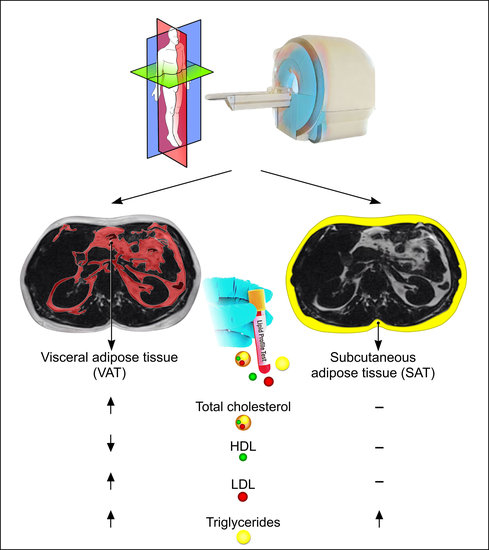
Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Whole-Body MR Imaging
2.3. MR-Image Analysis for Adipose Tissue Depots
2.4. Assessment of Lipid Profile
2.5. Other Risk Factors
- Prediabetes
- Diabetes
2.6. Statistical Analysis
3. Results
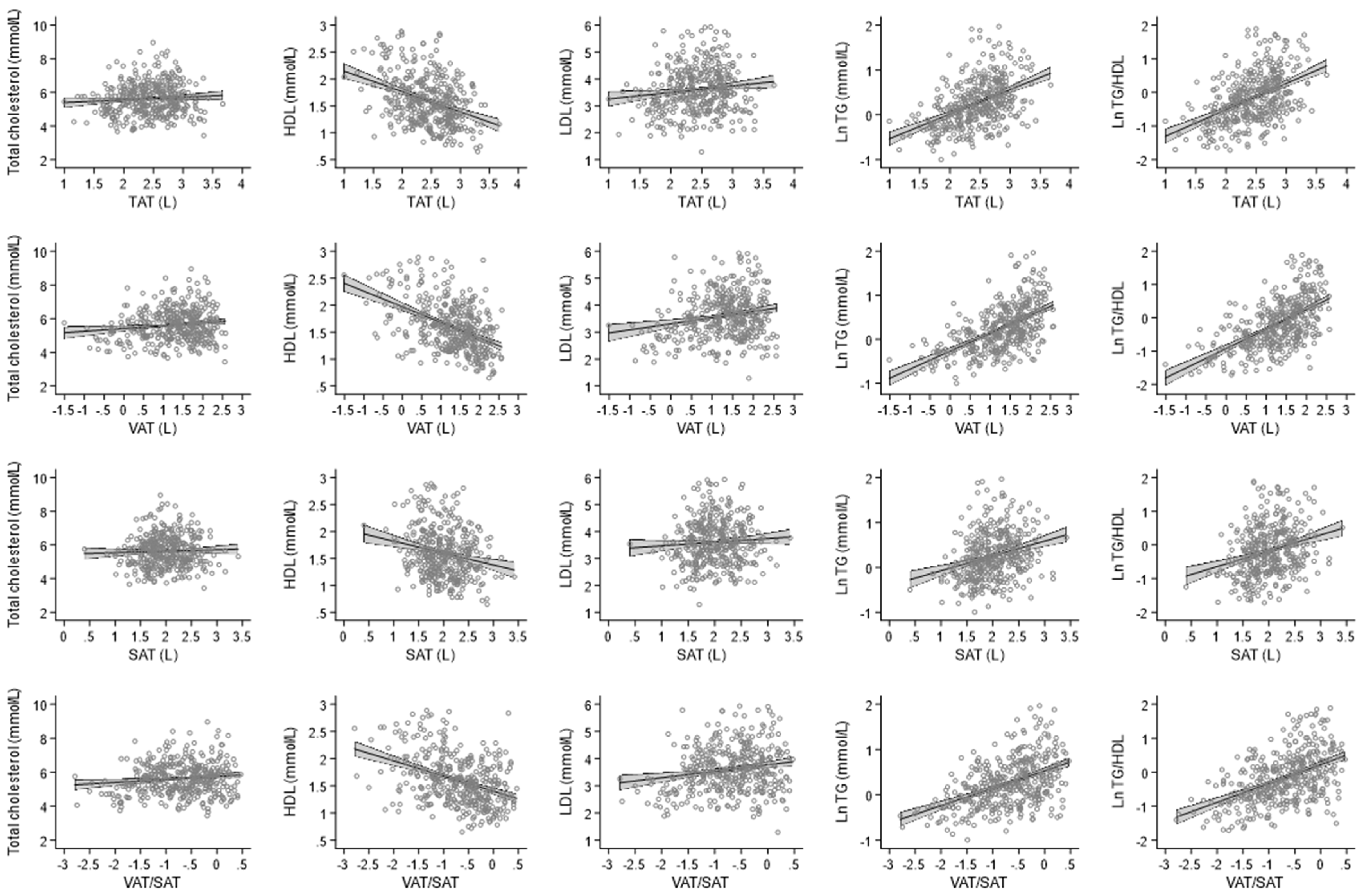
3.1. Association between Adipose Tissue with Lipid Profile
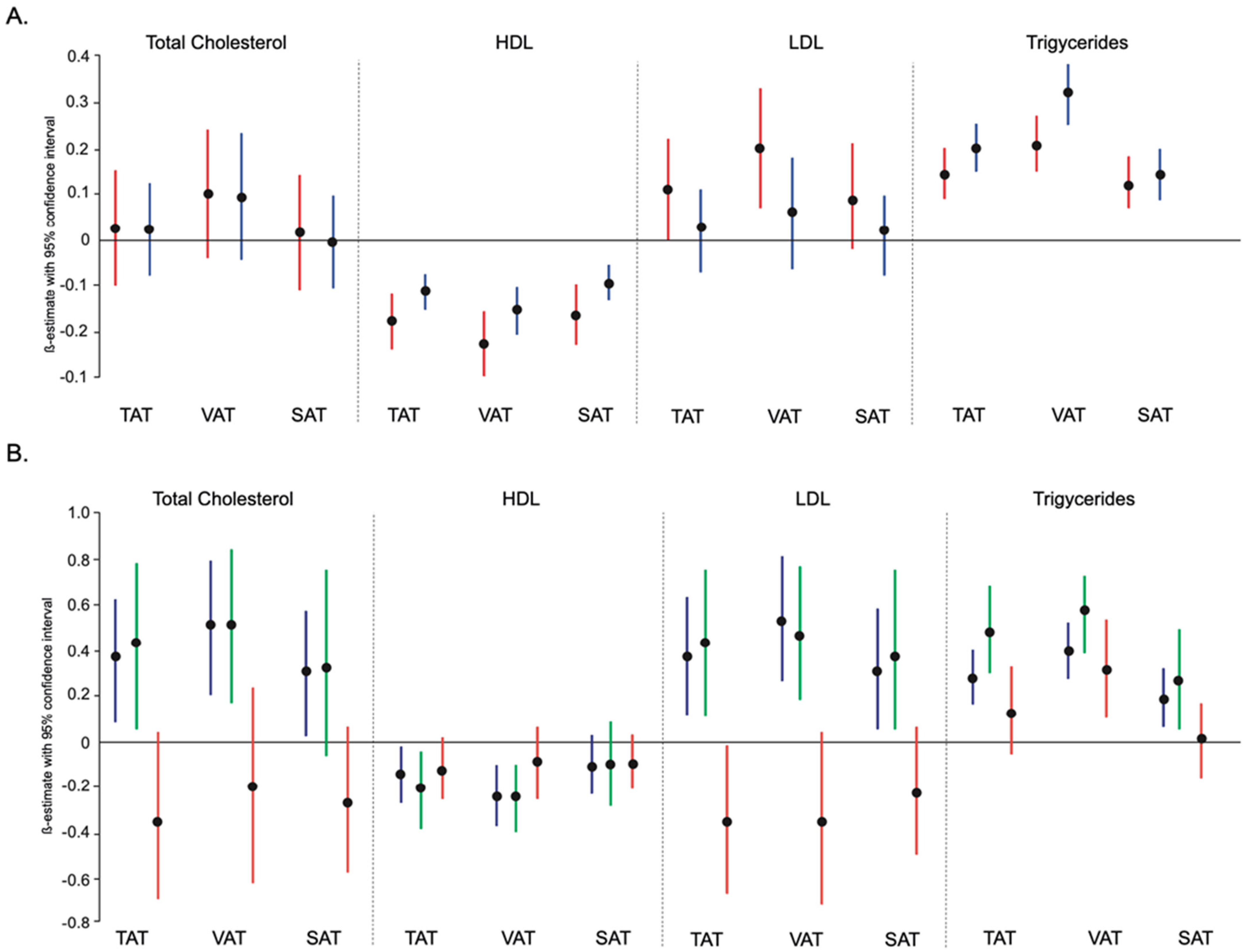
3.2. Association between Adipose Tissue with Lipid Profile According to Sex
3.3. Association between Adipose Tissue with Lipid Profile according to Body Mass Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; McNally, E.M.; Ackerman, M.J.; Baty, L.C.; Day, S.M.; Kullo, I.J.; Madueme, P.C.; Maron, M.S.; Martinez, M.W.; Salberg, L.; et al. Establishment of Specialized Clinical Cardiovascular Genetics Programs: Recognizing the Need and Meeting Standards: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2019, 12, e000054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rospleszcz, S.; Lorbeer, R.; Storz, C.; Schlett, C.L.; Meisinger, C.; Thorand, B.; Rathmann, W.; Bamberg, F.; Lieb, W.; Peters, A. Association of longitudinal risk profile trajectory clusters with adipose tissue depots measured by