Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Blood and Urinary Cadmium Measurements
2.3. Sociodemographic Characteristics
2.4. Definition for Pre-Existing Comorbidities
2.5. Statistical Analyses
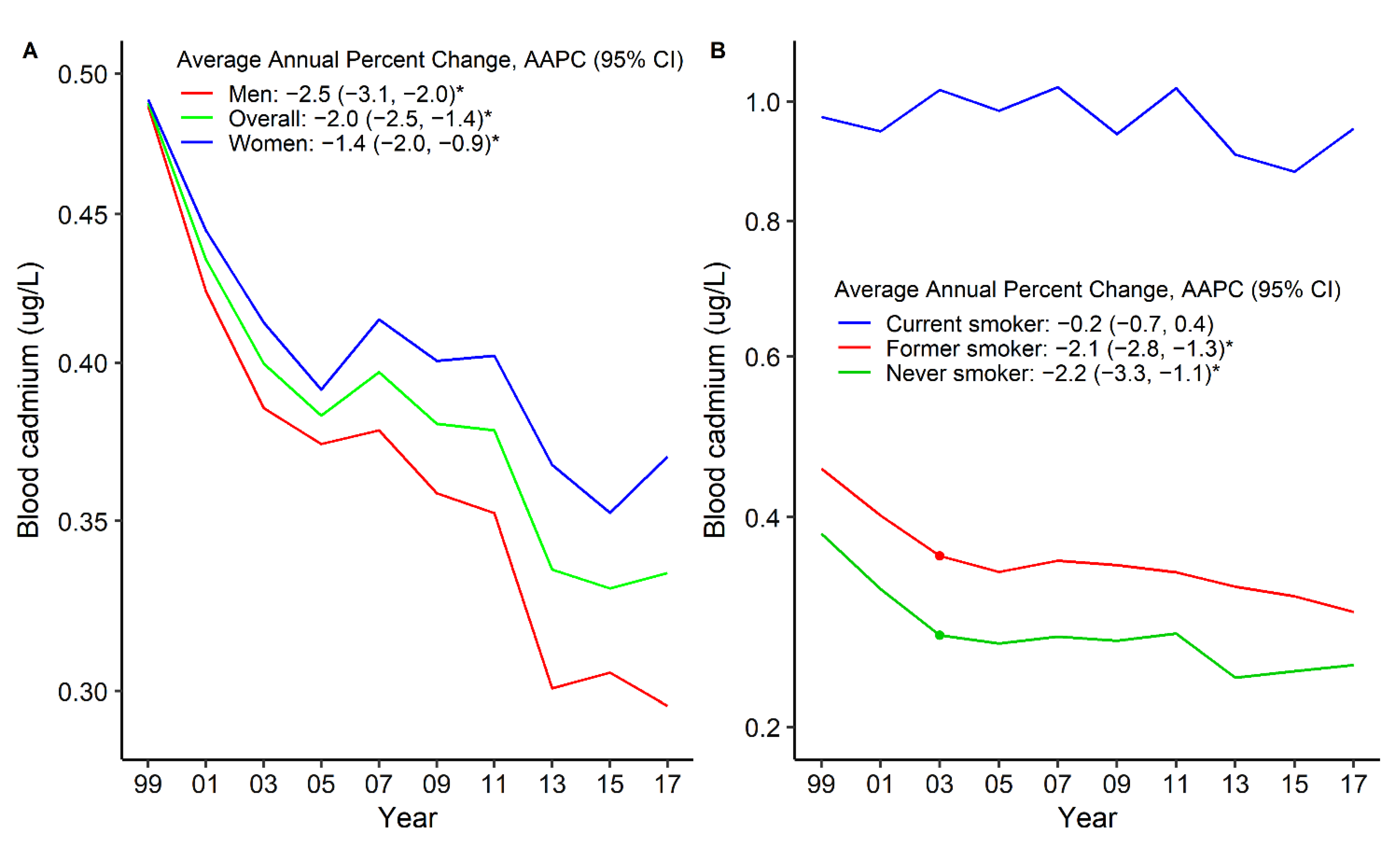
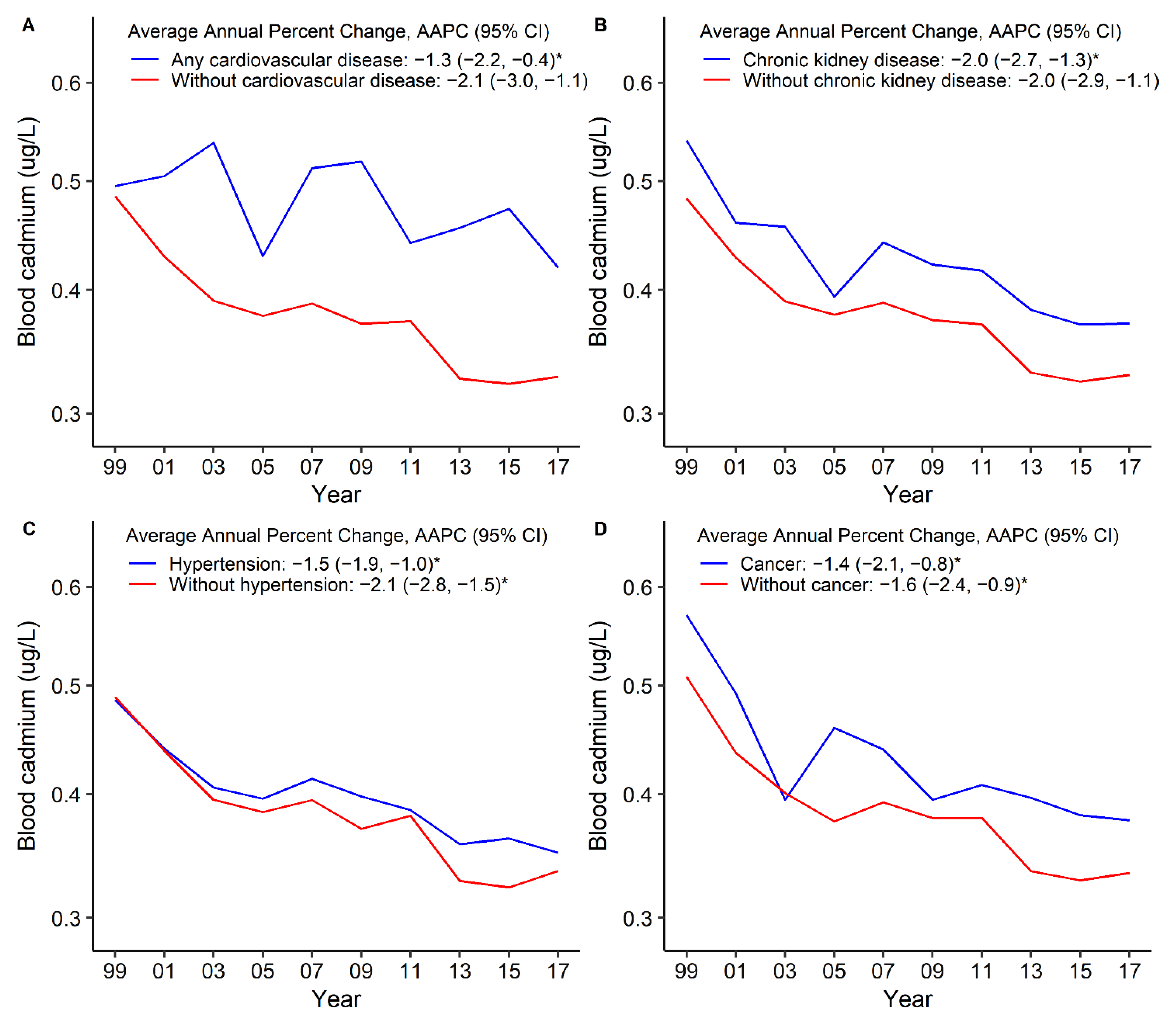
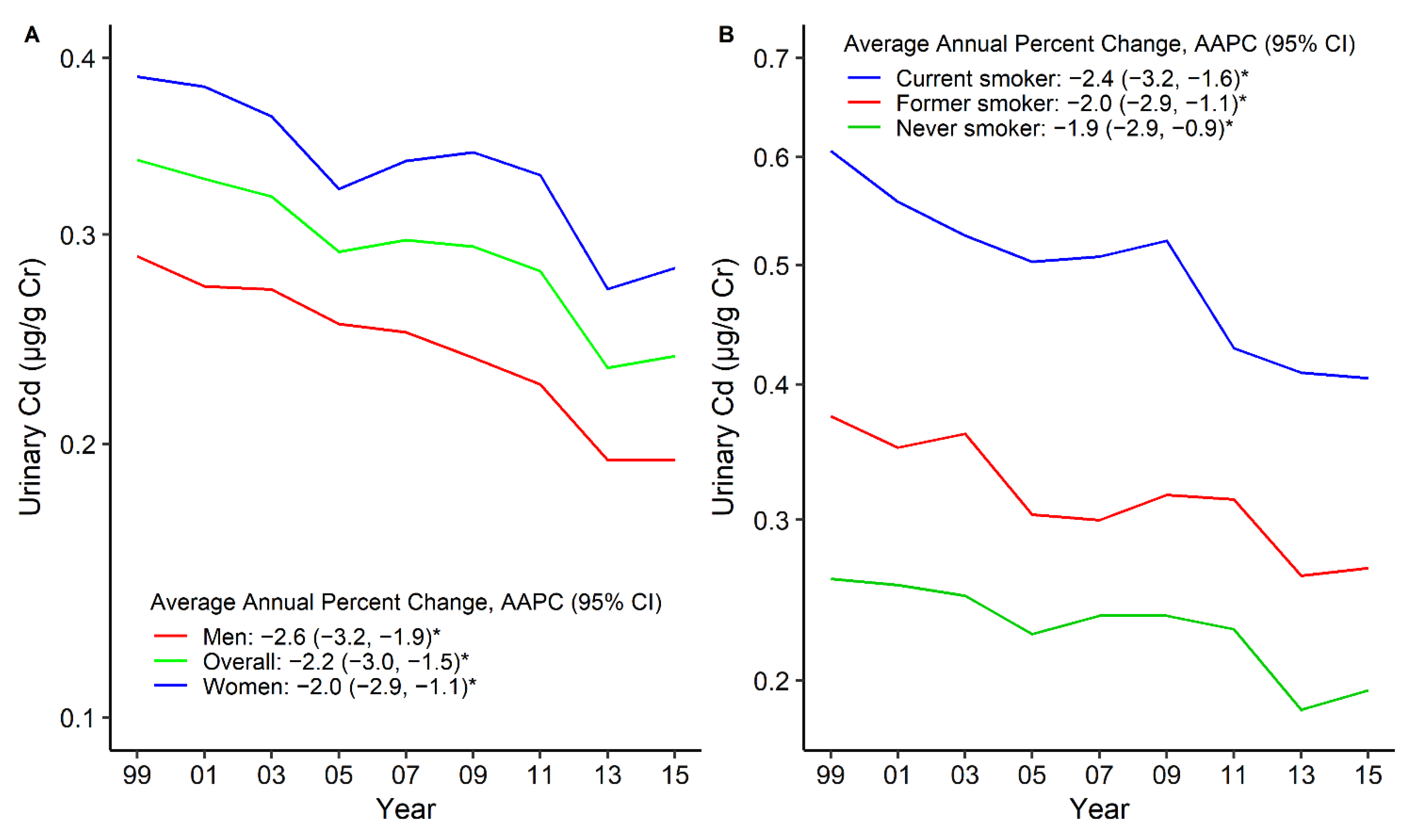
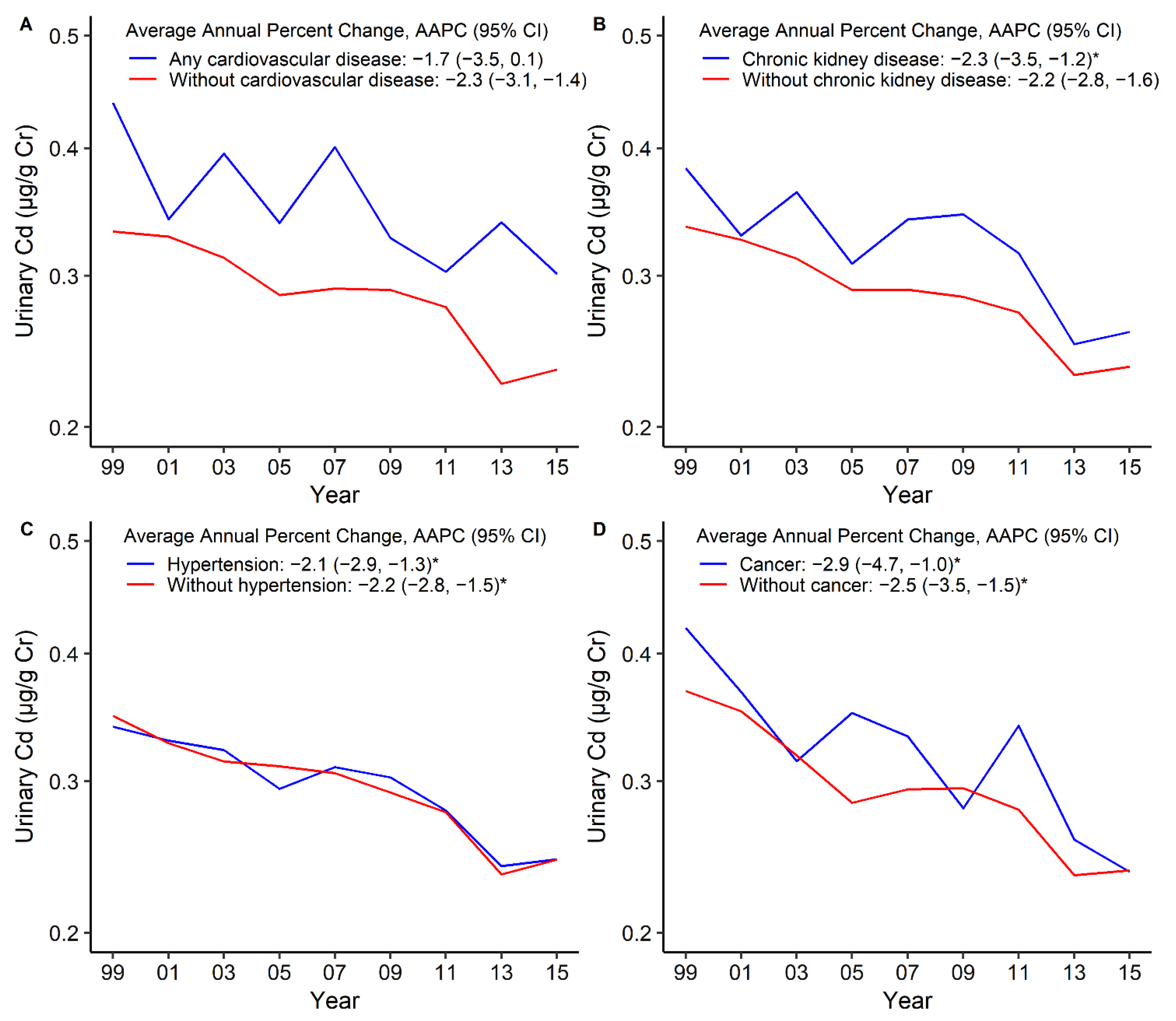
