Early Introduction of Multi-Allergen Mixture for Prevention of Food Allergy: Pilot Study
Abstract
:1. Introduction
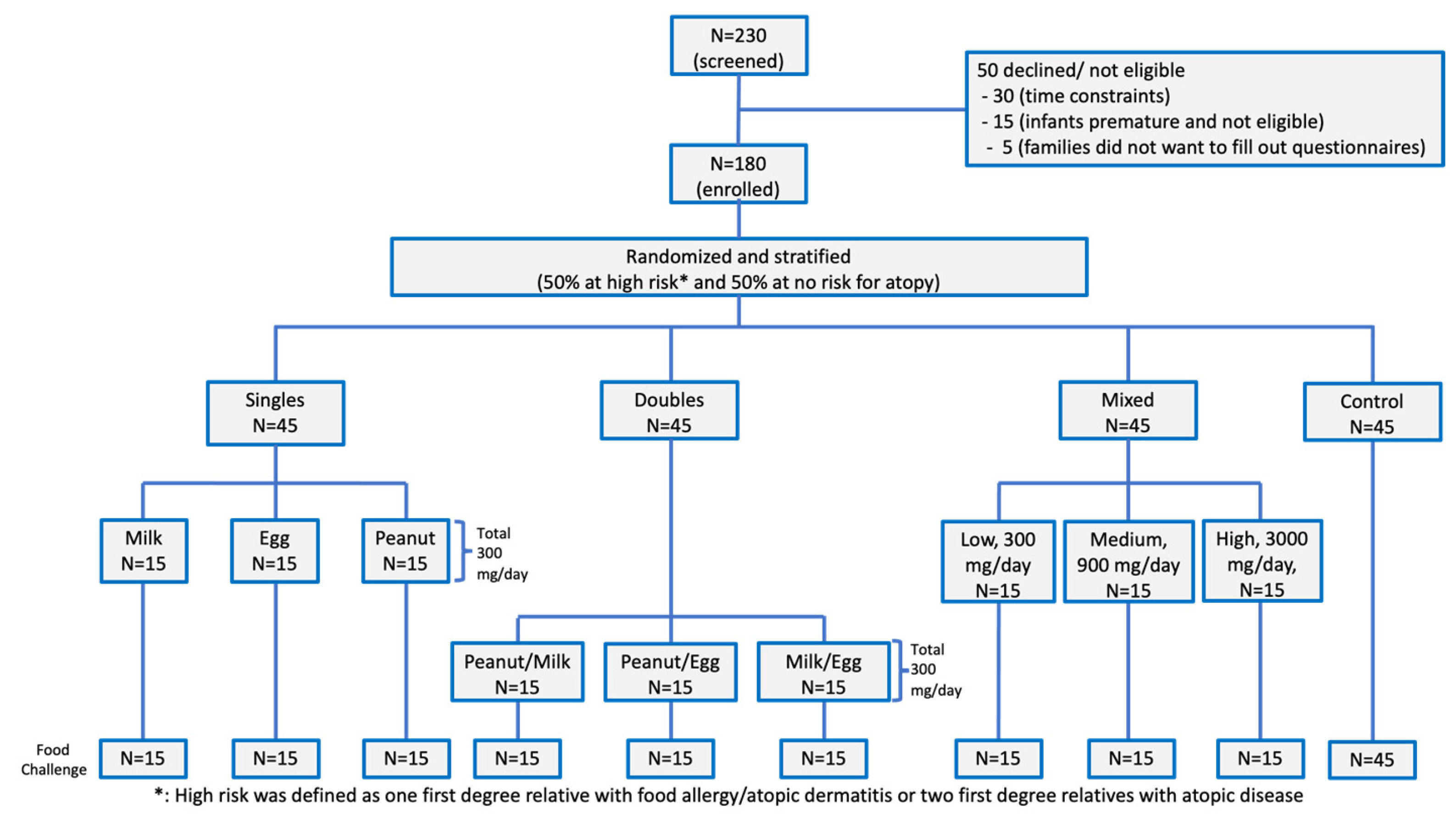
2. Materials and Methods
2.1. Substance Identity & Protein Composition
2.2. Statistical Analysis
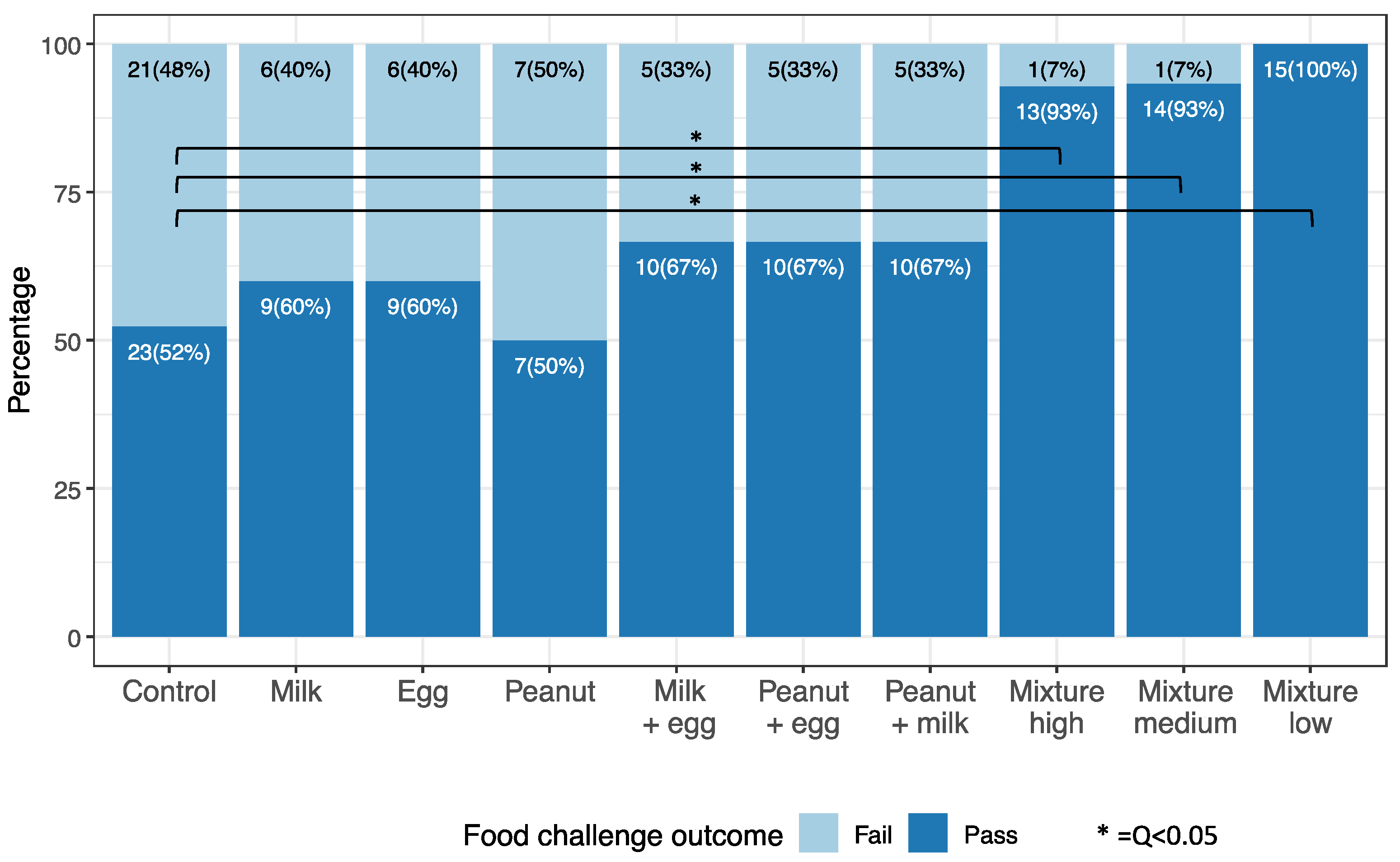
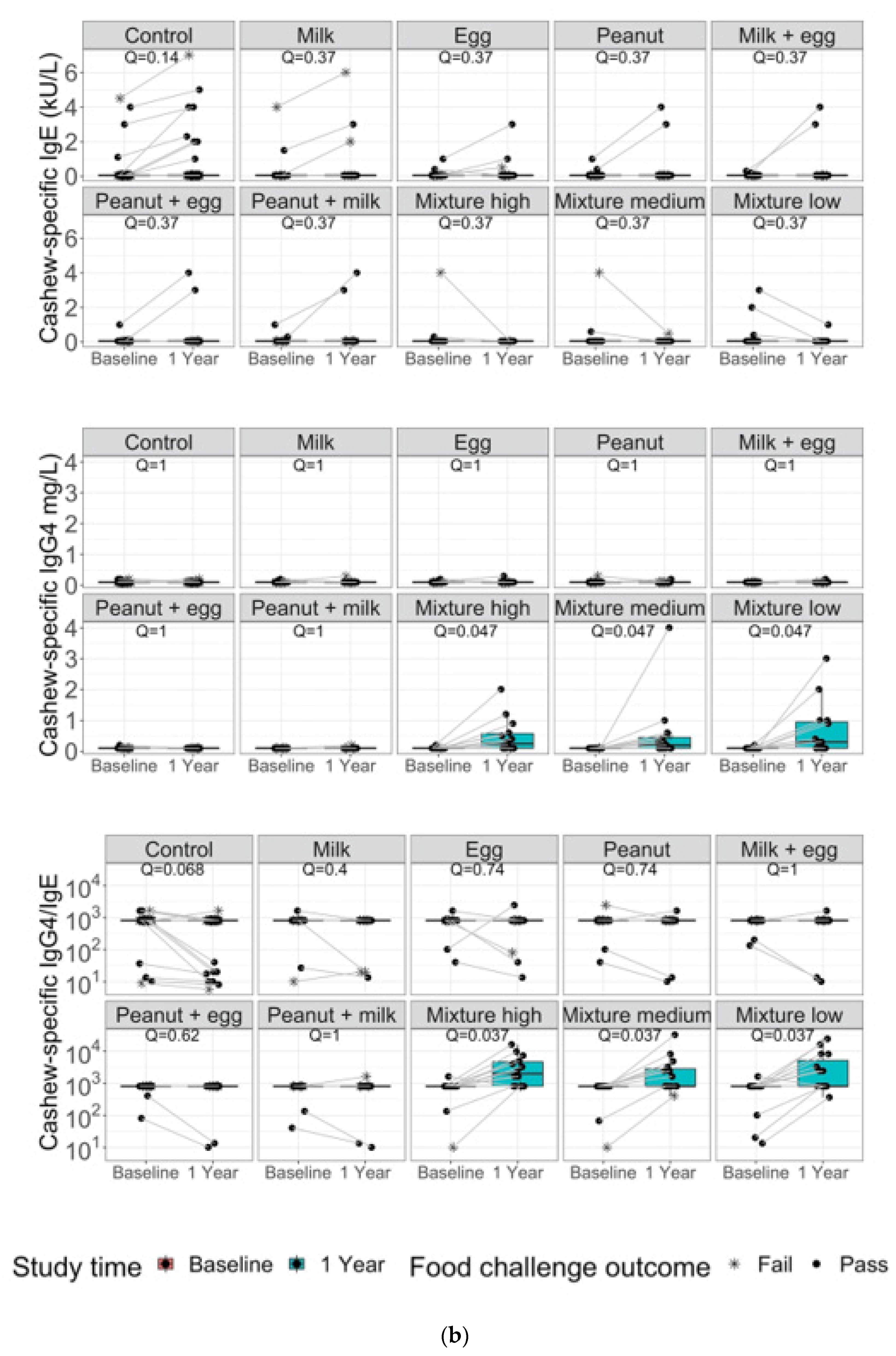
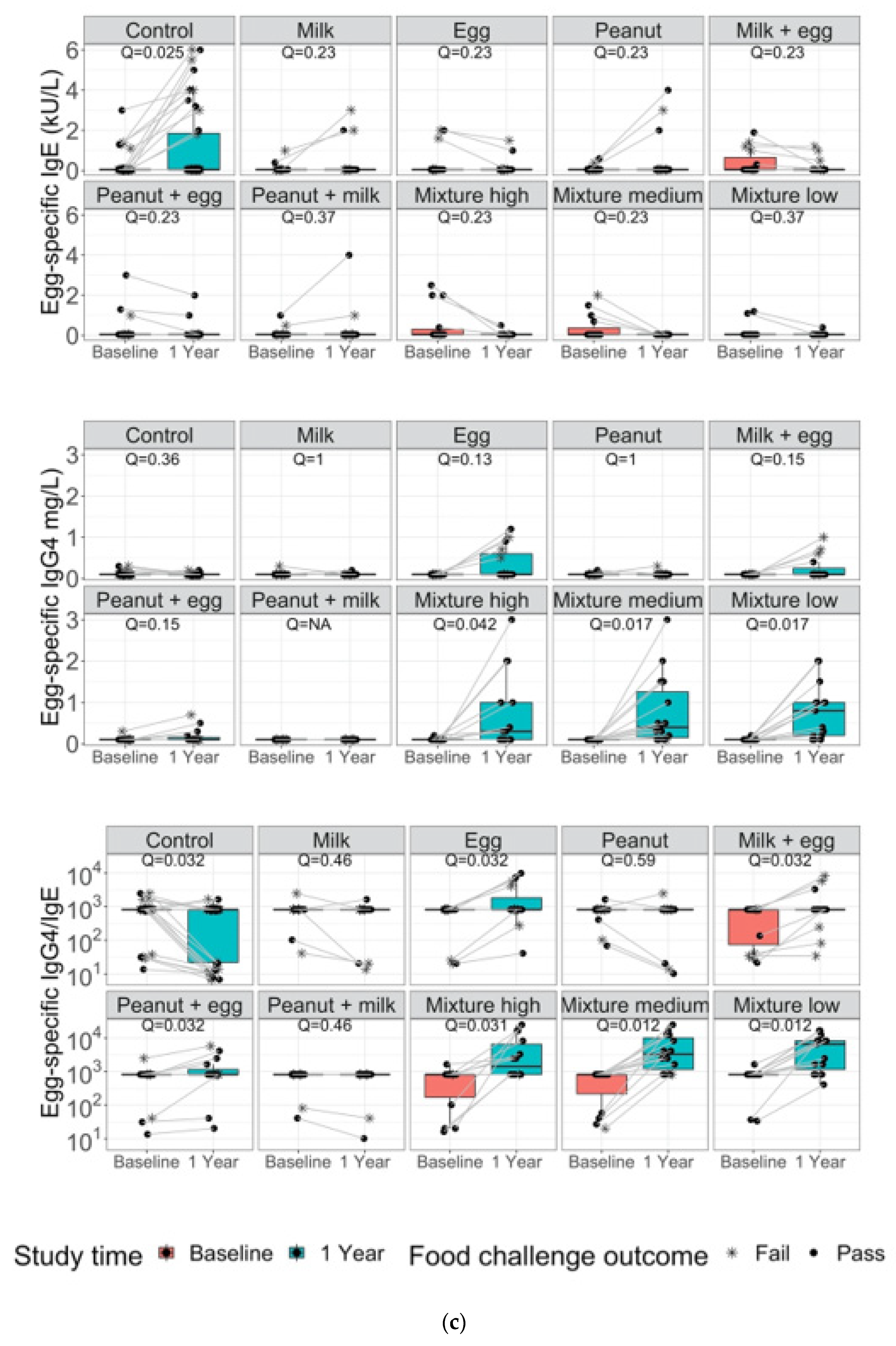
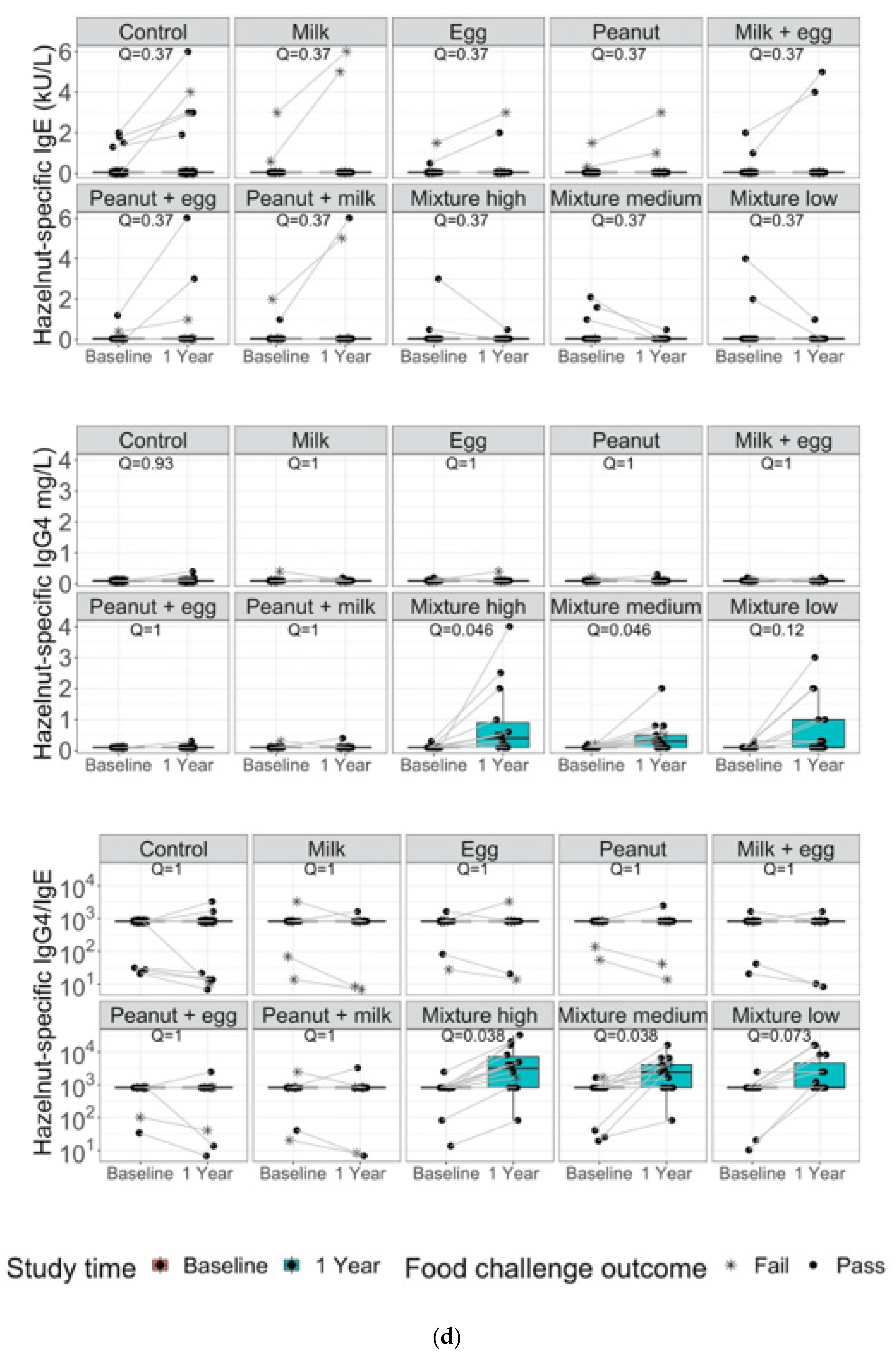
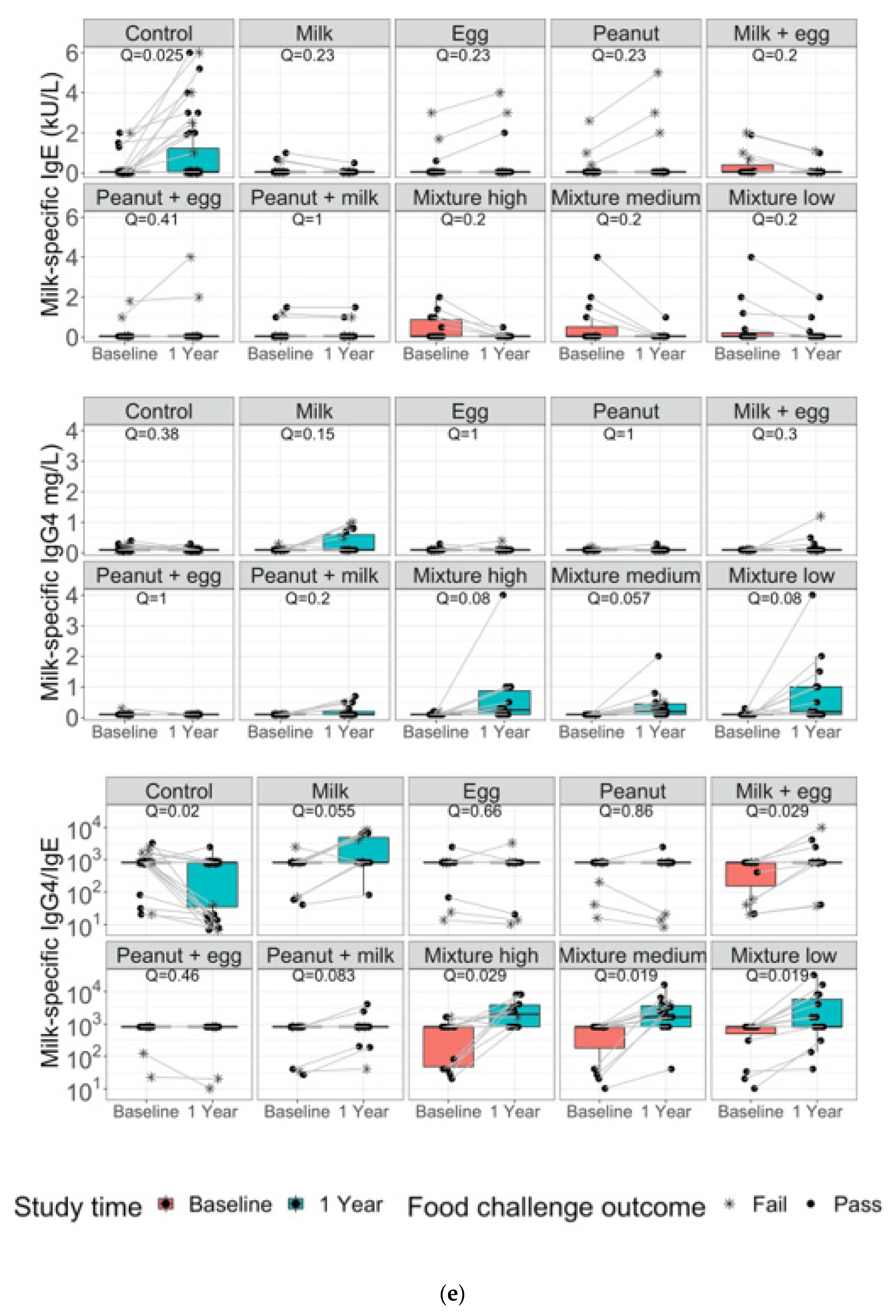
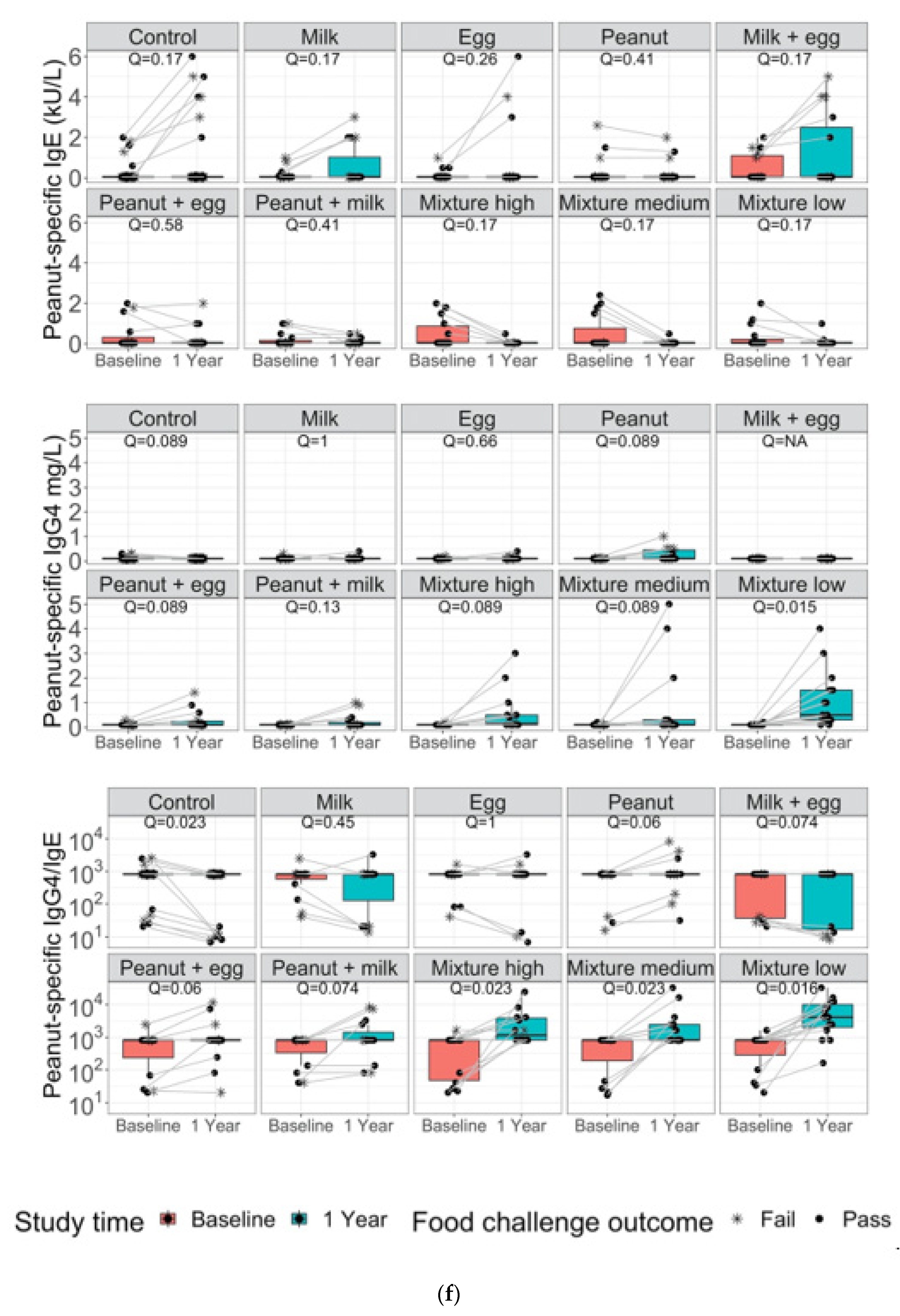
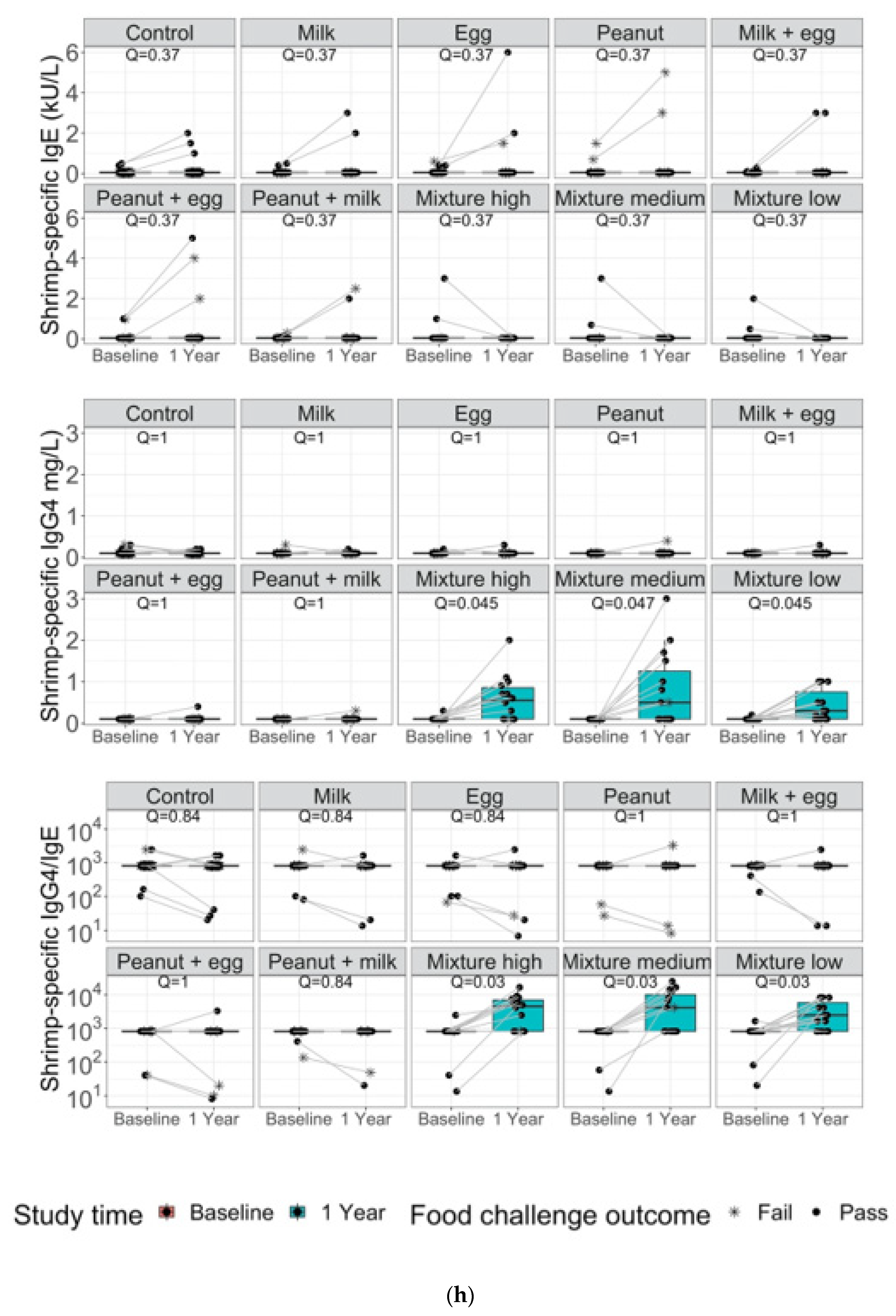
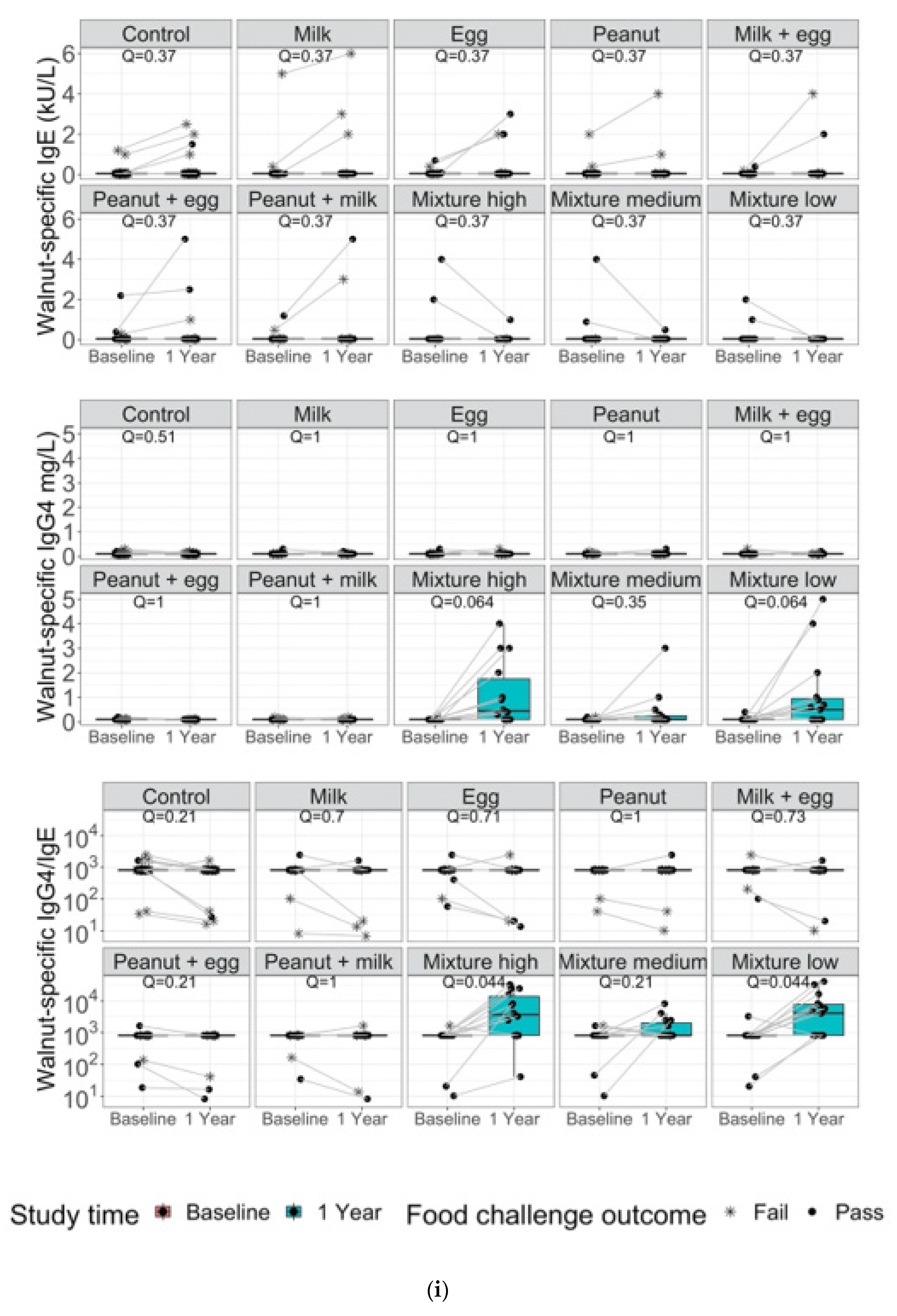
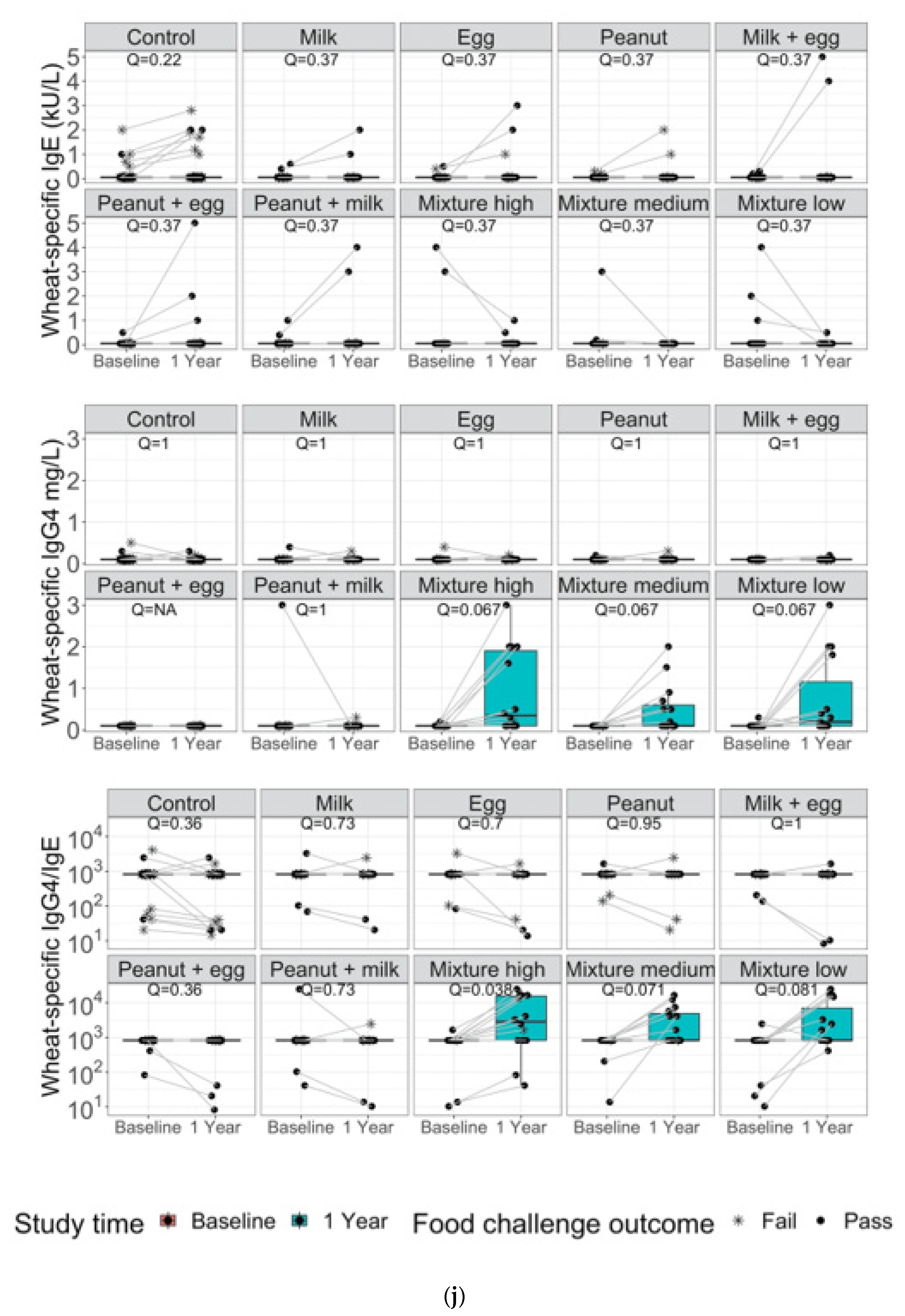
3. Results
4. Discussion and Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Allergen Group | N |
|---|---|
| Mixture (low dose) | 15 (100%) |
| Mixture (medium dose) | 14 (93%) |
| Mixture (high dose) | 15 (100%) |
| Control | 7 (15.5%) |
| Peanut | 10 (66.7%) |
| Milk | 8 (53.3%) |
| Egg | 9 (60.0%) |
| Peanut/milk | 11 (73.3%) |
| Milk/egg | 10 (66.7%) |
| Egg/peanut | 8 (53.3%) |
References
- Allen, K.J.; Koplin, J.J. The Epidemiology of IgE-Mediated Food Allergy and Anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.D.; Howie, L.D.; Akinbami, L.J. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief 2013, 121, 1–8. [Google Scholar]
