Vitamin D Is Associated with Clinical Outcomes in Patients with Primary Biliary Cholangitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Evaluations
2.3. Diagnosis of Cirrhosis at Baselines or during Follow-Up
2.4. Incomplete Response to UDCA Therapy
2.5. Liver-Related Events
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Response to UDCA
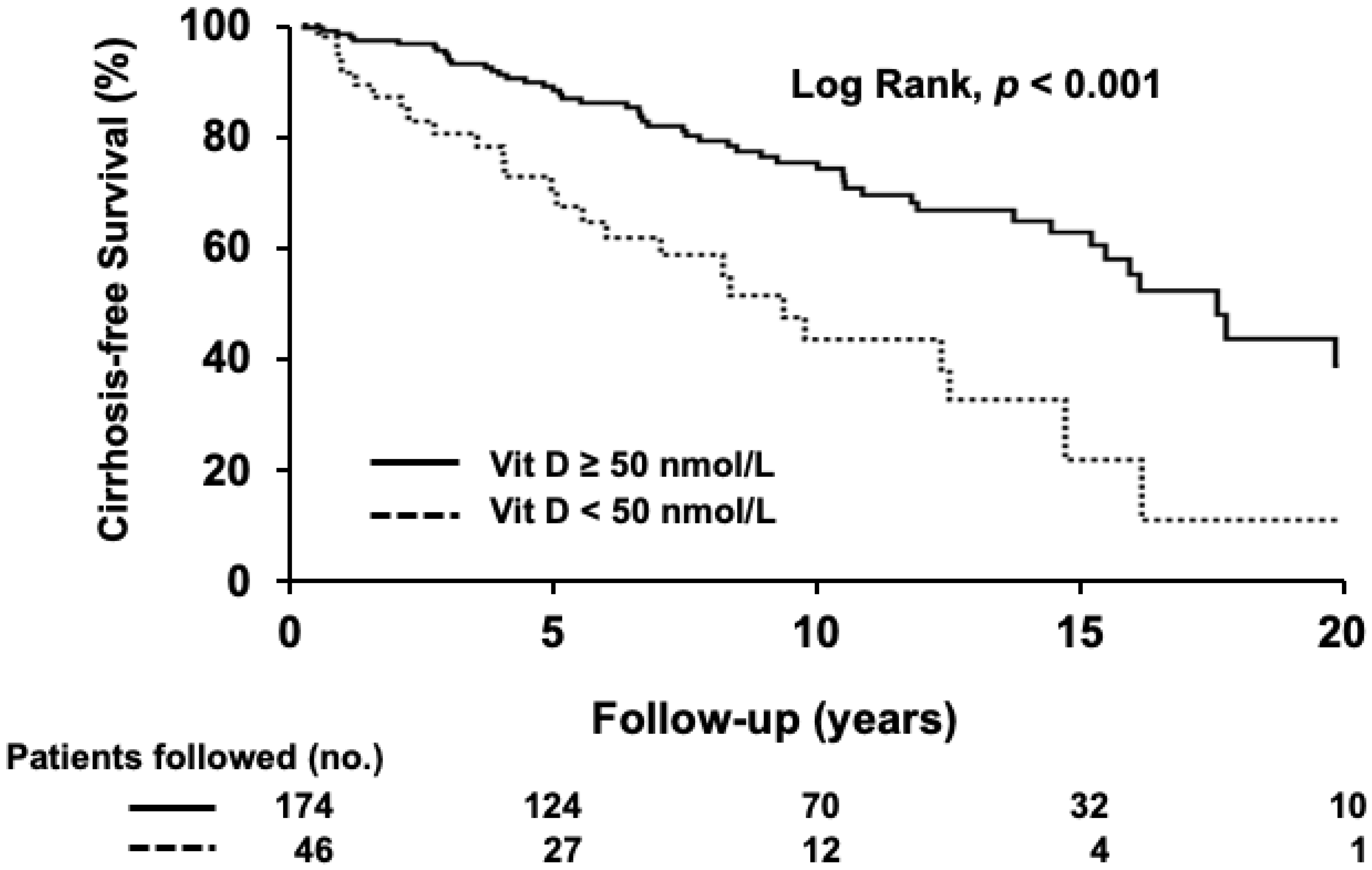
3.3. Development of Cirrhosis
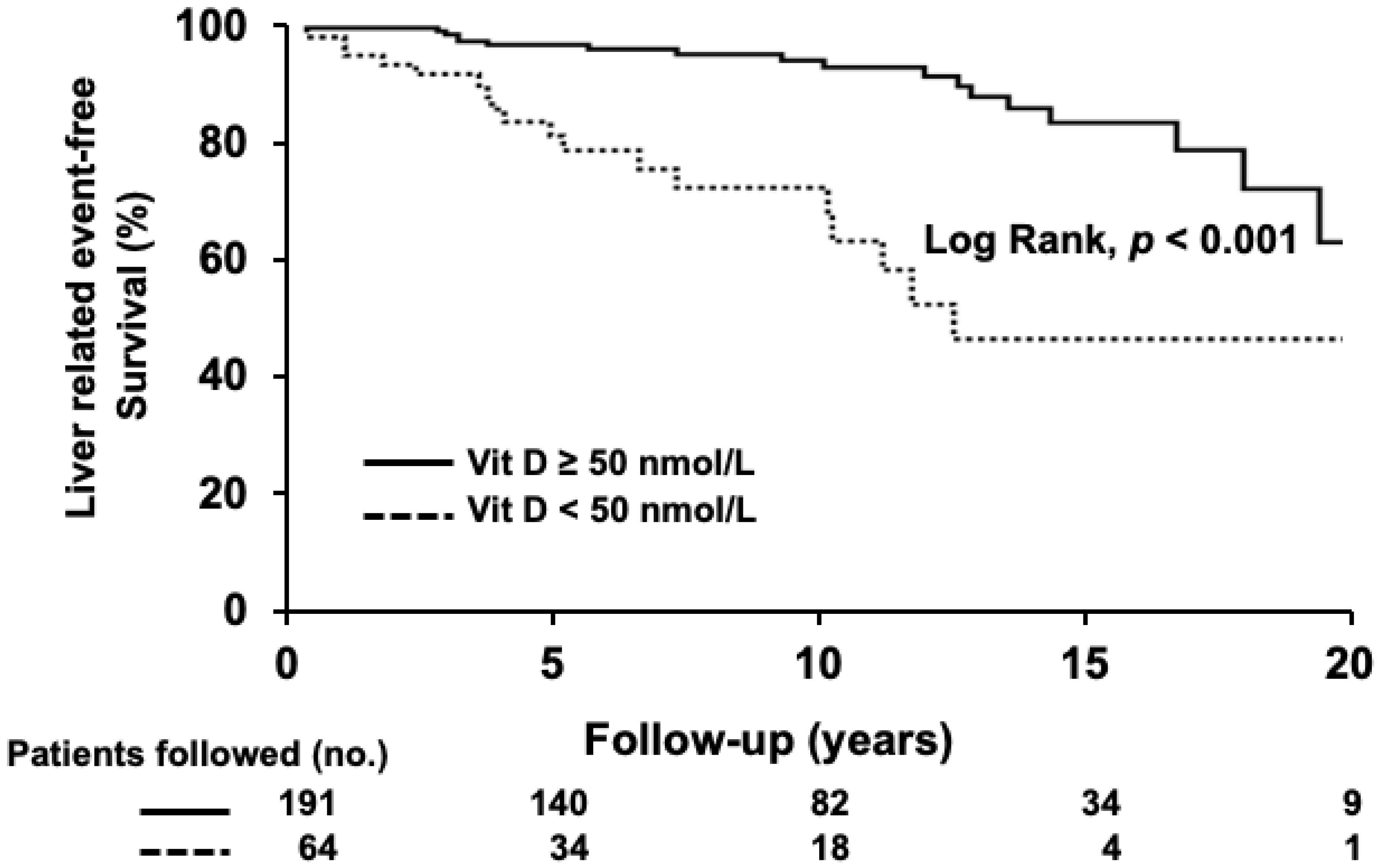
3.4. Liver-Related Mortality or Liver Transplantation
3.5. Response to VD Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Lytvyak, E.; Mason, A.; Czaja, A.J.; Montano-Loza, A.J. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis. Aliment. Pharmacol. Ther. 2019, 49, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Kopilov, R.; Selmi, C.; Nussinovitch, U.; Sanchez-Castanon, M.; Lopez-Hoyos, M.; Amital, H.; Kivity, S.; Gershwin, E.M.; Shoenfeld, Y. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol. Res. 2015, 61, 141–146. [Google Scholar] [CrossRef]
- Efe, C.; Kav, T.; Aydin, C.; Cengiz, M.; Imga, N.N.; Purnak, T.; Smyk, D.S.; Torgutalp, M.; Turhan, T.; Ozenirler, S.; et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig. Dis. Sci. 2014, 59, 3035–3042. [Google Scholar] [CrossRef]
- Smyk, D.S.; Orfanidou, T.; Invernizzi, P.; Bogdanos, D.P.; Lenzi, M. Vitamin D in autoimmune liver disease. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 535–545. [Google Scholar] [CrossRef]
- Luong, K.V.; Nguyen, L.T. The role of vitamin d in autoimmune hepatitis. J. Clin. Med. Res. 2013, 5, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J.; Montano-Loza, A.J. Evolving Role of Vitamin D in Immune-Mediated Disease and Its Implications in Autoimmune Hepatitis. Dig. Dis. Sci. 2019, 64, 324–344. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef]
- Beyazit, Y.; Kocak, E.; Tanoglu, A.; Kekilli, M. Oxidative stress might play a role in low serum vitamin D associated liver fibrosis among patients with autoimmune hepatitis. Dig. Dis. Sci. 2015, 60, 1106–1108. [Google Scholar] [CrossRef]