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poulsen, A.H.; Sears, C.G.; Harrington, J.; Howe, C.J.; James, K.A.; Roswall, N.; Overvad, K.; Tjønneland, A.; Wellenius, G.A.; Meliker, J.; et al. Urinary cadmium and stroke—A case-cohort study in Danish never-smokers. Environ. Res. 2021, 200, 111394. [Google Scholar] [CrossRef] [PubMed]
- Toxicological Profile for Cadmium. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=48&tid=15 (accessed on 12 July 2021).
- Ahn, J.; Kim, N.S.; Lee, B.K.; Oh, I.; Kim, Y. Changes of atmospheric and blood concentrations of lead and cadmium in the general population of south korea from 2008 to 2017. Int. J. Environ. Res. Public Health 2019, 16, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riederer, A.M.; Belova, A.; George, B.J.; Anastas, P.T. Urinary cadmium in the 1999–2008 U.S. National health and nutrition examination survey (nhanes). Environ. Sci. Technol. 2013, 47, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Test Definition: CDB. Available online: https://www.mayocliniclabs.com/test-catalog/overview/8682#Clinical-and-Interpretive (accessed on 10 July 2021).
- Heavy Metal/Creatinine Ratio, with Reflex, Urine. Available online: https://www.mayocliniclabs.com/test-catalog/overview/608899#Clinical-and-Interpretive (accessed on 10 July 2021).