- Tang, M.L.K.; Mullins, R.J. Food allergy: Is prevalence increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M. Food allergy quality of life and living with food allergy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 284–290. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.-C.; Boguniewicz, M.; Eigenmann, P. Evaluation of Food Allergy in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef]
- Cox, A.; Sicherer, S.H. Peanut and Tree Nut Allergy. Monoclonal Antibody Therapy 2015, 101, 131–144. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T. Japanese guidelines for food allergy 2017. Allergol. Int. 2017, 66, 248–264. [Google Scholar] [CrossRef]
- Zeiger, R.S. Food allergen avoidance in the prevention of food allergy in infants and children. Pediatrics 2003, 111, 1662–1671. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.; Brough, H.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Larsen, V.G.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease. JAMA J. Am. Med. Assoc. 2016, 316, 1181–1192. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Venter, C.; Maslin, K.; Holloway, J.; Silveira, L.J.; Fleischer, D.M.; Dean, T.; Arshad, S.H. Different Measures of Diet Diversity During Infancy and the Association with Childhood Food Allergy in a UK Birth Cohort Study. J. Allergy Clin. Immunol. Pract. 2020, 8, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Greenhawt, M.; Meyer, R.W.; Agostoni, C.; Reese, I.; du Toit, G.; Feeney, M.; Maslin, K.; Nwaru, B.I.; Roduit, C.; et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: Novel concepts and implications for studies in allergy and asthma. Allergy 2020, 75, 497–523. [Google Scholar] [CrossRef]
- Koplin, J.J.; Allen, K.J.; Tang, M.L.K. Important risk factors for the development of food allergy and potential options for prevention. Expert Rev. Clin. Immunol. 2019, 15, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef]
- USDA. Infant Nutrition and Feeding. 2019. Available online: https://wicworks.fns.usda.gov/resources/infant-nutrition-and-feeding-guide (accessed on 12 January 2022).
- Holl, J.L.; Bilaver, L.A.; Finn, D.J.; Savio, K. A randomized trial of the acceptability of a daily multi-allergen food supplement for infants. Pediatr. Allergy Immunol. 2020, 31, 418–420. [Google Scholar] [CrossRef]