magnetic resonance imaging. Sci. Rep. 2019, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Kastelein, J.J.P.; Catapano, A.L. Lipids and Lipoproteins in 2020. JAMA 2020, 324, 595–596. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP); Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin, Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Berliner, J.A.; Navab, M.; Fogelman, A.M.; Frank, J.S.; Demer, L.; Edwards, P.A.; Watson, A.D.; Lusis, A.J. Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation 1995, 91, 2488–2496. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.-H.; Chu, C.-S.; Lee, K.-T.; Lin, T.-H.; Hsieh, C.-C.; Chiu, C.-C.; Voon, W.-C.; Sheu, S.-H.; Lai, W.-T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2007, 32, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, F.; Tong, N. The association of visceral adipose tissue and subcutaneous adipose tissue with metabolic risk factors in a large population of Chinese adults. Clin. Endocrinol. 2016, 85, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk fac-tors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of abdominal visceral and subcu-taneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-C.; Arthur, R.; Iyengar, N.M.; Kamensky, V.; Xue, X.; Wassertheil-Smoller, S.; Allison, M.A.; Shadyab, A.H.; Wild, R.A.; Sun, Y.; et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur. Heart J. 2019, 40, 2849–2855. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Celis-Morales, C.A.; Lyall, D.M.; Anderson, J.; Gray, S.R.; Mackay, D.F.; Nelson, S.M.; Welsh, P.; Pell, J.P.; Gill, J.M.R.; et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovas-cular disease: A cohort study of 296,535 adults of white European descent. Eur. Heart J. 2018, 39, 1514–1520. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects With Prediabetes, Subjects With Diabetes, and Normal Control Subjects From the General Population: The KORA-MRI Study. Diabetes 2016, 66, 158–169. [Google Scholar] [CrossRef] [Green Version]
- von Krüchten, R.; Lorbeer, R.; Schuppert, C.; Storz, C.; Mujaj, B.; Schulz, H.; Kauczor, H.U.; Peters, A.; Bamberg, F.; Karrasch, S.; et al. Serum insulin is associated with right ventricle function parameters and lung volumes in subjects free of car-diovascular disease. Eur. J. Endocrinol. 2021, 184, 289–298. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Available online: https://apps.who.int/iris/handle/10665/43588 (accessed on 16 January 2022).