- Abramovitch, S.; Sharvit, E.; Weisman, Y.; Bentov, A.; Brazowski, E.; Cohen, G.; Volovelsky, O.; Reif, S. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, 112–120. [Google Scholar] [CrossRef]
- Reiter, F.P.; Hohenester, S.; Nagel, J.M.; Wimmer, R.; Artmann, R.; Wottke, L.; Makeschin, M.C.; Mayr, D.; Rust, C.; Trauner, M.; et al. 1,25-(OH)2-vitamin D3 prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4−/− model. Biochem. Biophys. Res. Commun. 2015, 459, 227–233. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689–1696. [Google Scholar] [CrossRef]
- Miroliaee, A.; Nasiri-Toosi, M.; Khalilzadeh, O.; Esteghamati, A.; Abdollahi, A.; Mazloumi, M. Disturbances of parathyroid hormone-vitamin D axis in non-cholestatic chronic liver disease: A cross-sectional study. Hepatol. Int. 2010, 4, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Yamasaki, T. Eight cytochrome P450s catalyze vitamin D metabolism. Front. Biosci. 2004, 9, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.; Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 21, 1073–1083. [Google Scholar] [CrossRef]
- Stokes, C.S.; Volmer, D.A.; Grunhage, F.; Lammert, F. Vitamin D in chronic liver disease. Liver Int. 2013, 33, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.S.; Krawczyk, M.; Reichel, C.; Lammert, F.; Grunhage, F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur. J. Clin. Invest. 2014, 44, 176–183. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Kronenberger, B.; Zeuzem, S.; Piiper, A.; Waidmann, O. Low 25-Hydroxyvitamin D Levels Are Associated with Infections and Mortality in Patients with Cirrhosis. PLoS ONE 2015, 10, e0132119. [Google Scholar] [CrossRef]
- Guo, G.Y.; Shi, Y.Q.; Wang, L.; Ren, X.; Han, Z.Y.; Guo, C.C.; Cui, L.N.; Wang, J.B.; Zhu, J.; Wang, N.; et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment. Pharm. Ther. 2015, 42, 221–230. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Trepo, E.; Ouziel, R.; Pradat, P.; Momozawa, Y.; Quertinmont, E.; Gervy, C.; Gustot, T.; Degre, D.; Vercruysse, V.; Deltenre, P.; et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J. Hepatol. 