- Ashley, S.; Dang, T.; Koplin, J.; Martino, D.; Prescott, S. Food for thought. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 237–242. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Chan, E.S.; Venter, C.; Spergel, J.M.; Abrams, E.M.; Stukus, D.; Groetch, M.; Shaker, M.; Greenhawt, M. A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J. Allergy Clin. Immunol. Pract. 2021, 9, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition, Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Allen, K.J.; Gurrin, L.C.; Peters, R.L.; Lowe, A.J.; Tan, H.-T.T.; Dharmage, S.C. The Impact of Family History of Allergy on Risk of Food Allergy: A Population-Based Study of Infants. Int. J. Environ. Res. Public Health 2013, 10, 5364–5377. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Long, A.; O’Laughlin, K.L.; Lyu, S.C.; Manohar, M.; Boyd, S.D.; Tibshirani, R.; Maecker, H.; et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): A large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019, 394, 1437–1449. [Google Scholar] [CrossRef]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef]
- Purington, N.; Chinthrajah, R.S.; Long, A.; Sindher, S.; Andorf, S.; O’Laughlin, K.; Woch, M.A.; Scheiber, A.; Assa’ad, A.; Pongracic, J.; et al. Eliciting Dose and Safety Outcomes From a Large Dataset of Standardized Multiple Food Challenges. Front. Immunol. 2018, 9, 2057. [Google Scholar] [CrossRef]
