The Influence of Roux-en-Y Gastric Bypass and Diet on NaCl and Sucrose Taste Detection Thresholds and Number of Circumvallate and Fungiform Taste Buds in Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Diet
2.3. COVID-19
2.4. Surgery
2.5. Recovery
2.6. Stimuli
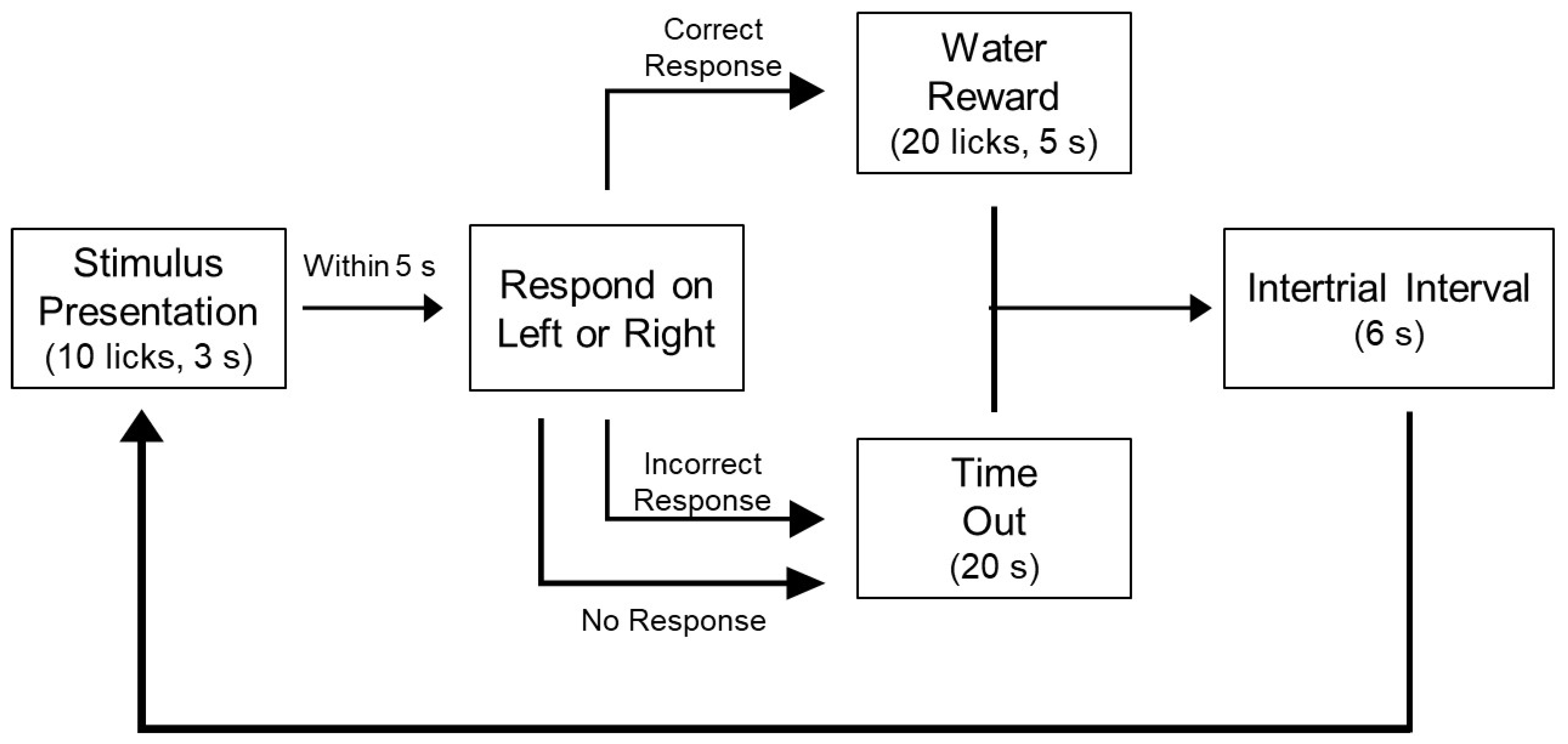
2.7. Apparatus
2.8. NaCl Training and Testing
2.9. Sucrose Training and Testing
2.10. Stimulus Control Test
2.11. EchoMRI
2.12. Hormone Analysis
2.13. Collection and Analysis of Fat Pads
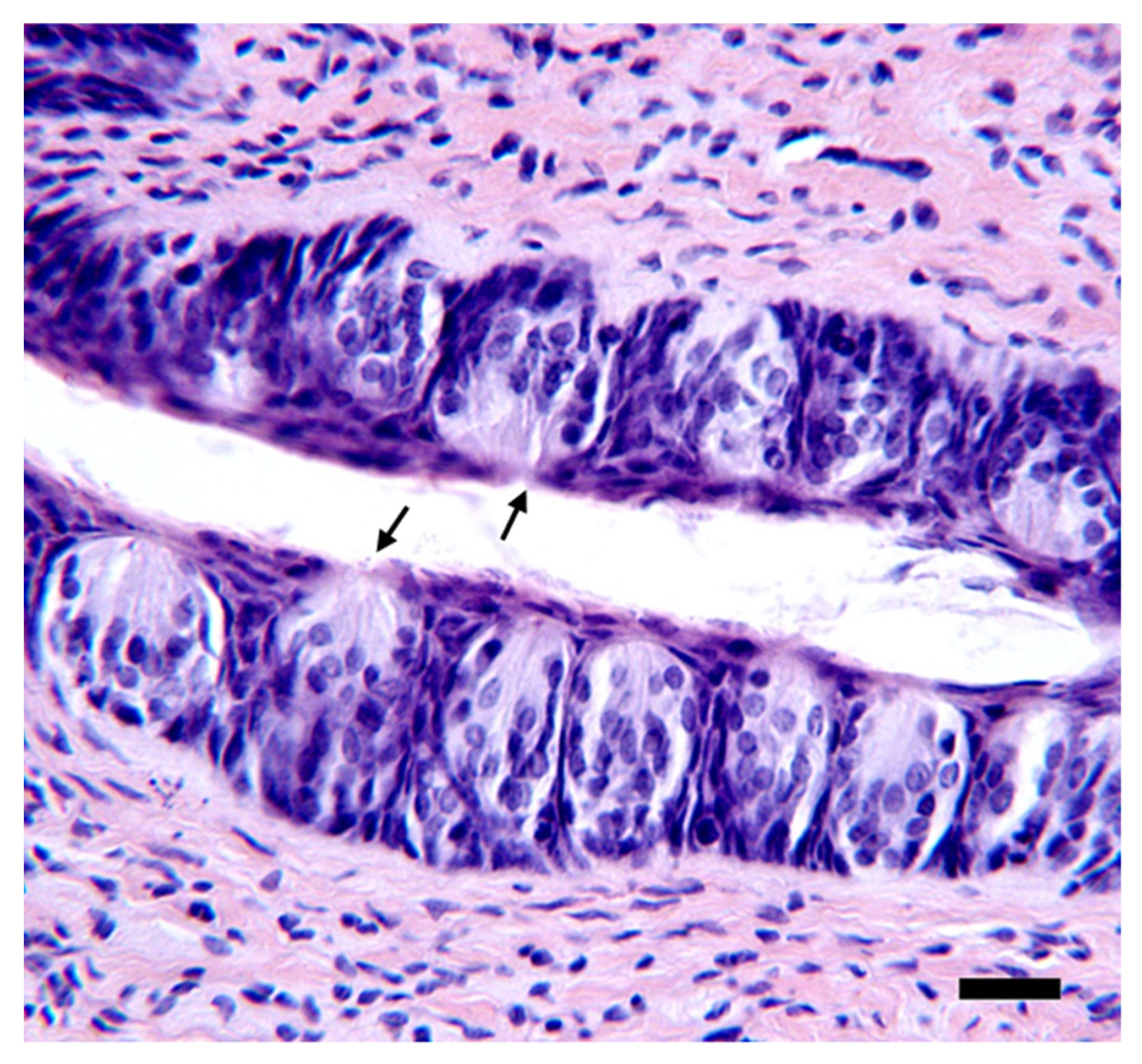
2.14. Histology
2.15. Data Analysis
3. Results
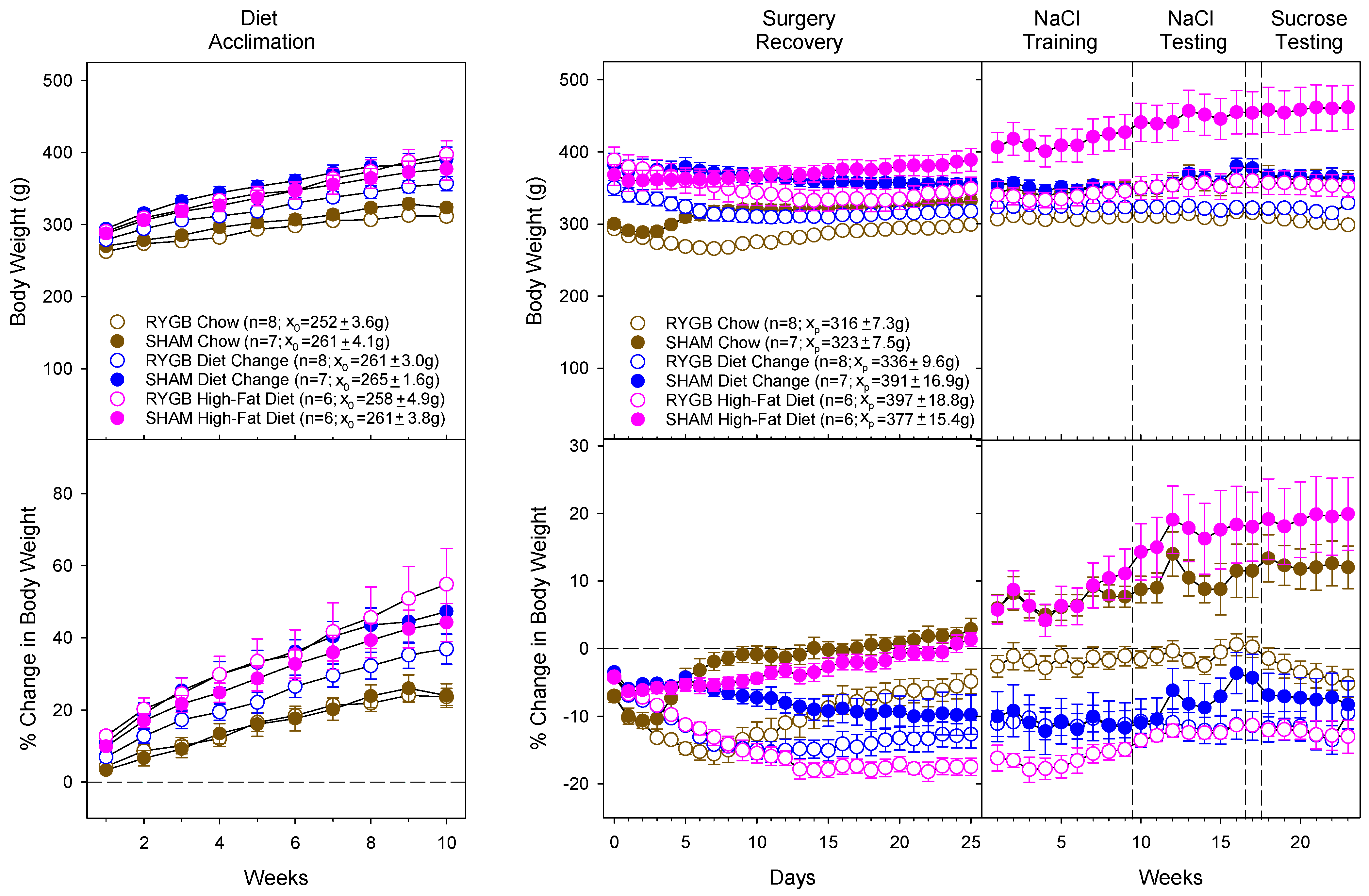
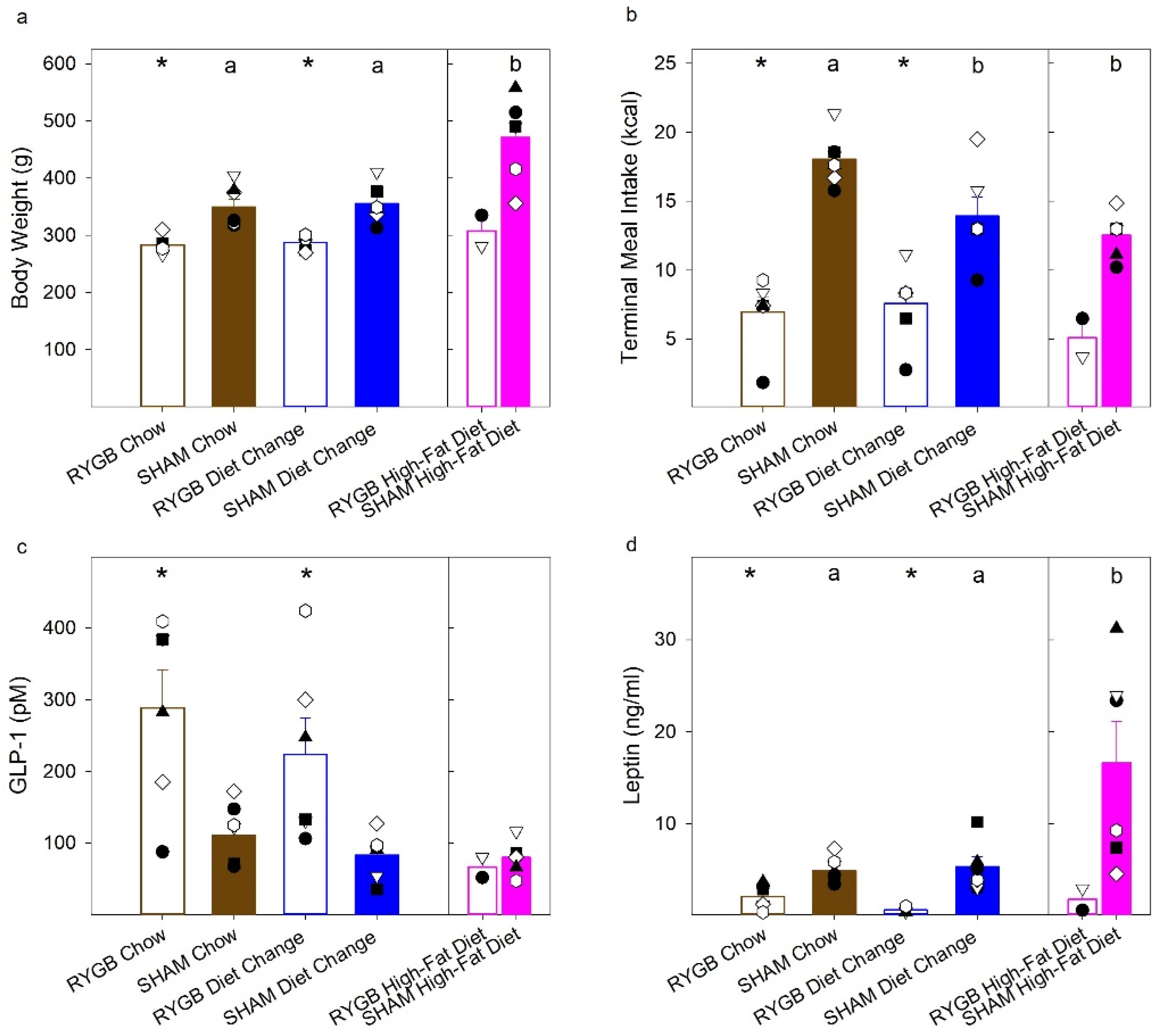
3.1. Diet and Surgical Influence of Body Weight and Fat Mass
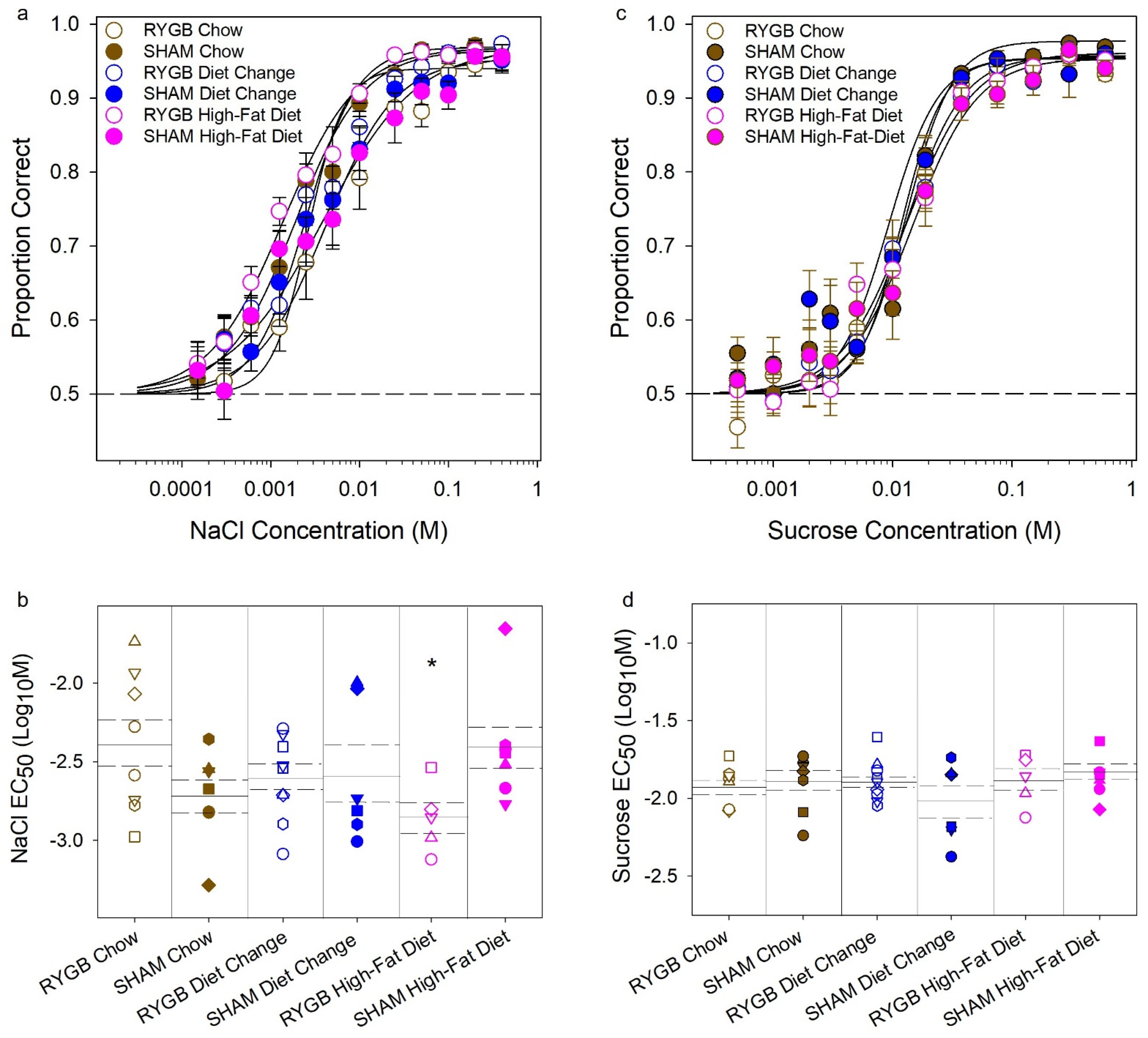
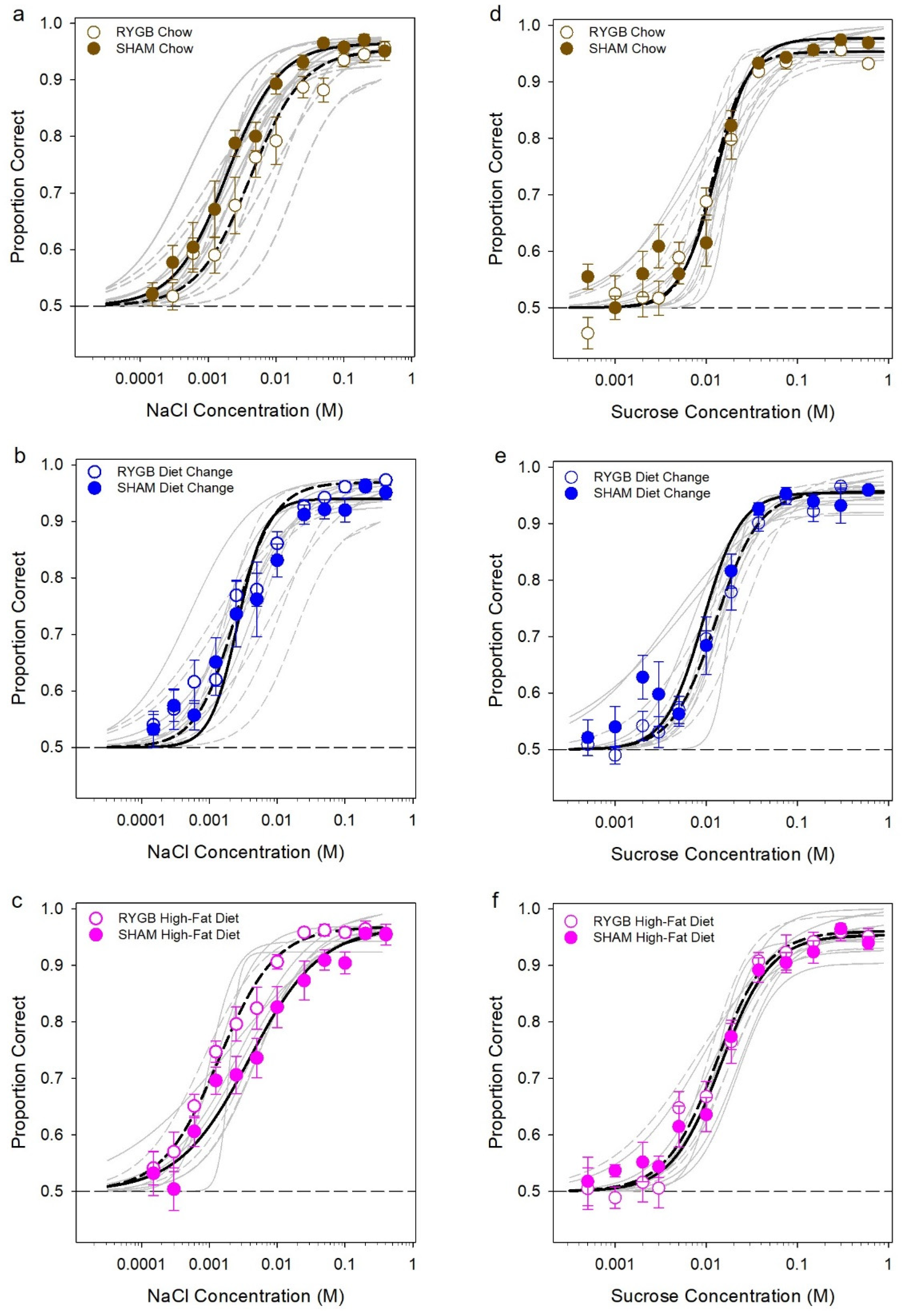
3.2. Effects of Diet and Surgery on Taste Dectection Thresholds
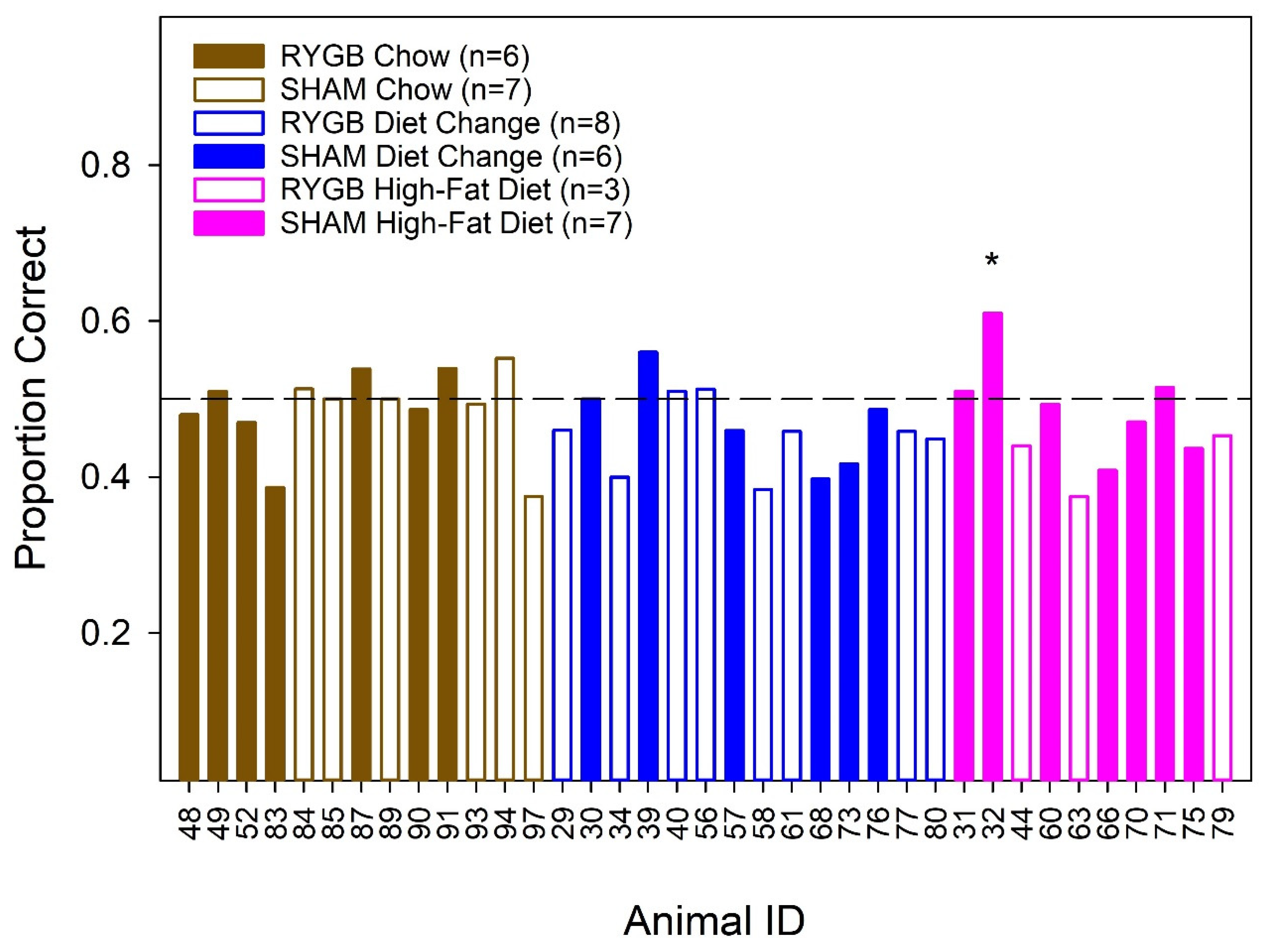
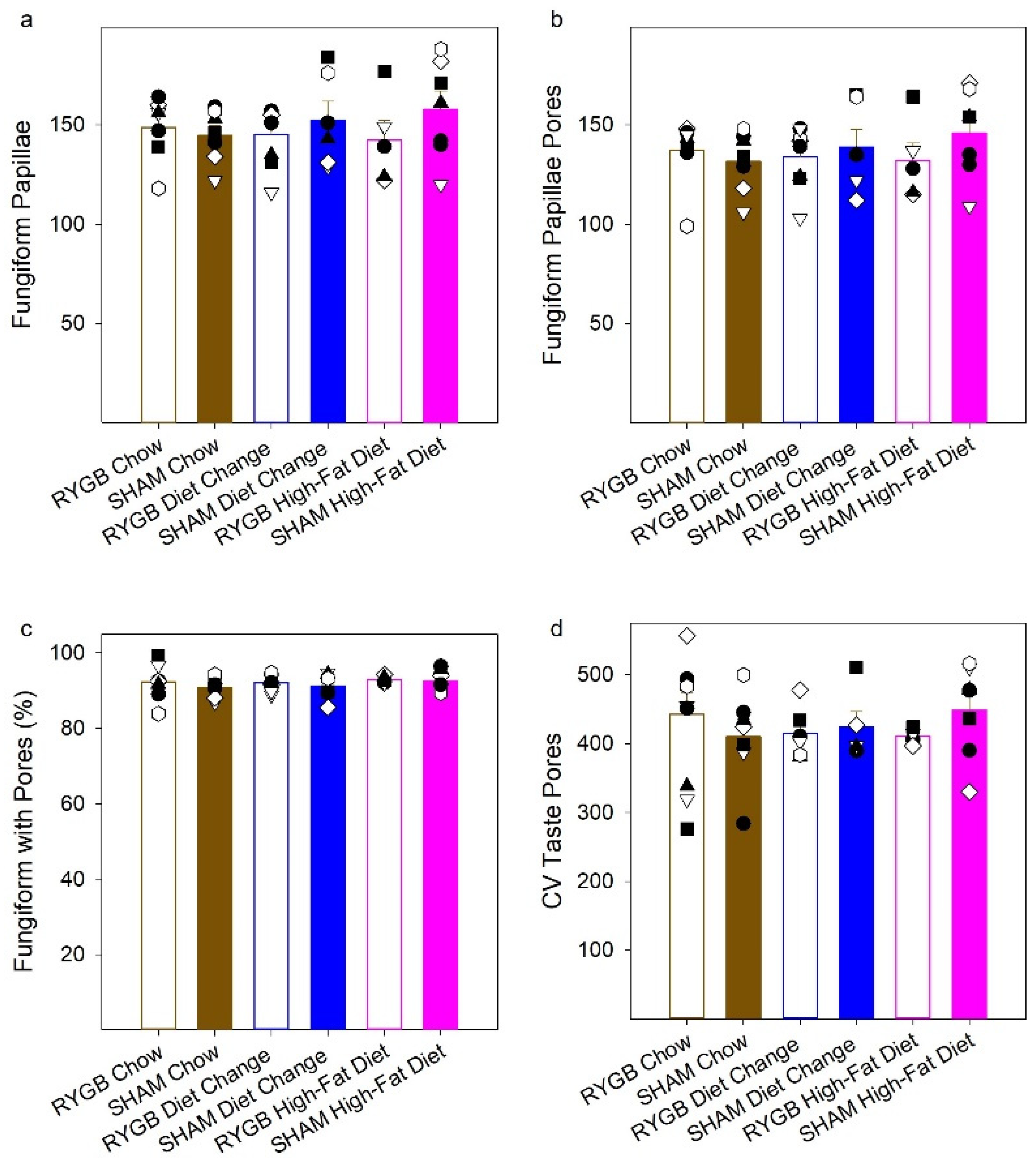
3.3. Quantification of Taste Pores in the Anterior Tongue and Circumvallate Papillae
3.4. Hormone Response following a Liquid Test Meal
3.5. Correlations between Key Experimental Measures
4. Discussion
4.1. Maintenance on HFD Does Not Affect Liminal Taste Sensitivity to NaCl or Sucrose in Female Rats
4.2. RYGB Has Little Effect on Liminal Taste Sensitivity to NaCl or Sucrose
4.3. Postprandial Circulating Levels of Leptin Were Not Related to Sucrose (or NaCl) Taste Detection Thresholds
4.4. Caveats Regarding Lack of Effects of Diet and RYGB on Taste Sensitivity
4.5. The Numbers of Fungiform Papillae and Fungiform and Circumvallate Taste Buds Were Unaffected by HFD Maintenance and RYGB
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef]
- Kourouniotis, S.; Keast, R.S.J.; Riddell, L.J.; Lacy, K.; Thorpe, M.G.; Cicerale, S. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Spector, A.C. Linking gustatory neurobiology to behavior in vertebrates. Neurosci. Biobehav. Rev. 2000, 24, 391–416. [Google Scholar] [CrossRef]
- Spector, A.C.; Glendinning, J.I. Linking Peripheral Taste Processes to Behavior. Curr. Opin. Neurobiol. 2009, 19, 370–377. [Google Scholar] [CrossRef]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28, 59–63. [Google Scholar] [CrossRef]
- Blundell, J.E.; Cooling, J. Routes to obesity: Phenotypes, food choices and activity. Br. J. Nutr. 2000, 83, 33–38. [Google Scholar] [CrossRef]
- Park, D.C.; Yeo, J.H.; Ryu, I.Y.; Kim, S.H.; Jung, J.; Yeo, S.G. Differences in taste detection thresholds between normal-weight and obese young adults. Acta Otolaryngol. 2015, 135, 478–483. [Google Scholar] [CrossRef]
- Tucker, R.M.; Edlinger, C.; Craig, B.A.; Mattes, R.D. Associations between BMI and Fat Taste Sensitivity in Humans. Chem. Senses 2014, 39, 349–357. [Google Scholar] [CrossRef]
- Asano, M.; Hong, G.; Matsuyama, Y.; Wang, W.; Izumi, S.; Izumi, M.; Toda, T.; Kudo, T.-A. Association of Oral Fat Sensitivity with Body Mass Index, Taste Preference, and Eating Habits in Healthy Japanese Young Adults. Tohoku J. Exp. Med. 2016, 238, 93–103. [Google Scholar] [CrossRef]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child. 2012, 97, 1048–1052. [Google Scholar] [CrossRef]
- Pasquet, P.; Frelut, M.L.; Simmen, B.; Hladik, C.M.; Monneuse, M.O. Taste perception in massively obese and in non-obese adolescents. Int. J. Pediatr. Obes. 2007, 2, 242–248. [Google Scholar] [CrossRef]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef]
- Heinze, J.M.; Costanzo, A.; Baselier, I.; Fritsche, A.; Frank-Podlech, S.; Keast, R. Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite 2018, 123, 7–13. [Google Scholar] [CrossRef]
- Scruggs, D.M.; Buffington, C.; Cowan, G.S.M. Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes. Surg. 1994, 4, 24–28. [Google Scholar] [CrossRef]