2013, 59, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Wagner, D.; Reiberger, T.; Mandorfer, M.; Schwarzer, R.; Ferlitsch, M.; Trauner, M.; Peck-Radosavljevic, M.; Ferlitsch, A. Low 25-OH-vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien. Klin. Wochenschr. 2017, 129, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Finkelmeier, F.; Kronenberger, B.; Koberle, V.; Bojunga, J.; Zeuzem, S.; Trojan, J.; Piiper, A.; Waidmann, O. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma—A prospective cohort study. Aliment. Pharmacol. Ther. 2014, 39, 1204–1212. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef]
- Ringe, J.D.; Kipshoven, C. Vitamin D-insufficiency: An estimate of the situation in Germany. Dermato-Endocrinology 2012, 4, 72–80. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Corpechot, C. Definition and Management of Patients with Primary Biliary Cholangitis and an Incomplete Response to Therapy. Clin. Gastroenterol. Hepatol. 2021, 19, 2241–2251. [Google Scholar] [CrossRef]
- Kumagi, T.; Guindi, M.; Fischer, S.E.; Arenovich, T.; Abdalian, R.; Coltescu, C.; Heathcote, E.J.; Hirschfield, G.M. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am. J. Gastroenterol. 2010, 105, 2186–2194. [Google Scholar] [CrossRef]
- Iruzubieta, P.; Teran, A.; Crespo, J.; Fabrega, E. Vitamin D deficiency in chronic liver disease. World J. Hepatol. 2014, 6, 901–915. [Google Scholar] [CrossRef]
- Lim, L.Y.; Chalasani, N. Vitamin d deficiency in patients with chronic liver disease and cirrhosis. Curr. Gastroenterol. Rep. 2012, 14, 67–73. [Google Scholar] [CrossRef]
- Artaza, J.N.; Sirad, F.; Ferrini, M.G.; Norris, K.C. 1,25(OH)2 vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J. Steroid Biochem. Mol. Biol. 2010, 119, 73–83. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.U.; Kang, I. 1,25-dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef]
- Vinh quoc Luong, K.; Nguyen, L.T.H. The role of vitamin d in primary biliary cirrhosis: Possible genetic and cell signaling mechanisms. Gastroenterol. Res. Pract. 2013, 2013, 602321. [Google Scholar] [CrossRef][Green Version]