- Schroer, B.; Groetch, M.; Mack, D.P.; Venter, C. Practical Challenges and Considerations for Early Introduction of Potential Food Allergens for Prevention of Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 44–56. [Google Scholar] [CrossRef]
- Cox, A.L.; Shah, A.; Groetch, M.; Sicherer, S.H. Allergic reactions in infants using commercial early allergen introduction products. J. Allergy Clin. Immunol. Pract. 2021, 9, 3517–3520. [Google Scholar] [CrossRef]
- Tan, J.W.-L.; Valerio, C.; Barnes, E.H.; Turner, P.J.; van Asperen, P.A.; Kakakios, A.M.; Campbell, D.E. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1621–1628. [Google Scholar] [CrossRef]
- Kulis, M.; Yue, X.; Guo, R.; Zhang, H.; Orgel, K.; Ye, P.; Li, Q.; Liu, Y.; Kim, E.; Burks, A.W.; et al. High- and low-dose oral immunotherapy similarly suppress pro-allergic cytokines and basophil activation in young children. Clin. Exp. Allergy 2019, 49, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Brough, H. Making the Most of In Vitro Tests to Diagnose Food Allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Pons, L.; Roberts, J.L.; Scurlock, A.M.; Perry, T.T.; Kulis, M.; Shreffler, W.G.; Steele, P.; Henry, K.A.; Adair, M.; et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J. Allergy Clin. Immunol. 