- Frijters, J.E.R.; Rasmussen-Conrad, E.L. Sensory Discrimination, Intensity Perception, and Affective Judgment of Sucrose-Sweetness in the Overweight. J. Gen. Psychol. 1982, 107, 233–247. [Google Scholar] [CrossRef]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef]
- Maliphol, A.B.; Garth, D.J.; Medler, K.F. Diet-Induced Obesity Reduces the Responsiveness of the Peripheral Taste Receptor Cells. PLoS ONE 2013, 8, e79403. [Google Scholar] [CrossRef]
- Harnischfeger, F.; O’connell, F.; Weiss, M.; Axelrod, B.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M.; Dando, R. Sprague dawley rats gaining weight on a high energy diet exhibit damage to taste tissue even after return to a healthy diet. Nutrients 2021, 13, 3062. [Google Scholar] [CrossRef]
- Kizy, S.; Jahansouz, C.; Downey, M.C.; Hevelone, N.; Ikramuddin, S.; Leslie, D. National Trends in Bariatric Surgery 2012–2015: Demographics, Procedure Selection, Readmissions, and Cost. Obes. Surg. 2017, 27, 2933–2939. [Google Scholar] [CrossRef]
- Sjöström, L. Surgical Intervention as a Strategy for Treatment of Obesity. Endocrine 2000, 13, 213–230. [Google Scholar] [CrossRef]
- Lutz, T.A.; Bueter, M. The physiology underlying Roux-en-Y gastric bypass: A status report. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 1275–1291. [Google Scholar] [CrossRef]
- Miras, A.D.; Le Roux, C.W. Bariatric surgery and taste: Novel mechanisms of weight loss. Curr. Opin. Gastroenterol. 2010, 26, 140–145. [Google Scholar] [CrossRef]
- le Roux, C.W.; Bueter, M. The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp. Physiol. 2014, 99, 1128–1132. [Google Scholar] [CrossRef]
- Zoon, H.F.A.; de Bruijn, S.E.M.; Jager, G.; Smeets, P.A.M.; de Graaf, C.; Janssen, I.M.C.; Schijns, W.; Deden, L.; Boesveldt, S. Altered neural inhibition responses to food cues after Roux-en-Y Gastric Bypass. Biol. Psychol. 2018, 137, 34–41. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Kittrell, H.; Graber, W.; Mariani, E.; Czaja, K.; Hajnal, A.; Di Lorenzo, P.M. Taste and odor preferences following roux-en-Y surgery in humans. PLoS ONE 2018, 13, e0199508. [Google Scholar] [CrossRef]
- Farias, G.; Silva, R.M.O.; da Silva, P.P.P.; Vilela, R.M.; Bettini, S.C.; Dâmaso, A.R.; Netto, B.D.M. Impact of dietary patterns according to NOVA food groups: 2 y after Roux-en-Y gastric bypass surgery. Nutrition 2020, 74, 746. [Google Scholar] [CrossRef]
- Olbers, T.; Björkman, S.; Lindroos, A.; Maleckas, A.; Lönn, L.; Sjöström, L.; Lönroth, H. Body Composition, Dietary Intake, and Energy Expenditure after Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Vertical Banded Gastroplasty. Ann. Surg. 2006, 244, 715–722. [Google Scholar] [CrossRef]
- Mathes, C.M.; Spector, A.C. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: A direct-measures approach. Physiol. Behav. 2012, 107, 476–483. [Google Scholar] [CrossRef]
- Gero, D.; Steinert, R.E.; le Roux, C.W.; Bueter, M. Do Food Preferences Change after Bariatric Surgery? Curr. Atheroscler. Rep. 2017, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Nance, K.; Acevedo, M.B.; Pepino, M.Y. Changes in taste function and ingestive behavior following bariatric surgery. Appetite 2020, 146, 104423. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Najim, W.; Menezes, C.; Price, R.K.; Boyle, C.O.; Bodnar, Z.; Spector, A.C.; Docherty, N.G.; Roux, C.W. A Comparison of Total Food Intake at a Personalised Buffet in People with Obesity, before and 24 Months after Roux-en-Y-Gastric Bypass Surgery. Nutrients 2021, 13, 3873. [Google Scholar] [CrossRef] [PubMed]
