month follow-up by psychiatric comorbidity. Australas. Psychiatry 2020, 28, 148–152. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Beglin, S.J. Eating disorder examination questionnaire (6.0). In Cognitive Behavior Therapy And Eating Disorders; Fairburn, C.G., Ed.; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Berg, K.C.; Peterson, C.B.; Frazier, P.; Crow, S.J. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. Int. J. Eat. Disord. 2012, 45, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Bohn, K.; Doll, H.A.; Cooper, Z.; O’Connor, M.; Palmer, R.L.; Fairburn, C.G. The measurement of impairment due to eating disorder psychopathology. Behav. Res. Ther. 2008, 46, 1105–1110. [Google Scholar] [CrossRef]
- Jenkins, P.E. Psychometric validation of the Clinical Impairment Assessment in a UK eating disorder service. Eat. Behav. 2013, 14, 241–243. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales; Psychology Foundation: Sydney, Australia, 1995. [Google Scholar]
- Henry, D.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Vall, E.; Wade, T.D. Predictors of treatment outcome in individuals with eating disorders: A systematic review and meta-analysis. Int. J. Eat. Disord. 2015, 48, 946–971. [Google Scholar] [CrossRef]
- Morris, S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for The Behavioural Sciences, 2nd ed.; Hillsdale: Erlbaum, NJ, USA, 1988. [Google Scholar]
- Steiger, H.; Sansfaçon, J.; Thaler, L.; Leonard, N.; Cottier, D.; Kahan, E.; Fletcher, E.; Rossi, E.; Israel, M.; Gauvin, L. eAutonomy support and autonomous motivation in the outpatient treatment of adults with an eating disorder. Int. J. Eat. Disord. 2017, 50, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.S.; Schafer, K.J.; Crowley, M.E.J.; Olivardia, R. A qualitative analysis of job burnout in eating disorder treatment providers. Eat. Disord. 2012, 20, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.S.; Schafer, K.J.; Crowley, M.E.J.; Olivardia, R. Demographic and work-related correlates of job burnout in professional eating disorder treatment providers. Psychotherapy 2013, 50, 553–564. [Google Scholar] [CrossRef] [PubMed]
| Meal | Content |
|---|---|
| Breakfast |
|
| |
| |
| Morning Tea | Snack item(s) between 250 and 350 calories |
| Lunch |
|
| |
| |
| Afternoon Tea | Snack item(s) between 250 and 350 calories |
| Dinner | A balanced hot meal will include |
| Guidelines for appearance of meal |
|
| |
| |
| Guidelines for serves of protein | 1 red meat steak (palm size in length and thickness), 2 lamb loin chops, 1 med pork chop, 3 slices roast meat, ¼ of a chicken, 1 small chicken breast. |
| 1 × ’hand’ size piece of fish (150–200 g) | |
| 1 cup (when cooked) chickpeas/lentils/soybeans/baked beans | |
| 2 vegetable/lentil burgers/patties | |
| Guidelines for serves of carbohydrate foods | Tofu 150 g; Quorn 150g mince/2 patties/3 sausages/1 cutlet |
| Dessert | 1 cup of cooked rice /pasta/quinoa/couscous/noodles/mashed potato |
| 1 medium potato cooked in any way, 2 slices of bread or 1 crusty roll | |
| Dessert as per current snack list | |
| Supper | Snack item(s) between 250 and 350 calories |
| Group Name | Description |
|---|---|
| Review | First group of each day. Review of meal plan adherence and ED behaviours since last in DP, problem-solving of challenges experienced. |
| Planning | Planning for time between leaving DP and until next day due to return, with respect to meal plan compliance and management of ED behaviours. Using problem-solving and motivational interviewing approach. |
| Nutrition Group | Session delivered by dietitian. Providing information, education and skill development on relevant topics, e.g., starvation syndrome, regular and adequate eating, fluids, macronutrients, food rules, feared foods, metabolism, cooking, meal preparation, evaluating nutritional advice, and social eating. |
| Physical Recovery | Collaborative weighing, review of meal plan adherence and risk assessment (carried out individually). |
| Goal Review | Reviewing achievement of individual goals set over previous week and problem-solving. |
| Goal Setting | Setting 5 specific goals for week ahead to address ED behaviours, including setting a challenge snack to eat within DP supported meal and a goal to get back into normal life. |
| Flexible Thinking | Psychology-based group covering such topics as body image, perfectionism, self-compassion and also cognitive remediation therapy. |