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Bonjour, J.P.; Payen, F.D.; Rousseau, B. Moderate amounts of vitamin D3 in supplements are effective in raising serum 25-hydroxyvitamin D from low baseline levels in adults: A systematic review. Nutrients 2015, 7, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, F.A.; Itkonen, S.T.; Ohman, T.; Kiely, M.; Cashman, K.D.; Lamberg-Allardt, C.; Cashman, K.; Kiely, M.; Andersen, R.; Sempos, C.; et al. Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies. Foods 2021, 10, 3065. [Google Scholar] [CrossRef] [PubMed]
- Bleizgys, A. Vitamin D Dosing: Basic Principles and a Brief Algorithm (2021 Update). Nutrients. 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Farooqui, K.J.; Batra, C.M.; Marwaha, R.K.; Mithal, A. Effect of oral versus intramuscular Vitamin D replacement in apparently healthy adults with Vitamin D deficiency. Indian J. Endocrino.l Metab. 2017, 21, 131–136. [Google Scholar] [CrossRef]
- Ataide, F.L.; Carvalho Bastos, L.M.; Vicente Matias, M.F.; Skare, T.L.; Freire de Carvalho, J. Safety and effectiveness of vitamin D mega-dose: A systematic review. Clin. Nutr. ESPEN 2021, 46, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef]
- Keane, J.T.; Elangovan, H.; Stokes, R.A.; Gunton, J.E. Vitamin D and the Liver-Correlation or Cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Grunhage, F.; Hochrath, K.; Krawczyk, M.; Hoblinger, A.; Obermayer-Pietsch, B.; Geisel, J.; Trauner, M.; Sauerbruch, T.; Lammert, F. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology 2012, 56, 1883–1891. [Google Scholar] [CrossRef]
| Characteristics | All (n = 255) | <50 nmol/L (n = 64) | ≥50 nmol/L (n = 191) | p-Value |
|---|---|---|---|---|
| Age at diagnosis, years | 53 ± 12 | 52 ± 13 | 53 ± 12 | 0.73 |
| Sex, male | 32 (13) | 9 (14) | 23 (12) | 0.67 |
| BMI, kg/m2 | 27 ± 6 | 26 ± 6 | 27 ± 5 | 0.10 |
| BMI ≥ 25 kg/m2 | 186 (73) | 42 (66) | 144 (75) | 0.14 |
| Hypertension | 78 (31) | 20 (31) | 58 (30) | 0.88 |
| Diabetes | 38 (15) | 16 (25) | 22 (12) | 0.01 |