- Shoar, S.; Naderan, M.; Shoar, N.; Modukuru, V.R.; Mahmoodzadeh, H. Alteration Pattern of Taste Perception after Bariatric Surgery: A Systematic Review of Four Taste Domains. Obes. Surg. 2019, 29, 1542–1550. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Andersen, I.N.S.K.; Lange, B.; Ritz, C.; le Roux, C.W.; Schmidt, J.B.; Sjödin, A.; Bredie, W.L.P. Bariatric Surgery Leads to Short-Term Effects on Sweet Taste Sensitivity and Hedonic Evaluation of Fatty Food Stimuli. Obesity 2019, 27, 1796–1804. [Google Scholar] [CrossRef]
- Bueter, M.; Miras, A.D.; Chichger, H.; Fenske, W.; Ghatei, M.A.; Bloom, S.R.; Unwin, R.J.; Lutz, T.A.; Spector, A.C.; Le Roux, C.W. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, 13–20. [Google Scholar] [CrossRef]
- Burge, J.C.; Schaumburg, J.Z.; Choban, P.S.; DiSilvestro, R.A.; Flancbaum, L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J. Am. Diet. Assoc. 1995, 95, 666–670. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Boughter, J.D.; Madan, A.K. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg. Obes. Relat. Dis. 2006, 2, 440–444. [Google Scholar] [CrossRef]
- Zerrweck, C.; Zurita, L.; Álvarez, G.; Maydón, H.G.; Sepúlveda, E.M.; Campos, F.; Caviedes, A.; Guilbert, L. Taste and Olfactory Changes Following Laparoscopic Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2016, 26, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- El Labban, S.; Safadi, B.; Olabi, A. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on taste acuity and sweetness acceptability in postsurgical subjects. Nutrition 2016, 32, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Nozaki, T.; Sawamoto, R.; Komaki, G.; Miyata, N.; Hosoi, M.; Sudo, N. Effects of Weight Loss on Sweet Taste Preference and Palatability following Cognitive Behavioral Therapy for Women with Obesity. Obes. Facts 2019, 12, 529–542. [Google Scholar] [CrossRef]
- Sauer, H.; Ohla, K.; Dammann, D.; Teufel, M.; Zipfel, S.; Enck, P.; Mack, I. Changes in Gustatory Function and Taste Preference Following Weight Loss. J. Pediatr. 2017, 182, 120–126. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Bray, G.A.; Paeratakul, S.; Popkin, B.M. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol. Behav. 2004, 83, 549–555. [Google Scholar] [CrossRef]
- Oscai, L.B. Dietary-induced severe obesity: A rat model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1982, 11, 212–215. [Google Scholar] [CrossRef]
- Seyfried, F.; Le Roux, C.W.; Bueter, M. Lessons Learned from Gastric Bypass Operations in Rats. Obes. Facts 2011, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Bueter, M. The Use of Rat and Mouse Models in Bariatric Surgery Experiments. Front. Nutr. 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.M.; Bohnenkamp, R.A.; le Roux, C.W.; Spector, A.C. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, 864–874. [Google Scholar] [CrossRef]
- Mathes, C.M.; Bueter, M.; Smith, K.R.; Lutz, T.A.; le Roux, C.W.; Spector, A.C. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, 751–767. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, H.; Shin, A.C.; Lenard, N.R.; Townsend, R.L.; Patterson, L.M.; Sigalet, D.L.; Berthoud, H.-R. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, N.; Nestoridi, E.; Kucharczyk, J.; Uygun, M.K.; Yarmush, M.L.; Stylopoulos, N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int. J. Obes. 2012, 36, 1396–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathes, C.M.; Bohnenkamp, R.A.; Blonde, G.D.; Letourneau, C.; Corteville, C.; Bueter, M.; Lutz, T.A.; le Roux, C.W.; Spector, A.C. Gastric Bypass in Rats Does Not Decrease Appetitive Behavior towards Sweet or Fatty Fluids Despite Blunting Preferential Intake of Sugar and Fat. Physiol. Behav. 