- Oka, R.; Miura, K.; Sakurai, M.; Nakamura, K.; Yagi, K.; Miyamoto, S.; Moriuchi, T.; Mabuchi, H.; Koizumi, J.; Nomura, H.; et al. Impacts of Visceral Adipose Tissue and Subcutaneous Adipose Tissue on Metabolic Risk Factors in Middle-aged Japanese. Obesity 2010, 18, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Fujimoto, W.Y.; Hayashi, T.; Kahn, S.E.; Leonetti, D.L.; Boyko, E.J. Increased Visceral Adipose Tissue Is an In-dependent Predictor for Future Development of Atherogenic Dyslipidemia. J. Clin. Endocrinol. Metab. 2016, 101, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, Y.; Nakagawa, T.; Yamamoto, S.; Takahashi, Y.; Yokoyama, T.; Mizoue, T.; Noda, M. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men: The Hitachi Health Study. Diabetes Care 2012, 35, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Pierre, J.; Lemieux, I.; Vohl, M.-C.; Perron, P.; Tremblay, G.; Després, J.-P.; Gaudet, D. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease. Am. J. Cardiol. 2002, 90, 15–18. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. 2003, 27, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Mujaj, B.; Bos, D.; Selwaness, M.; Leening, M.J.; Kavousi, M.; Wentzel, J.J.; van der Lugt, A.; Hofman, A.; Stricker, B.H.; Vernooij, M.W.; et al. Statin use is associated with carotid plaque composition: The Rotterdam Study. Int. J. Cardiol. 2018, 260, 213–218. [Google Scholar] [CrossRef]
- Mujaj, B.; Bos, D.; Muka, T.; van der Lugt, A.; Ikram, M.A.; Vernooij, M.W.; Stricker, B.H.; Franco, O. Antithrombotic treatment is associated with intraplaque haemorrhage in the atherosclerotic carotid artery: A cross-sectional analysis of The Rotterdam Study. Eur. Heart J. 2018, 39, 3369–3376. [Google Scholar] [CrossRef]
- Rydén, M.; Arner, P. Subcutaneous Adipocyte Lipolysis Contributes to Circulating Lipid Levels. Arter. Thromb. Vasc. Biol. 2017, 37, 1782–1787. [Google Scholar] [CrossRef] [Green Version]
| All | Female | Male | p Value | |
|---|---|---|---|---|
| n = 384 | n = 161 | n = 223 | ||
| Age, years | 56.2 (±9.2) | 56.3 (±8.9) | 56.2 (±9.4) | 0.929 |
| Women | 161 (41.9%) | - | - | |
| Body mass index, kg/m2 | 27.9 (±4.7) | 27.4 (±5.3) | 28.3 (±4.2) | 0.081 |
| Body surface area, m2 | 1.95 (±0.21) | 1.78 (±0.16) | 2.07 (±0.16) | <0.001 |
| Waist circumference, cm | 98 (±13.8) | 91.3 (±13.7) | 102.9 (±11.7) | <0.001 |
| Hip circumference, cm | 106.6 (±8.6) | 106.5 (±10.2) | 106.6 (±7.2) | 0.921 |
| Waist-to-hip ratio | 0.92 (±0.09) | 0.86 (±0.08) | 0.96 (±0.07) | <0.001 |
| Smoking status | 0.083 | |||