- Bergström, G.; Fagerberg, B.; Sallsten, G.; Lundh, T.; Barregard, L. Is cadmium exposure associated with the burden, vulnerability and rupture of human atherosclerotic plaques? PLoS ONE 2015, 10, e0121240. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Wu, H.-B.; Niu, Q.-S.; Jia, P.-P.; Qin, Q.-R.; Wang, X.-D.; He, J.-L.; Yang, W.-J.; Huang, F. Exposure to multiple metals and the risk of hypertension in adults: A prospective cohort study in a local area on the Yangtze River, China. Environ. Int. 2021, 153, 106538. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: Systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2016, 45, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.R. Nutritional influences on metal toxicity: Cadmium as a model toxic element. Environ. Health Perspect. 1979, 29, 95–104. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, X.; Li, T.; Xu, X. Cadmium exposure in US adults, research based on the national health and nutrition examination survey from 1988 to 2018. Environ. Sci. Pollut. Res. Int. 2021, in press. [Google Scholar] [CrossRef]
- Yang, J.; Yang, A.; Cheng, N.; Huang, W.; Huang, P.; Liu, N.; Bai, Y. Sex-specific associations of blood and urinary manganese levels with glucose levels, insulin resistance and kidney function in US adults: National health and nutrition examination survey 2011–2016. Chemosphere 2020, 258, 126940. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, K.R.; Sozio, S.M.; Chen, J.; Sang, Y.; Shafi, T. Resistant hypertension and cardiovascular disease mortality in the US: Results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol. 2019, 20, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, G.; Madeddu, R.; Tolu, P.; Asara, Y.; Marchal, J.A.; Bocca, B. Reference intervals for blood Cd and Pb in the general population of Sardinia (Italy). Int. J. Hyg. Environ. Health 2011, 214, 102–109. [Google Scholar] [CrossRef]
- Heitland, P.; Köster, H.D. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP-MS. J. Trace Elem. Med. Biol. 2006, 20, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Saravanabhavan, G.; Werry, K.; Walker, M.; Haines, D.; Malowany, M.; Khoury, C. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. Int. J. Hyg. Environ. Health 2017, 220, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, T.; McKeating, D.; Perkins, A.V.; Tinggi, U. Trace element analysis in whole blood and plasma for reference levels in a selected Queensland population, Australia. Int. J. Environ. Res. Public Health 2021, 18, 2652. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.K.; Moon, C.S.; Choi, I.J.; Lee, K.J.; Yi, S.-M.; Jang, B.-K.; Yoon, B.J.; Kim, D.S.; Peak, D.; et al. Korea national survey for environmental pollutants in the human body 2008: Heavy metals in the blood or urine of the Korean population. Int. J. Hyg. Environ. Health 2012, 215, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Nisse, C.; Tagne-Fotso, R.; Howsam, M.; Richeval, C.; Labat, L.; Leroyer, A. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: The IMEPOGE study, 2008–2010. Int. J. Hyg. Environ. Health 2017, 220, 341–363. [Google Scholar] [CrossRef]
- Butler-Dawson, J.; James, K.A.; Krisher, L.; Jaramillo, D.; Dally, M.; Neumann, N.; Pilloni, D.; Cruz, A.; Asensio, C.; Johnson, R.J.; et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J. Expo. Sci. Environ. Epidemiol. 2021, in press. [Google Scholar] [CrossRef]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.-T.; et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef]