2015, 142, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.M.; Letourneau, C.; Blonde, G.D.; le Roux, C.W.; Spector, A.C. Roux-en-Y gastric bypass in rats progressively decreases the proportion of fat calories selected from a palatable cafeteria diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.M.; Blonde, G.D.; Bueter, M.; le Roux, C.W.; Spector, A.C. Gastric Bypass in Female Rats Lowers Concentrated Sugar Solution Intake and Preference without Affecting Brief Access Licking after Long-Term Sugar Exposure. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R870–R885. [Google Scholar] [CrossRef]
- Hajnal, A.; Kovacs, P.; Ahmed, T.; Meirelles, K.; Lynch, C.J.; Cooney, R.N. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 967–979. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Rebecca Glatt, A.; Madan, A.K.; Harper, J.; Tokita, K.; Boughter, J.D. Decrease in sweet taste in rats after gastric bypass surgery. Surg. Endosc. 2011, 25, 1176–1181. [Google Scholar] [CrossRef]
- Shin, A.C.; Berthoud, H.R. Food reward functions as affected by obesity and bariatric surgery. Int. J. Obes. 2011, 35, 40–44. [Google Scholar] [CrossRef]
- le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Lowenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 1057–1066. [Google Scholar] [CrossRef]
- Aly, S.; Hachey, K.; Pernar, L.I.M. Gender disparities in weight loss surgery. Mini-Invasive Surg. 2020, 4, 21. [Google Scholar] [CrossRef]
- Dagan, S.S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Sandbank, G.K.; Ben-Porat, T.; Sinai, T. Nutritional recommendations for adult bariatric surgery patients: Clinical practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.M.; Nuessle, T.M.; Garneau, N.L.; Smutzer, G.; Mattes, R.D. No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem. Senses 2015, 40, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Pittman, D.W.; Labban, C.E.; Anderson, A.A.; O’Connor, H.E. Linoleic and Oleic Acids Alter the Licking Responses to Sweet, Salt, Sour, and Bitter Tastants in Rats. Chem. Senses 2006, 31, 835–843. [Google Scholar] [CrossRef]
- Chen, C.S.-Y.; Bench, E.M.; Allerton, T.D.; Schreiber, A.L.; Arceneaux, K.P.; Primeaux, S.D. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 1346–1355. [Google Scholar] [CrossRef]
- McCormack, D.N.; Clyburn, V.L.; Pittman, D.W. Detection of free fatty acids following a conditioned taste aversion in rats. Physiol. Behav. 2006, 87, 582–594. [Google Scholar] [CrossRef]
- Mattes, R.D. Effects of linoleic acid on sweet, sour, salty, and bitter taste thresholds and intensity ratings of adults. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 1243–1248. [Google Scholar] [CrossRef]
- Spector, A.C.; Blonde, G.D.; Henderson, R.P.; Treesukosol, Y.; Hendrick, P.; Newsome, R.; Fletcher, F.H.; Tang, T.; Donaldson, J.A. A New Gustometer for Taste Testing in Rodents. Chem. Senses 2015, 40, 187–196. [Google Scholar] [CrossRef]
- Bales, M.B.; Schier, L.A.; Blonde, G.D.; Spector, A.C. Extensive Gustatory Cortex Lesions Significantly Impair Taste Sensitivity to KCl and Quinine but Not to Sucrose in Rats. PLoS ONE 2015, 10, e0143419. [Google Scholar] [CrossRef]
- Blonde, G.D.; Bales, M.B.; Spector, A.C. Extensive Lesions in Rat Insular Cortex Significantly Disrupt Taste Sensitivity to NaCl and KCl and Slow Salt Discrimination Learning. PLoS ONE 2015, 10, e0117515. [Google Scholar] [CrossRef]
- St. John, S.; Spector, A. Behavioral Analysis of Taste Function in Rodent Models. In The Senses, Olfaction and Taste; Fritzsch, B., Meyerhof, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume III. [Google Scholar]