| Never | 141 (36.7%) | 68 (42.2%) | 73 (32.7%) | |
| Past | 165 (43%) | 59 (36.7%) | 106 (47.5%) | |
| Current | 78 (20.3%) | 34 (21.1%) | 44 (19.7%) | |
| Alcohol use, (g/day) | 18.6 (±24) | 8.5 (±13.8) | 25.9 (±27) | <0.001 |
| Physical activity | 229 (59.6%) | 103 (64%) | 126 (56.5%) | 0.141 |
| Diabetes | 52 (13.5%) | 13 (8.1%) | 39 (17.5%) | 0.008 |
| HbA1c, % | 5.57 (±0.72) | 5.54 (±0.51) | 5.59 (±0.84) | 0.463 |
| Fasting glucose, mmol/L | 5.50 (5.11;6.11) | 5.27 (4.88;5.88) | 5.66 (5.27;6.27) | <0.001 |
| Fasting insulin, pmol/L | 54.6 (37.2;81.0) | 49.9 (34.8;74.9) | 60.0 (38.4;87.1) | 0.004 |
| Antidiabetic medication | 31 (8.1%) | 11 (6.8%) | 20 (9%) | 0.448 |
| Hypertension | 128 (33.3%) | 45 (28%) | 83 (37.2%) | 0.057 |
| Systolic, mm/Hg | 120.6 (±16.4) | 113.2 (±14) | 126 (±15.9) | <0.001 |
| Diastolic, mm/Hg | 75.3 (±9.9) | 72.1 (±8.5) | 77.7 (±10.2) | <0.001 |
| Antihypertensive medication | 94 (24.5%) | 43 (26.7%) | 51 (22.9%) | 0.388 |
| Total cholesterol, mmol/L | 5.63 (±0.94) | 5.67 (±0.88) | 5.60 (±0.97) | 0.463 |
| HDL, mmol/L | 1.60 (±0.46) | 1.82 (±0.46) | 1.44 (±0.39) | <0.001 |
| LDL, mmol/L | 3.60 (±0.84) | 3.54 (±0.81) | 3.65 (±0.86) | 0.199 |
| Triglycerides, mmol/L | 1.22 (0.88;1.78) | 1.07 (0.77;1.37) | 1.42 (0.99;2.13) | <0.001 |
| Ratio TG/HDL | 0.78 (0.48;1.37) | 0.58 (0.42;0.91) | 1.02 (0.61;1.75) | <0.001 |
| Lipid lowering medication | 41 (10.7%) | 17 (10.6%) | 24 (10.8%) | 0.949 |
| GGT, U/L | 28.8 (17.4;45) | 19.8 (13.8;31.8) | 35.4 (24;57) | <0.001 |
| AST, U/L | 22.8 (19.2;28.8) | 19.8 (16.8;25.8) | 25.2 (21;30) | <0.001 |
| ALP, U/L | 27 (19.8;37.2) | 21 (16.2;28.2) | 31.2 (25.2;40.8) | <0.001 |
| Serum creatinine, µmol/L | 78.1 (±13.7) | 68.3 (±10.5) | 85.1 (±11.2) | <0.001 |
| TAT, L | 11.9 (8.57;16.11) | 10.79 (7.3;16.17) | 12.03 (9.18;16.11) | 0.039 |
| VAT, L | 4.03 (2.43;6.1) | 2.69 (1.39;4.06) | 5.42 (3.47;7.32) | <0.001 |
| SAT, L | 7.12 (5.44;9.94) | 8.41(5.74;11.79) | 6.66 (5.37;8.72) | <0.001 |
| Ratio VAT/SAT | 0.52 (0.31;0.81) | 0.29 (0.21;0.39) | 0.74 (0.58;0.96) | <0.001 |
| TAT | Low Tertile | Medium Tertile | High Tertile | p Value |
|---|---|---|---|---|
| Total cholesterol | 5.49 (±0.89) | 5.74 (±0.98) | 5.64 (±0.92) | 0.261 |
| HDL | 1.78 (±0.49) | 1.60 (±0.45) | 1.42 (±0.36) | <0.001 |
| LDL | 3.44 (±0.83) | 3.71 (±0.87) | 3.66 (±0.81) | 0.031 |
| Triglycerides | 0.89 (0.73; 1.20) | 1.27 (0.99;1.80) | 1.58 (1.19; 2.30) | <0.001 |
| Ratio TG/HDL | 0.52 (0.38; 0.83) | 0.86 (0.53; 1.35) | 1.19 (0.7; 1.94) | <0.001 |
| VAT | ||||
| Total cholesterol | 5.46 (±0.94) | 5.78 (±0.84) | 5.64 (±1.00) | 0.202 |