| Coping Skills | Delivering dialectical behavioural therapy skills modules of mindfulness, distress tolerance, emotion regulation and interpersonal effectiveness. |
| Life and Relationships | Issues that impact life and relationships in people with an ED, e.g., sleep, assertive communication skills, motivation for recovering, social media literacy, reviewing values, vocational choices. |
| Mind | Cognitive-behaviour therapy (CBT) strategies for EDs, e.g., developing an understanding of how the ED is maintained, identifying and addressing unhelpful thinking styles, developing understanding of thought, feelings and behaviour connections. |
| Distress Tolerance | Independent practice of strategies to manage distress with clinician available to provide support and engagement. For example, playing games, practising mindfulness, distraction activities, art. |
| Mindfulness | Education on mindfulness and practising various mindfulness activities. |
| Creative Writing | Delivered by professional writer, ‘writer in residence’. Guiding patients through various creative writing tasks, including poetry. |
| Sensory | Developing skills and knowledge to use sensory approaches in developing regulation skills (e.g., self-soothing or alerting/arousal using the 5 senses). |
| Group Processes | Review group dynamics and norms, to identify and address any issues. |
| Program Descriptors | Format 1 | Format 2 | Format 3 |
|---|---|---|---|
| Date | August 2018 to August 2019 | September 2019 to September 2020 | October 2020 to July 2021 |
| Admission lengths | Determined on an individual case-by-case basis, typically during admission | Pre-determined typically prior to commencement | |
| Meal support | Morning tea at start of admission. | ||
| Lunch and afternoon tea on each DP day | |||
| Dinner meal support | 3 per week | 1 per week | |
| 1 take home meal per week | |||
| Crisis support | Provided on an individual basis | Limiting provision of crisis support. Participants required to cease and resume once manageable and able to re-engage. | |
| Patient relationships | Not encouraged, no formal requirements | Not permitting socialising with other patients outside of DP. | |
| Total DP hours | 25.25 | 22.25 | 22.25 |
| Variable | Group 1 | Group 2 | Group 3 | ANOVA Comparison |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F, p | |
| n = 28 | n = 17 | n = 11 | ||
| Age | 25.78 (8.65) | 26.74 (10.41) | 21.70 (5.23) | 1.20, 0.31 |
| Duration of admission (weeks) | 9.79 (5.95) | 12.53 (5.48) | 10.40 (4.17) | 1.33, 0.28 |
| BMI: underweight | 17.64 (0.79) | 15.15 (5.42) | 16.57 (1.18) | 1.18, 0.33 |
| Motivated to recover | 73.96 (17.96) | 76.25 (21.35) | 79.00 (20.41) | 0.27, 0.76 |
| Ready to change | 63.19 (26.23) | 56.81 (29.11) | 57.70 (30.68) | 0.31, 0.73 |
| Confidence | 55.33 (24.43) | 70.56 (27.05) | 62.82 (22.59) | 1.90, 0.16 |
| N (%) | N (%) | N (%) | X2 (df), p | |
| BMI < 18.5 | 9 (50) | 10 (75) | 6 (55) | 4.60 (2), 0.10 |
| Female | 26 (93) | 17 (100) | 11 (100) | 2.07 (2), 0.36 |
| Anorexia Nervosa | 13 (46) | 14 (82) | 8 (73) | 8.63 (6), 0.20 |
| Variable | Admission | Discharge | Cohen’s d Effect Size |
|---|---|---|---|
| Mean (SE) | Mean (SE) | (95% Confidence Intervals) | |
| EDE-Q | 4.23 (0.19) | 2.82 (0.22) | −1.16 (−1.56: −0.76) |
| CIA | 34.86 (1.48) | 23.99 (1.80) | −0.99 (−1.39: −0.60) |
| DASS | 74.35 (3.80) | 58.00 (4.57) | −0.68 (−1.06: −0.30) |
| BMI < 18.5 (n = 24) | 16.45 (0.73) | 19.30 (0.37) | 0.80 (0.21:1.39) |
| Weight (kg) | 47.10 (1.03) | 53.13 (1.11) | 1.66 (1.56:2.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshkevari, E.; Ferraro, I.; McGregor, A.; Wade, T. Eating Disorder Day Programs: Is There a Best Format? Nutrients 2022, 14, 879. https://doi.org/10.3390/nu14040879
Eshkevari E, Ferraro I, McGregor A, Wade T. Eating Disorder Day Programs: Is There a Best Format? Nutrients. 2022; 14(4):879. https://doi.org/10.3390/nu14040879
Chicago/Turabian StyleEshkevari, Ertimiss, Isabella Ferraro, Andrew McGregor, and Tracey Wade. 2022. "Eating Disorder Day Programs: Is There a Best Format?" Nutrients 14, no. 4: 879. https://doi.org/10.3390/nu14040879
APA StyleEshkevari, E., Ferraro, I., McGregor, A., & Wade, T. (2022). Eating Disorder Day Programs: Is There a Best Format? Nutrients, 14(4), 879. https://doi.org/10.3390/nu14040879