- Hayes, M.R.; De Jonghe, B.C.; Kanoski, S.E. Role of the Glucagon-Like-Peptide-1 Receptor in the Control of Energy Balance. Physiol. Behav. 2010, 100, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; le Roux, C.W. Metabolic surgery and gut hormones—A review of bariatric entero-humoral modulation. Physiol. Behav. 2009, 97, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, C.; Jørgensen, N.; Bojsen-Møller, K.; Kielgast, U.; Jacobsen, S.; Clausen, T.; Worm, D.; Hartmann, B.; Rehfeld, J.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut Hormones as Mediators of Appetite and Weight Loss After Roux-en-Y Gastric Bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Shigemura, N.; Yasumatsu, K.; Ohta, R.; Sugimoto, K.; Nakashima, K.; Lindemann, B. Leptin and sweet taste. Vitam. Horm. 2002, 64, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin and the Regulation of Body Weight. Keio Med. Sci. 2011, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- St John, S.J.; Markison, S.; Spector, A.C. Salt discriminability is related to number of regenerated taste buds after chorda tympani nerve section in rats. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, 141–153. [Google Scholar] [CrossRef]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste loss with obesity in mice and men. Int. J. Obes. 2020, 44, 739–743. [Google Scholar] [CrossRef]
- Sun, H.; Yan, J.; Sun, B.; Song, L.; Yan, J. Taste sensitivity to sucrose is lower in outbred Sprague-Dawley phenotypic obesity-prone rats than obesity-resistant rats. Biochem. Biophys. Res. Commun. 2017, 489, 155–163. [Google Scholar] [CrossRef]
- Shin, A.C.; Townsend, R.L.; Patterson, L.M.; Berthoud, H. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 1267–1280. [Google Scholar] [CrossRef]
- Duca, F.A.; Swartz, T.D.; Covasa, M. Effect of Diet on Preference and Intake of Sucrose in Obese Prone and Resistant Rats. PLoS ONE 2014, 9, e111232. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W. Dietary manipulations influence sucrose acceptance in diet induced obese mice. Appetite 2012, 58, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Sugimoto, K.; Nakashima, K.; Miura, H.; Ninomiya, Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Sako, N.; Imai, Y. Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Sanematsu, K.; Nakamura, Y.; Nomura, M.; Shigemura, N.; Ninomiya, Y. Diurnal variation of sweet taste recognition thresholds is absent in overweight and obese humans. Nutrients 2018, 10, 297. [Google Scholar] [CrossRef]
- Lu, B.; Breza, J.M.; Nikonov, A.A.; Paedae, A.B.; Contreras, R.J. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol. Behav. 2012, 107, 533–539. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Elson, A.E.T.; Kalik, S.; Sosa, Y.; Patterson, C.M.; Myers, M.G.; Munger, S.D. Taste responsiveness to sweeteners is resistant to elevations in plasma leptin. Chem. Senses 2015, 40, 223–231. [Google Scholar] [CrossRef]
- Blonde, G.D.; Spector, A.C. Masking the Detection of Taste Stimuli in Rats: NaCl and Sucrose. Chem. Senses 2020, 45, 359–370. [Google Scholar] [CrossRef]
- Smith, K.R.; Spector, A.C. The Importance of the Presence of a 5-Ribonucleotide and the Contribution of the T1R1 T1R3 Heterodimer and an Additional Low-Affinity Receptor in the Taste Detection of L-Glutamate as Assessed Psychophysically. J. Neurosci. 2014, 34, 13234–13245. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Spector, A.C. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol 2012, 303, 218–235. [Google Scholar] [CrossRef]