- Wen, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhou, L.; Di, L.; Xie, C.; Lv, Z.; Guo, Y.; Ke, Y.; et al. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ. Int. 2019, 125, 125–134. [Google Scholar] [CrossRef]
- Martins, A.C.; Almeida Lopes, A.C.B.; Urbano, M.R.; Carvalho, M.d.F.H.; Silva, A.M.R.; Tinkov, A.A.; Aschner, M.; Mesas, A.E.; Silbergeld, E.K.; Paoliello, M.M.B. An updated systematic review on the association between Cd exposure, blood pressure and hypertension. Ecotoxicol. Environ. Saf. 2021, 208, 111636. [Google Scholar] [CrossRef]
- Jeong, J.; Yun, S.-M.; Kim, M.; Koh, Y.H. Association of blood cadmium with cardiovascular disease in Korea: From the Korea national health and nutrition examination survey 2008–2013 and 2016. Int. J. Environ. Res. Public Health 2020, 17, 6288. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baillon, L.; Sow, M.; Gotreau, S.; Gonzalez, P. Effect of low-dose cadmium exposure on DNA methylation in the endangered European eel. Environ. Sci. Technol. 2014, 48, 797–803. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Asara, Y.; Marchal, J.A.; Carrasco, E.; Boulaiz, H.; Solinas, G.; Bandiera, P.; Garcia, M.A.; Farace, C.; Montella, A.; Madeddu, R. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int. J. Mol. Sci. 2013, 14, 16600–16616. [Google Scholar] [CrossRef]
- López-Herranz, A.; Cutanda, F.; Esteban, M.; Pollán, M.; Calvo, E.; Pérez-Gómez, B.; Victoria Cortes, M.; Castaño, A. Cadmium levels in a representative sample of the Spanish adult population: The BIOAMBIENT.ES project. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Nishijo, M.; Satarug, S.; Honda, R.; Tsuritani, I.; Aoshima, K. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol. Cell Biochem 2004, 255, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention. Tobacco product use among adults—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 | 2017–2018 | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood cadmium analysis | |||||||||||
| N | 4207 | 4772 | 4525 | 4509 | 5364 | 5765 | 5030 | 2695 | 2610 | 5021 | 44,498 |
| Men, n (%) | 1966 (46.7) | 2259 (47.3) | 2182 (48.2) | 2158 (47.9) | 2622 (48.9) | 2784 (48.3) | 2480 (49.3) | 1290 (47.9) | 1277 (48.9) | 2417 (48.1) | 21,435 (48.2) |
| Smoking status, n (%) | |||||||||||
| Never smoker | 2234 (53.1) | 2445 (51.2) | 2267 (50.1) | 2374 (52.7) | 2816 (52.5) | 3085 (53.5) | 2872 (57.1) | 1533 (56.9) | 1522 (58.3) | 2905 (57.9) | 24,053 (54.1) |
| Former smoker | 1116 (26.5) | 1262 (26.4) | 1238 (27.4) | 1145 (25.4) | 1357 (25.3) | 1417 (24.6) | 1161 (23.1) | 627 (23.3) | 608 (23.3) | 1210 (24.1) | 11,141 (25.0) |
| Current smoker | 857 (20.4) | 1065 (22.3) | 1020 (22.5) | 990 (21.9) | 1191 (22.2) | 1263 (21.9) | 997 (19.8) | 535 (19.8) | 480 (18.4) | 906 (18.0) | 9304 (20.9) |
| Diabetes, n (%) | 509 (12.1) | 562 (11.8) | 622 (13.7) | 655 (14.5) | 1029 (19.2) | 1043 (18.1) | 951 (18.9) | 465 (17.3) | 564 (21.6) | 1094 (21.8) | 7494 (16.8) |
| Chronic kidney disease, n (%) | 569 (13.5) | 588 (12.3) | 527 (11.6) | 549 (12.2) | 719 (13.4) | 614 (10.7) | 641 (12.7) | 291 (10.8) | 331 (12.7) | 692 (13.8) | 5521 (12.4) |
| Hypertension, n (%) | 1729 (41.1) | 1879 (39.4) | 1945 (43.0) | 1747 (38.7) | 2314 (43.1) | 2359 (40.9) | 2102 (41.8) | 1144 (42.4) | 1127 (43.2) | 2357 (46.9) | 18,703 (42.0) |
| Any cardiovascular disease, n (%) | 444 (10.6) | 508 (10.6) | 603 (13.3) | 498 (11.0) | 632 (11.8) | 614 (10.7) | 514 (10.2) | 252 (9.4) | 276 (10.6) | 619 (12.3) | 4960 (11.1) |
| Coronary heart disease, n (%) | 315 (7.5) | 378 (7.9) | 420 (9.3) | 337 (7.5) | 410 (7.6) | 428 (7.4) | 313 (6.2) | 172 (6.4) | 180 (6.9) | 418 (8.3) | 3371 (7.6) |