| Albumin (g/L) | 40 ± 5 | 38 ± 6 | 41 ± 4 | <0.001 |
| Alkaline phosphatase (IU/L) | 209 ± 160 | 299 ± 209 | 179 ± 127 | <0.001 |
| Bilirubin (µmol/L) | 14 ± 14 | 21 ± 23 | 12 ± 8 | 0.005 |
| VD (nmol/L) | 77 ± 39 | 30 ± 13 | 93 ± 31 | <0.001 |
| Hepatic encephalopathy | 19 (7) | 11 (17) | 8 (4) | 0.002 |
| Ascites | 51 (20) | 24 (38) | 27 (14) | <0.001 |
| Cirrhosis at diagnosis | 35 (14) | 18 (28) | 17 (9) | <0.001 |
| UDCA-incomplete response | 70 (28) | 29 (45) | 41 (22) | <0.001 |
| Development of cirrhosis | 80 (31) | 25 (54) | 55 (32) | 0.006 |
| Liver-related events | 37 (15) | 19 (30) | 18 (9) | <0.001 |
| Seasonal variation | ||||
| Spring–summer | 122 (48) | 31 (48) | 91 (48) | 1.00 |
| Fall–winter | 133 (52) | 33 (52) | 100 (52) | 1.00 |
| Univariate | Multivariate Serum VD Level (nmol/L) | Multivariate (VD Deficiency) | ||||
|---|---|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age at diagnosis, years | 0.97 (0.95–0.99) | 0.009 | 0.97 (0.95–0.99) | 0.01 | 0.97 (0.95–0.99) | 0.009 |
| Sex, male | 0.57(0.23–1.46) | 0.24 | ||||
| Hypertension | 0.65(0.35–1.22) | 0.18 | ||||
| Diabetes | 0.79 (0.36–1.77) | 0.57 | ||||
| Cirrhosis at diagnosis | 0.76 (0.33–1.75) | 0.51 | ||||
| BMI ≥ 25 kg/m2 | 0.25 (0.14–0.45) | <0.001 | 0.24 (0.13–0.45) | <0.001 | 0.25 (0.13–0.46) | <0.001 |
| Serum VD level, nmol/L | 0.99 (0.98–0.996) | 0.002 | 0.99 (0.98–0.997) | 0.01 | ||
| VD deficiency | 3.03 (1.66–5.53) | <0.001 | 2.92 (1.53–5.56) | 0.001 | ||
| Univariate | Multivariate Serum VD Level (nmol/L) | Multivariate (VD Deficiency) | ||||
|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at diagnosis, years | 1.03 (1.01–1.05) | 0.002 | 1.03 (1.01–1.05) | 0.004 | 1.03 (1.01–1.05) | 0.009 |
| Sex, male | 2.02 (1.11–3.68) | 0.02 | 1.26 (0.67–2.39) | 0.48 | 1.48 (0.79–2.79) | 0.22 |
| Hypertension | 1.34 (0.83–2.14) | 0.23 | ||||
| Diabetes | 2.39 (1.33–4.32) | 0.004 | 2.21 (1.20–4.06) | 0.01 | 2.19 (1.19–4.04) | 0.01 |
| UDCA-incomplete response | 1.99 (1.27–3.14) | 0.003 | 2.07 (1.29–3.34) | 0.003 | 2.02 (1.25–3.28) | 0.004 |
| BMI ≥ 25 kg/m2 | 0.79 (0.49–1.28) | 0.34 | ||||