2009, 124, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134. [Google Scholar] [CrossRef]
- Varshney, P.; Jones, S.M.; Scurlock, A.M.; Perry, T.T.; Kemper, A.; Steele, P.; Hiegel, A.; Kamilaris, J.; Carlisle, S.; Yue, X.; et al. A randomized controlled study of peanut oral immunotherapy: Clinical desensitization and modulation of the allergic response. J. Allergy Clin. Immunol. 2011, 127, 654–660. [Google Scholar] [CrossRef]
- Sampath, V.; Nadeau, K.C. Newly identified T cell subsets in mechanistic studies of food immunotherapy. J. Clin. Investig. 2019, 129, 1431–1440. [Google Scholar] [CrossRef]


| Category | Analysis | Reference Method |
|---|---|---|
| Protein Characterization | SDS-PAGE | Bio-Rad Laboratories or Thermofisher Scientific—Pierce Protein Methodology |
| Protein Quantitation | Kjeldahl Titration | AOAC 991.20 |
| Bioburden | Total Aerobic Microbial Count—Pour Plate | USP/NF <61> or FDA BAM |
| Total Yeast and Mold Count—Pour Plate | USP/NF <61> or FDA BAM | |
| Escherichia coli—Direct Inoculation | USP/NF <62> or FDA BAM | |