- Blonde, G.; Jiang, E.; Garcea, M.; Spector, A.C. Learning-based recovery from perceptual impairment in salt discrimination after permanently altered peripheral gustatory input. Am J Physiol Regul Integr Comp Physiol 2010, 299, 1027–1036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geran, L.C.; Spector, A.C. Sodium taste detectability in rats is dependent of anion size: The psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav. Neurosci. 2000, 114, 1229. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.E.; Kay, K.E.; Torregrossa, A.-M. Bitter-Induced Salivary Proteins Increase Detection Threshold of Quinine, But Not Sucrose. Chem. Senses 2019, 44, 379–388. [Google Scholar] [CrossRef] [PubMed]
- St John, S.J.; Markison, S.; Guagliardo, N.A.; Hackenberg, T.D.; Spector, A.C. Chorda Tympani Transection and Selective Desalivation Differentially Disrupt Two-Lever Salt Discrimination Performance in Rats. Behav. Neurosci. 1997, 111, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Nikonova, L.; Kay, K.; Paedae, A.B.; Contreras, R.J.; Torregrossa, M. Salivary Proteins Alter Taste-Guided Behaviors and Taste Nerve Signaling in Rat. Physiol. Behav. 2018, 184, 150–161. [Google Scholar] [CrossRef]
- Archer, N.; Shaw, J.; Cochet-Broch, M.; Bunch, R.; Poelman, A.; Barendse, W.; Duesing, K. Obesity is associated with altered gene expression in human tastebuds. Int. J. Obes. 2019, 43, 1475–1484. [Google Scholar] [CrossRef] [PubMed]

| Phase | Duration |
|---|---|
| Acclimation 1 | 10 weeks |
| Surgery and Recovery | 4–6 weeks |
| Training 2 | 10 weeks |
| Spout | 6–15 sessions |
| Side | 6–10 sessions |
| Alternation | 4 sessions |
| Random I | 7–14 sessions |
| Random II | 2–15 sessions |
| Test NaCl | 6 weeks |
| Train Sucrose | 1 week |
| Test Sucrose 3 | 7 weeks |
| Stimulus Control Test | 1 session/animal |
| EchoMRI 4 | 1 day |
| Tissue Collection 5 | 2 weeks |
| HFD | DC | Chow | |
|---|---|---|---|
| NaCl Testing | R = 5; S = 7 | R = 9; S = 6 | R = 8; S = 7 |
| Sucrose Testing | R = 5; S = 7 | R = 9; S = 6 | R = 8; S = 7 |
| EchoMRI | R = 1; S = 4 | R = 4; S = 4 | R = 6; S = 4 |
| Fat Pads | R = 3; S = 5 | R = 4; S = 4 | R = 6; S = 4 |
| Anterior Taste Pores | R = 5; S = 7 | R = 8; S = 6 | R = 8; S = 7 |
| CV Taste Pores | R = 4; S = 7 | R = 6; S = 5 | R = 7; S = 7 |
| GLP-1 ELISA * | R = 2; S = 6 | R = 6; S = 6 | R = 6; S = 7 |
| Leptin ELISA | R = 2; S = 6 | R = 3; S = 6 | R = 6; S = 7 |
| Acclimation | Recovery | Detection Training | NaCl Testing | Sucrose Training | Sucrose Testing | |
|---|---|---|---|---|---|---|
| Surg | F (1,36) = 2.97, p = 0.094 | F (1,36) = 21.35, p < 0.001 | F (1,36) = 24.50, p < 0.001 | F (1,36) = 25.90, p < 0.001 | F (1,36) = 30.76, p < 0.001 | F (1,36) = 30.76, p < 0.001 |
| Diet | F (2,36) = 21.58, p < 0.001 | F (2,36) = 21.95, p < 0.001 | F (2,36) = 9.59, p < 0.001 | F (2,36) = 13.23, p < 0.001 | F (2,36) = 12.82, p < 0.001 | F (2,36) = 12.82, p < 0.001 |
| Time | F (9,324) = 234.55, p < 0.001 | F (25,900) = 6.83, p < 0.001 | F (8,288) = 7.62, p < 0.001 | F (5,180) = 18.24, p < 0.001 | F (6,216) = 1.49, p = 0.18 | |
| Surg × Diet | F (2,36) = 2.74, p = 0.078 | F (2,36) = 0.71, p = 0.50 | F (2,36) = 2.33, p = 0.11 | F (2,36) = 2.30, p = 0.12 | F (2,36) = 1.86, p = 0.17 | F (2,36) = 1.86, p = 0.17 |
| Surg × Time | F (9,324) = 0.54, p = 0.83 | F (25,900) = 15.94, p < 0.001 | F (8, 288) = 1.45, p = 0.18 | F (5,180) = 8.19, p < 0.001 | F (6,216) = 1.20, p = 0.31 | |
| Diet × Time | F (18,324) 5.91, p < 0.001 | F (50,900) = 14.65, p < 0.001 | F (16,288) = 2.49, p < 0.001 | F (10,180) = 0.72, p = 0.71 | F (12,216) = 0.77, p = 0.68 | |
| Surg × Diet × Time | F (18,324) = 1.04, p = 0.41 | F (50,900) = 6.84, p < 0.001 | F (16,288) = 0.71, p = 0.79 | F (10,180) = 0.55, p = 0.89 | F (12,216) = 2.03, p = 0.023 |
| Acclimation | Recovery | Detection Training | NaCl Testing | Sucrose Training | Sucrose Testing | |