| HDL | 1.84 (±0.48) | 1.58 (±0.37) | 1.38 (±0.39) | <0.001 |
| LDL | 3.40 (±0.84) | 3.76 (±0.76) | 3.65 (±0.88) | 0.015 |
| Triglycerides | 0.88 (0.73; 1.20) | 1.26 (0.97; 1.60) | 1.75 (1.25; 2.50) | <0.001 |
| Ratio TG/HDL | 0.50 (0.38; 0.71) | 0.82 (0.54; 1.17) | 1.40 (0.76; 2.28) | <0.001 |
| SAT | ||||
| Total cholesterol | 5.47 (±0.9) | 5.81 (±0.96) | 5.61 (±0.92) | 0.292 |
| HDL | 1.71 (±0.48) | 1.57 (±0.44) | 1.52 (±0.43) | 0.002 |
| LDL | 3.43 (±0.85) | 3.79 (±0.82) | 3.59 (±0.82) | 0.179 |
| Triglycerides | 0.99 (0.77; 1.40) | 1.27 (0.97; 2.00) | 1.45 (1.09; 2.00) | <0.001 |
| Ratio TG/HDL | 0.61 (0.41; 1.01) | 0.83 (0.53; 1.42) | 1.00 (0.61; 1.56) | <0.001 |
| VAT/SAT ratio | ||||
| Total cholesterol | 5.54 (±0.84) | 5.68 (±0.96) | 5.66 (±0.99) | 0.444 |
| HDL | 1.85 (±0.47) | 1.53 (±0.40) | 1.42 (±0.38) | <0.001 |
| LDL | 3.44 (±0.78) | 3.71 (±0.84) | 3.66 (±0.89) | 0.055 |
| Triglycerides | 0.96 (0.75; 1.27) | 1.28 (0.98; 1.75) | 1.56 (1.06; 2.32) | <0.001 |
| Ratio TG/HDL | 0.52 (0.36; 0.78) | 0.85 (0.56; 1.32) | 1.19 (0.70; 1.96) | <0.001 |
| Per SD Log Transformed | Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | Model 4 | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total Cholesterol | ||||||||
| TAT | 0.05 (−0.04; 0.15) | 0.294 | 0.35 (0.21; 0.49) | <0.001 | 0.32 (0.16; 0.49) | <0.001 | 0.35 (0.20; 0.51) | <0.001 |
| VAT | 0.15 (0.03; 0.27) | 0.012 | 0.41 (0.26; 0.55) | <0.001 | 0.36 (0.20; 0.52) | <0.001 | 0.39 (0.23; 0.55) | <0.001 |
| SAT | 0.03 (−0.07; 0.12) | 0.598 | 0.31 (0.17; 0.45) | <0.001 | 0.29 (0.13; 0.45) | <0.001 | 0.30 (0.15; 0.45) | <0.001 |
| VAT/SAT ratio | 0.07 (−0.06; 0.21) | 0.294 | 0.50 (0.31; 0.70) | <0.001 | 0.46 (0.23; 0.69) | <0.001 | 0.51 (0.28; 0.73) | <0.001 |
| HDL | ||||||||
| TAT | −0.16 (−0.20; −0.12) | <0.001 | −0.14 (−0.20; −0.09) | <0.001 | −0.09 (−0.15; −0.02) | 0.009 | −0.09 (−0.16; −0.02) | 0.009 |
| VAT | −0.21 (−0.26; −0.16) | <0.001 | −0.18 (−0.24; −0.12) | <0.001 | −0.13 (−0.20; −0.07) | <0.001 | −0.14 (−0.20; −0.07) | <0.001 |
| SAT | −0.14 (−0.18; −0.10) | <0.001 | −0.10 (−0.16; −0.04) | 0.001 | −0.04 (−0.10; 0.03) | 0.273 | −0.04 (−0.10; 0.03) | 0.273 |
| VAT/SAT ratio | −0.23 (−0.29; −0.17) | <0.001 | −0.21 (−0.29; −0.13) | <0.001 | −0.13 (−0.22; −0.03) | 0.009 | −0.13 (−0.22; −0.03) | 0.009 |
| LDL | ||||||||
| TAT | 0.09 (0.00; 0.17) | 0.048 | 0.30 (0.17; 0.43) | <0.001 | 0.29 (0.14; 0.44) | <0.001 | 0.32 (0.18; 0.46) | <0.001 |
| VAT | 0.18 (0.07; 0.28) | 0.001 | 0.36 (0.23; 0.49) | <0.001 | 0.34 (0.19; 0.49) | <0.001 | 0.37 (0.23; 0.52) | <0.001 |