| Serum VD level, nmol/L | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.98–0.996) | 0.002 | ||
| VD deficiency | 2.43 (1.51–3.92) | <0.001 | 1.93 (1.17–3.19) | 0.01 | ||
| Univariate | Multivariate Serum VD Level (nmol/L) | Multivariate (VD Deficiency) | ||||
|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at diagnosis, years | 0.99 (0.97–1.02) | 0.69 | ||||
| Sex, male | 1.71 (0.71–4.12) | 0.23 | ||||
| Hypertension | 1.30 (0.65–2.60) | 0.47 | ||||
| Diabetes | 2.79 (1.30–6.00) | 0.009 | 2.57 (1.15–5.70) | 0.02 | 2.64 (1.19–5.86) | 0.02 |
| Cirrhosis at diagnosis | 6.04 (2.66–13.72) | <0.001 | 4.69 (1.89–11.63) | <0.001 | 5.32 (2.13–13.30) | <0.001 |
| UDCA-incomplete response | 2.64 (1.37–5.10) | 0.004 | 1.95 (0.91–4.20) | 0.09 | 1.98 (0.93–4.23) | 0.08 |
| BMI ≥ 25 kg/m2 | 0.56 (0.29–1.10) | 0.09 | 0.80 (0.36–1.75) | 0.57 | 0.75 (0.35–1.59) | 0.45 |
| Serum VD level, nmol/L | 0.97 (0.96–0.98) | <0.001 | 0.98 (0.97–0.99) | <0.001 | ||
| VD deficiency | 5.38 (2.76–10.47) | <0.001 | 3.33 (1.57–7.07) | 0.002 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebadi, M.; Ip, S.; Lytvyak, E.; Asghari, S.; Rider, E.; Mason, A.; Montano-Loza, A.J. Vitamin D Is Associated with Clinical Outcomes in Patients with Primary Biliary Cholangitis. Nutrients 2022, 14, 878. https://doi.org/10.3390/nu14040878
Ebadi M, Ip S, Lytvyak E, Asghari S, Rider E, Mason A, Montano-Loza AJ. Vitamin D Is Associated with Clinical Outcomes in Patients with Primary Biliary Cholangitis. Nutrients. 2022; 14(4):878. https://doi.org/10.3390/nu14040878
Chicago/Turabian StyleEbadi, Maryam, Stephen Ip, Ellina Lytvyak, Somayyeh Asghari, Elora Rider, Andrew Mason, and Aldo J. Montano-Loza. 2022. "Vitamin D Is Associated with Clinical Outcomes in Patients with Primary Biliary Cholangitis" Nutrients 14, no. 4: 878. https://doi.org/10.3390/nu14040878
APA StyleEbadi, M., Ip, S., Lytvyak, E., Asghari, S., Rider, E., Mason, A., & Montano-Loza, A. J. (2022). Vitamin D Is Associated with Clinical Outcomes in Patients with Primary Biliary Cholangitis. Nutrients, 14(4), 878. https://doi.org/10.3390/nu14040878