|---|---|---|---|---|---|---|
| Surg | F (1,36) = 0.22, p = 0.65 | F (1,36) = 74.50, p < 0.001 | F (1,36) = 36.14, p < 0.001 | F (1,36) = 39.42, p < 0.001 | F (1,36) = 33.99, p < 0.001 | F (1,36) = 43.22, p < 0.001 |
| Diet | F (2,36) = 13.29, p < 0.001 | F (2,36) = 7.60, p = 0.002 | F (2,36) = 19.81, p < 0.001 | F (2,36) = 15.77, p < 0.001 | F (2,36) = 9.54, p < 0.001 | F (2,36) = 12.27, p < 0.001 |
| Time | F (9,324) = 224.98, p < 0.001 | F (25,900) = 6.64, p < 0.001 | F (8,288) = 7.75, p < 0.001 | F (5,180) = 8.95, p < 0.001 | F (6,216) = 1.65, p = 0.13 | |
| Surg × Diet | F (2,36) = 2.34, p = 0.11 | F (2,36) = 3.60, p = 0.037 | F (2,36) = 13.87, p < 0.001 | F (2,36) = 10.69, p < 0.001 | F (2,36) = 5.74, p = 0.007 | F (2,36) = 8.36, p = 0.001 |
| Surg × Time | F (9,324) = 0.43, p = 0.92 | F (25, 900) = 16.30, p < 0.001 | F (8, 288) = 1.58, p = 0.13 | F (5,180) = 4.62, p = 0.001 | F (6,216) = 1.23, p = 0.29 | |
| Diet × Time | F (18, 324) = 5.46, p < 0.001 | F (50, 900) = 15.93, p < 0.001 | F (16, 288) = 2.24, p = 0.004 | F (10,180) = 0.72, p = 0.71 | F (12,216) = 0.90, p = 0.55 | |
| Surg × Diet × Time | F (18, 324) = 1.06, p = 0.39 | F (50, 900) = 6.03, p < 0.001 | F (16, 288) = 0.72, p = 0.79 | F (10,180) = 0.94, p = 0.50 | F (12,216) = 1.87, p = 0.039 |
| Between Chow | Between Diet Change | Between RYGB | Between SHAM | ||||
|---|---|---|---|---|---|---|---|
| RYGB vs. SHAM | RYGB vs. SHAM | Chow vs. Diet Change | ANOVA | Chow vs. Diet Change | Chow vs. High-Fat Diet | Diet Change vs. High-Fat Diet | |
| Total Fat Pad Weight | t(8) = 7.16, p < 0.001 | t(6) = 6.89, p < 0.001 | t(8) = 1.89, p = 0.096 | F (2,10) = 5.01, p= 0.031 | t(6) = 0.53, p = 0.62 | t(7) = 2.21, p = 0.063 | t(7) = 2.33, p = 0.053 |
| Retro Peritoneal Fat Pad | t(8) = 6.99, p < 0.001 | t(6) = 8.48, p < 0.001 | t(8) = 1.76, p = 0.12 | F (2,10) = 4.76, p = 0.035 | t(6) = 0.41, p = 0.69 | t(7) = 2.23, p = 0.061 | t(7) = 2.17, p = 0.066 |
| Gonadal Fat Pad | t(8) = 6.10, p < 0.001 | t(6) = 3.93, p = 0.008 | t(8) = 1.69, p = 0.13 | F (2,10) = 2.87, p = 0.10 | t(6) = 1.0, p = 0.35 | t(7) = 1.52, p = 0.17 | t(7) = 1.95, p = 0.093 |
| Perirenal Fat Pad | t(8) = 6.30, p < 0.001 | t(6) = 5.43, p < 0.001 | t(8) = 1.70, p = 0.13 | F (2,10) = 5.53, p = 0.024 | t(6) = 1.80, p = 0.12 | t(7) = 2.07, p = 0.077 | t(7) = 2.67, p = 0.032 |
| Body Weight on MRI Scan | t(8) = 4.83, p = 0.001 | t(6) = 3.63, p = 0.011 | t(8) = 0.23, p = 0.82 | F (2,9) = 2.67, p = 0.12 | t(6) = 0.09, p = 0.93 | t(6) = 1.67, p = 0.15 | t(6) = 1.69, p = 0.14 |
| %Fat Mass | t(8) = 3.23, p = 0.012 | t(6) = 5.41, p = 0.002 | t(8) = 1.91, p = 0.093 | F (2,9) = 4.98, p = 0.035 | t(6) = 0.11, p = 0.91 | t(6) = 2.22, p = 0.068 | t(6) = 2.34, p = 0.058 |
| %Lean Mass | t(8) = 3.03, p = 0.016 | t(6) = 4.40, p = 0.005 | t(8) = 1.59, p = 0.15 | F (2,9) = 4.71, p = 0.040 | t(6) = 0.24, p = 0.82 | t(6) = 2.22, p = 0.068 | t(6) = 2.22, p = 0.068 |
| %Bone Mass | t(8) = 0.76, p = 0.47 | t(6) = 0.37, p = 0.72 | t(8) = 0.23, p = 0.83 | F (2,9) = 2.25, p = 0.16 | t(6) = 1.05, p = 0.34 | t(6) = 1.17, p = 0.29 | t(6) = 1.98, p = 0.096 |
| Chow | Diet Change | High-Fat Diet | |||||
|---|---|---|---|---|---|---|---|
| RYGB | SHAM | RYGB | SHAM | RYGB | SHAM | ||
| NaCl Curve Parameters | Asymptote (A) | 0.95 (0.009) | 0.97 (0.007) | 0.97 (0.008) | 0.94 (0.008) | 0.97 (0.007) | 0.97 (0.010) |
| Threshold (EC50) | −2.39 (0.16) | −2.72 (0.11) | −2.61 (0.089) | −2.58 (0.18) | −2.86 (0.098) | −2.41 (0.14) | |
| Slope (B) | −1.11 (0.12) | −1.12 (0.09) | −1.50 (0.53) | −2.14 (1.21) | −1.04 (0.14) | −0.83 (0.089) | |
| R-squared | 0.93 (0.022) | 0.93 (0.013) | 0.91 (0.019) | 0.93 (0.016) | 0.95 (0.008) | 0.87 (0.015) | |