| Stroke, n (%) | 141 (3.4) | 148 (3.1) | 186 (4.1) | 175 (3.9) | 222 (4.1) | 206 (3.6) | 202 (4.0) | 83 (3.1) | 102 (3.9) | 242 (4.8) | 1707 (3.8) |
| Heart failure, n (%) | 126 (3.0) | 138 (2.9) | 166 (3.7) | 152 (3.4) | 187 (3.5) | 158 (2.7) | 170 (3.4) | 74 (2.7) | 80 (3.1) | 179 (3.6) | 1430 (3.2) |
| Cancer, n (%) | 56 (1.3) | 78 (1.6) | 176 (3.9) | 266 (5.9) | 485 (9.0) | 567 (9.8) | 421 (8.4) | 219 (8.1) | 225 (8.6) | 461 (9.2) | 2954 (6.6) |
| Urinary cadmium analysis | |||||||||||
| N | 1299 | 1560 | 1532 | 1520 | 1857 | 2019 | 1715 | 1811 | 1794 | NA | 15,107 |
| Men, n (%) | 620 (47.7) | 737 (47.2) | 743 (48.5) | 736 (48.4) | 922 (49.6) | 976 (48.3) | 867 (50.6) | 878 (48.5) | 886 (49.4) | NA | 7365 (48.8) |
| Smoking status, n (%) | |||||||||||
| Never smoker | 671 (51.6) | 787 (50.5) | 792 (51.7) | 799 (52.6) | 985 (53.1) | 1085 (53.7) | 986 (57.5) | 1019 (56.3) | 1030 (57.4) | NA | 8154 (54.0) |
| Former smoker | 349 (26.9) | 411 (26.3) | 420 (27.4) | 397 (26.1) | 460 (24.8) | 503 (24.9) | 395 (23.0) | 426 (23.5) | 427 (23.8) | NA | 3788 (25.1) |
| Current smoker | 279 (21.5) | 362 (23.2) | 320 (20.9) | 324 (21.3) | 412 (22.2) | 431 (21.3) | 334 (19.5) | 366 (20.2) | 337 (18.8) | NA | 3165 (20.9) |
| Diabetes, n (%) | 159 (12.2) | 191 (12.2) | 222 (14.5) | 217 (14.3) | 346 (18.6) | 377 (18.7) | 327 (19.1) | 305 (16.8) | 366 (20.4) | NA | 2510 (16.6) |
| Chronic kidney disease, n (%) | 173 (13.3) | 199 (12.8) | 187 (12.2) | 199 (13.1) | 256 (13.8) | 230 (11.4) | 221 (12.9) | 201 (11.1) | 224 (12.5) | NA | 1890 (12.5) |
| Hypertension, n (%) | 525 (40.4) | 615 (39.4) | 697 (45.5) | 560 (36.8) | 785 (42.3) | 824 (40.8) | 709 (41.3) | 754 (41.6) | 753 (42.0) | NA | 6222 (41.2) |
| Any cardiovascular disease, n (%) | 146 (11.2) | 163 (10.4) | 213 (13.9) | 170 (11.2) | 204 (11.0) | 186 (9.2) | 157 (9.2) | 170 (9.4) | 181 (10.1) | NA | 1590 (10.5) |
| Coronary heart disease, n (%) | 107 (8.2) | 127 (8.1) | 144 (9.4) | 117 (7.7) | 129 (6.9) | 124 (6.1) | 104 (6.1) | 113 (6.2) | 120 (6.7) | NA | 1085 (7.2) |
| Stroke, n (%) | 43 (3.3) | 45 (2.9) | 67 (4.4) | 63 (4.1) | 75 (4.0) | 65 (3.2) | 56 (3.3) | 58 (3.2) | 69 (3.8) | NA | 541 (3.6) |
| Heart failure, n (%) | 46 (3.5) | 46 (2.9) | 56 (3.7) | 53 (3.5) | 58 (3.1) | 49 (2.4) | 51 (3.0) | 46 (2.5) | 49 (2.7) | NA | 454 (3.0) |
| Cancer, n (%) | 18 (1.4) | 22 (1.4) | 63 (4.1) | 98 (6.4) | 162 (8.7) | 222 (11.0) | 138 (8.0) | 146 (8.1) | 161 (9.0) | NA | 1030 (6.8) |
| Blood Cadmium, μg/L | Urinary Cadmium, μg/g Creatinine | |||||
|---|---|---|---|---|---|---|
| Group, n (%) | <5.0 | ≥5.0 | p Values | <0.6 | ≥0.6 | p Values |
| Overall | 44,429 (99.8) | 69 (0.2) | -- | 12,507 (82.8) | 2595 (17.2) | -- |
| Sex | 0.307 | <0.001 | ||||
| Men | 21,406 (99.9) | 29 (0.1) | 6407 (87.0) | 955 (13.0) | ||
| Women | 23,023 (99.8) | 40 (0.2) | 6100 (78.8) | 1640 (21.2) | ||
| Smoking status | <0.001 | <0.001 | ||||
| Never smoker | 24,031 (100) | 0 (0.0) | 7345 (90.2) | 798 (9.8) | ||
| Former smoker | 11,129 (100) | 0 (0.0) | 2952 (78.0) | 832 (22.0) | ||
| Current smoker | 9229 (99.3) | 69 (0.7) | 2198 (69.5) | 964 (30.5) | ||
| Chronic kidney disease | 0.385 | <0.001 | ||||
| Yes | 5510 (99.8) | 11 (0.2) | 1385 (73.3) | 505 (26.7) | ||
| No | 38,641 (99.9) | 58 (0.1) | 11,122 (84.2) | 2090 (15.8) | ||
| Hypertension | 0.087 | <0.001 | ||||
| Yes | 18,681 (99.9) | 22 (0.1) | 4756 (76.5) | 1463 (23.5) | ||
| No | 25,735 (99.8) | 47 (0.2) | 7747 (87.3) | 1130 (12.7) | ||
| Diabetes | 0.244 | <0.001 | ||||
| Yes | 7486 (99.9) | 8 (0.1) | 1927 (84.0) | 582 (16.0) | ||
| No | 36,943 (99.8) | 61 (0.2) | 10,580 (76.8) | 2013 (23.2) | ||