| Salmonella species—Direct Inoculation | USP/NF <62> or FDA BAM | |
| Listeria species—Absent/Present | AOAC-RI 061702 | |
| Listeria monocytogenes—Absent/Present | AOAC-RI 061701 | |
| Listeria species Confirmation (Genus 48 h) | FDA BAM Chapter 10 | |
| Aflatoxin Panel | AOAC 2005.8 or Modified AOAC 999.07 |
| Allergen | Marker Protein | Published Weight (kDa) | Observed Reference Data | Initial Acceptance Criteria, Inclusive | |||
|---|---|---|---|---|---|---|---|
| Weight (kDa) | Intensity (10 µg Load) | Weight (kDa) | Intensity (10 µg Load) | Total Protein | |||
| Almond | Pru du 6 (Amandin) | 40 | 38.5–41.7 | 1,142,948–4,526,868 | 34.7–45.9 | 800,064–5,884,928 | 10–70% |
| Cashew | Ana o 2 (Anacardein) | 33 | 26.5–34.1 | 752,512–6,028,437 | 23.9–37.5 | 526,758–7,836,968 | 10–55% |
| Egg | Gal d 2 (Ovalbumin) | 43–45 | 39.0–44.6 | 6,627,824–14,047,568 | 35.1–49.1 | 4,639,477–18,261,838 | 50–95% |
| Hazelnut | Cor a 9 (Corylin) | 35–40 | 31.7–34.8 | 1,963,532–6,958,956 | 28.5–38.3 | 1,374,472–9,046,643 | 10–65% |
| Milk | Bos d 5 (β-Lactoglobulin) | 18–18.3 | 14.7–16.9 | 1,007,988–4,842,720 | 13.2–18.6 | 705,592–6,295,536 | 10–65% |