| Sucrose Curve Parameters | Asymptote (A) | 0.95 (0.003) | 0.98 (0.009) | 0.96 (0.009) | 0.96 (0.009) | 0.96 (0.01) | 0.95 (0.013) |
| Threshold (EC50) | −1.93 (0.044) | −1.89 (0.068) | −1.89 (0.045) | −2.04 (0.11) | −1.89 (0.069) | −1.83 (0.053) | |
| Slope (B) | −2.47 (0.53) | −2.23 (0.55) | −1.81 (0.13) | −2.09 (0.64) | −1.55 (0.17) | −1.60 (0.27) | |
| R-squared | 0.93 (0.019) | 0.92 (0.031) | 0.94 (0.014) | 0.88 (0.038) | 0.95 (0.018) | 0.92 (0.026) | |
| Surgery | Diet | Interaction | ||
|---|---|---|---|---|
| NaCl Curve Parameters | Asymptote (A) | F (1,36) = 0.61, p = 0.44 | F (2,36) = 1.26, p = 0.30 | F (2,36) = 3.11, p = 0.057 |
| Threshold (EC50) | F (1,36) = 0.19, p = 0.67 | F (2,36) = 0.17, p = 0.85 | F (2,36) = 4.06, p = 0.026 | |
| Between RYGB | F (2,19) = 3.04, p = 0.072 | |||
| Between SHAM | F (2,17) = 1.27, p = 0.31 | |||
| Between Chow | t (13) = 1.68, p = 0.12 | |||
| Between DC | t (13) = 0.16, p = 0.88 | |||
| Between HFD | t (10) = 2.46, p = 0.034 | |||
| Slope (B) | F (1,36) = 0.12, p = 0.73 | F (2,36) = 1.61, p = 0.21 | F (2,36) = 0.36, p = 0.70 | |
| Sucrose Curve Parameters | Asymptote (A) | F (1,36) = 0.47, p = 0.50 | F (2,36) = 0.52, p = 0.60 | F (2,36) = 1.56, p = 0.22 |
| Threshold (EC50) | F (1,36) = 0.10, p = 0.75 | F (2,36) = 1.27, p = 0.29 | F (2,36) = 1.50, p = 0.24 | |
| Slope (B) | F (1,36) = 0.006, p = 0.94 | F (2,36) = 1.56, p = 0.22 | F (2,36) = 0.19, p = 0.83 |
| Chow | Diet Change | Between RYGB | Between SHAM | ||||
|---|---|---|---|---|---|---|---|
| RYGB vs. SHAM | RYGB vs. SHAM | Chow vs. Diet Change | ANOVA | Chow vs. Diet Change | Chow vs. High-Fat Diet | Diet Change vs. High-Fat Diet | |
| Body Weight | t (11) = 4.21, p = 0.001 | t (10) = 4.58, p = 0.001 | t (10) = 0.53, p = 0.61 | F (2,16) = 11.61, p = 0.001 | t (11) = 0.31, p = 0.76 | t (11) = 3.92, p = 0.002 | t (10) = 3.52, p = 0.006 |
| Caloric Intake | t (11) = 9.089, p < 0.001 | t (10) = 3.52, p = 0.006 | t (10) = 0.40, p = 0.70 | F (2,16) = 9.53, p = 0.002 | t (11) = 2.79, p = 0.018 | t (11) = 5.79, p < 0.001 | t (10) = 0.90, p = 0.39 |
| GLP-1 | t (11) = 3.46, p = 0.005 | t (10) = 2.68, p = 0.023 | t (10) = 0.89, p = 0.40 | F (2,16) = 1.63, p = 0.23 | t (11) = 1.29, p = 0.22 | t (11) = 1.61, p = 0.14 | t (10) = 0.22, p = 0.83 |
| Leptin | t (11) = 3.68, p = 0.004 | t (7) = 3.036, p = 0.019 | t (7) = 1.82, p = 0.11 | F (2,16) = 6.81, p = 0.007 | t (11) = 0.40, p = 0.70 | t (11) = 2.84, p = 0.016 | t (10) = 2.46, p = 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyde, K.M.; Blonde, G.D.; Nisi, A.V.; Spector, A.C. The Influence of Roux-en-Y Gastric Bypass and Diet on NaCl and Sucrose Taste Detection Thresholds and Number of Circumvallate and Fungiform Taste Buds in Female Rats. Nutrients 2022, 14, 877. https://doi.org/10.3390/nu14040877
Hyde KM, Blonde GD, Nisi AV, Spector AC. The Influence of Roux-en-Y Gastric Bypass and Diet on NaCl and Sucrose Taste Detection Thresholds and Number of Circumvallate and Fungiform Taste Buds in Female Rats. Nutrients. 2022; 14(4):877. https://doi.org/10.3390/nu14040877
Chicago/Turabian StyleHyde, Kellie M., Ginger D. Blonde, A. Valentina Nisi, and Alan C. Spector. 2022. "The Influence of Roux-en-Y Gastric Bypass and Diet on NaCl and Sucrose Taste Detection Thresholds and Number of Circumvallate and Fungiform Taste Buds in Female Rats" Nutrients 14, no. 4: 877. https://doi.org/10.3390/nu14040877
APA StyleHyde, K. M., Blonde, G. D., Nisi, A. V., & Spector, A. C. (2022). The Influence of Roux-en-Y Gastric Bypass and Diet on NaCl and Sucrose Taste Detection Thresholds and Number of Circumvallate and Fungiform Taste Buds in Female Rats. Nutrients, 14(4), 877. https://doi.org/10.3390/nu14040877