| SAT | 0.07 (−0.02; 0.15) | 0.114 | 0.26 (0.13; 0.39) | <0.001 | 0.25 (0.10; 0.39) | 0.001 | 0.26 (0.12; 0.40) | <0.001 |
| VAT/SAT ratio | 0.12 (0.00; 0.25) | 0.048 | 0.43 (0.25; 0.61) | <0.001 | 0.42 (0.21; 0.63) | <0.001 | 0.46 (0.25; 0.66) | <0.001 |
| Triglycerides | ||||||||
| TAT | 0.23 (0.18; 0.28) | <0.001 | 0.31 (0.24; 0.37) | <0.001 | 0.20 (0.12; 0.27) | <0.001 | 0.20 (0.12; 0.27) | <0.001 |
| VAT | 0.33 (0.27; 0.38) | <0.001 | 0.36 (0.29; 0.43) | <0.001 | 0.26 (0.19; 0.34) | <0.001 | 0.27 (0.19; 0.34) | <0.001 |
| SAT | 0.18 (0.13; 0.23) | <0.001 | 0.23 (0.16; 0.30) | <0.001 | 0.11 (0.04; 0.19) | 0.003 | 0.11 (0.04; 0.19) | 0.004 |
| VAT/SAT ratio | 0.33 (0.26; 0.39) | <0.001 | 0.44 (0.34; 0.53) | <0.001 | 0.28 (0.17; 0.39) | <0.001 | 0.28 (0.17; 0.39) | <0.001 |
| Ratio TG/HDL | ||||||||
| TAT | 0.33 (0.27; 0.39) | <0.001 | 0.39 (0.30; 0.48) | <0.001 | 0.24 (0.14; 0.34) | <0.001 | 0.24 (0.14; 0.35) | <0.001 |
| VAT | 0.46 (0.38; 0.54) | <0.001 | 0.47 (0.38; 0.56) | <0.001 | 0.33 (0.23; 0.44) | <0.001 | 0.34 (0.23; 0.44) | <0.001 |
| SAT | 0.27 (0.21; 0.34) | <0.001 | 0.28 (0.19; 0.38) | <0.001 | 0.13 (0.02; 0.23) | 0.016 | 0.12 (0.02; 0.23) | 0.018 |
| VAT/SAT ratio | 0.47 (0.38; 0.56) | <0.001 | 0.56 (0.43; 0.69) | <0.001 | 0.34 (0.20; 0.49) | <0.001 | 0.34 (0.19; 0.49) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Krüchten, R.; Lorbeer, R.; Müller-Peltzer, K.; Rospleszcz, S.; Storz, C.; Askani, E.; Kulka, C.; Schuppert, C.; Rathmann, W.; Peters, A.; et al. Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study. Nutrients 2022, 14, 797. https://doi.org/10.3390/nu14040797
von Krüchten R, Lorbeer R, Müller-Peltzer K, Rospleszcz S, Storz C, Askani E, Kulka C, Schuppert C, Rathmann W, Peters A, et al. Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study. Nutrients. 2022; 14(4):797. https://doi.org/10.3390/nu14040797
Chicago/Turabian Stylevon Krüchten, Ricarda, Roberto Lorbeer, Katharina Müller-Peltzer, Susanne Rospleszcz, Corinna Storz, Esther Askani, Charlotte Kulka, Christopher Schuppert, Wolfgang Rathmann, Annette Peters, and et al. 2022. "Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study" Nutrients 14, no. 4: 797. https://doi.org/10.3390/nu14040797
APA Stylevon Krüchten, R., Lorbeer, R., Müller-Peltzer, K., Rospleszcz, S., Storz, C., Askani, E., Kulka, C., Schuppert, C., Rathmann, W., Peters, A., Bamberg, F., Schlett, C. L., & Mujaj, B. (2022). Association between Adipose Tissue Depots and Dyslipidemia: The KORA-MRI Population-Based Study. Nutrients, 14(4), 797. https://doi.org/10.3390/nu14040797