| Cardiovascular disease | 0.099 | <0.001 | ||||
| Yes | 4948 (99.8) | 12 (0.2) | 1075 (67.7) | 514 (32.3) | ||
| No | 39,476 (99.9) | 57 (0.1) | 11,432 (84.6) | 2081 (15.4) | ||
| Coronary heart disease | 0.086 | <0.001 | ||||
| Yes | 3362 (99.7) | 9 (0.3) | 722 (66.6) | 362 (33.4) | ||
| No | 41,053 (99.9) | 60 (0.1) | 11,784 (84.1) | 2233 (15.9) | ||
| Stroke | 0.684 | <0.001 | ||||
| Yes | 1705 (99.9) | 2 (0.1) | 361 (66.9) | 179 (33.1) | ||
| No | 42,668 (99.8) | 67 (0.2) | 12,135 (83.4) | 2412 (16.6) | ||
| Heart failure | 0.010 | <0.001 | ||||
| Yes | 1424 (99.6) | 6 (0.4) | 305 (67.3) | 148 (32.7) | ||
| No | 42,858 (99.9) | 63 (0.1) | 12,174 (83.3) | 2432 (16.7) | ||
| Cancer | 0.182 | <0.001 | ||||
| Yes | 2947 (99.8) | 7 (0.2) | 791 (76.8) | 239 (23.2) | ||
| No | 28,906 (99.9) | 40 (0.1) | 8444 (84.6) | 1540 (15.4) | ||
| Cohort 1 | Cohort 2 | Numerator Degrees of Freedom | Denominator Degrees of Freedom | Number of Permutations | p-Value | Coincidence |
|---|---|---|---|---|---|---|
| Blood cadmium analysis | ||||||
| Men | Women | 2 | 16 | 4500 | 0.003 | Rejected |
| Never smoker | Former smoker | 4 | 12 | 4500 | <0.001 | Rejected |
| Never smoker | Current smoker | 4 | 12 | 4500 | <0.001 | Rejected |
| Former smoker | Current smoker | 4 | 12 | 4500 | <0.001 | Rejected |
| Chronic kidney disease | Without chronic kidney disease | 4 | 12 | 4500 | 0.002 | Rejected |
| Hypertension | Without hypertension | 4 | 12 | 4500 | <0.001 | Rejected |
| Diabetes | Without diabetes | 2 | 16 | 4500 | 0.051 | Failed to reject |
| Any cardiovascular disease | Without cardiovascular disease | 4 | 12 | 4500 | 0.003 | Rejected |
| Coronary heart disease | Without coronary heart disease | 2 | 16 | 4500 | 0.004 | Rejected |
| Stroke | Without stroke | 4 | 12 | 4500 | 0.003 | Reject |
| Heart failure | Without heart failure | 2 | 16 | 4500 | 0.054 | Failed to reject |
| Cancer | Without cancer | 2 | 16 | 4500 | 0.014 | Rejected |
| Urinary cadmium analysis | ||||||
| Men | Women | 2 | 14 | 4500 | 0.002 | Rejected |
| Never smoker | Former smoker | 2 | 14 | 4500 | <0.001 | Rejected |
| Never smoker | Current smoker | 2 | 14 | 4500 | 0.002 | Rejected |
| Former smoker | Current smoker | 2 | 14 | 4500 | 0.002 | Rejected |
| Chronic kidney disease | Without chronic kidney disease | 2 | 14 | 4500 | <0.001 | Rejected |
| Hypertension | Without hypertension | 4 | 10 | 4500 | 0.736 | Failed to reject |
| Diabetes | Without diabetes | 4 | 10 | 4500 | 0.357 | Failed to reject |
| Any cardiovascular disease | Without cardiovascular disease | 2 | 14 | 4500 | <0.001 | Rejected |
| Coronary heart disease | Without coronary heart disease | 2 | 14 | 4500 | <0.001 | Rejected |
| Stroke | Without stroke | 2 | 14 | 4500 | 0.009 | Rejected |
| Heart failure | Without heart failure | 2 | 14 | 4500 | 0.002 | Rejected |
| Cancer | Without cancer | 2 | 14 | 4500 | 0.038 | Rejected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Lo, K.; Yang, A. Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018. Nutrients 2022, 14, 802. https://doi.org/10.3390/nu14040802
Yang J, Lo K, Yang A. Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018. Nutrients. 2022; 14(4):802. https://doi.org/10.3390/nu14040802
Chicago/Turabian StyleYang, Jingli, Kenneth Lo, and Aimin Yang. 2022. "Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018" Nutrients 14, no. 4: 802. https://doi.org/10.3390/nu14040802
APA StyleYang, J., Lo, K., & Yang, A. (2022). Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018. Nutrients, 14(4), 802. https://doi.org/10.3390/nu14040802