| Peanut | Ara h 3 (Glycinin) | 37 | 35.0–40.4 | 1,125,819–3,025,396 | 31.5–44.4 | 788,073–3,933,015 | 15–75% |
| Salmon | Sal s 2 (β-enolase) | 47.3 | 41.2–47.4 | 147,088–529,396 | 37.1–52.1 | 102,962–688,215 | 35–99% |
| Shrimp | Pen a 1 (Tropomyosin) | 36 | 38.7–38.8 | 953,381–1,453,936 | 34.8–42.7 | 667,367–1,890,117 | 60–99% |
| Walnut | Jug r 4 (11S globulin) | 30–40 | 29.6–36.2 | 1,211,022–6,709,672 | 26.6–39.8 | 847,715–8,722,574 | 10–75% |
| Wheat | Tri a 26 (Glutenin) | 88 | 75.9–94.6 | 209,988–1,396,822 | 68.3–104.1 | 146,992–1,815,869 | 10–95% |
| Demographic Characteristics at Baseline | Control N = 45 | Active Milk (N = 15) | Active Egg (N = 15) | Active Peanut (N = 15) | Active Peanut/Milk (N = 15) | Active Milk/ Egg (N = 15) | Active Peanut/ Egg (N = 15) | Active Mixture Low (N = 15) | Active Mixture Medium (N = 15) | Active Mixture High (N = 15) |
|---|---|---|---|---|---|---|---|---|---|---|
| # Female | 23 | 8 | 7 | 8 | 7 | 8 | 7 | 8 | 8 | 7 |
| Age months (median and range) | 6 (2–12) | 5 (2–12) | 5 (2–12) | 6 (2–12) | 5 (2–12) | 5 (2–12) | 6 (2–12) | 6 (2–12) | 6 (2–12) | 5 (2–12) |
| Weight in kg (median and range) | 7 (5–9) | 7 (5–9) | 6 (5–8) | 7 (5–9) | 6 (4–8) | 6 (5–9) | 7 (6–10) | 7 (5–9) | 8 (6–10) | 7 (5–9) |
| # breast feeding until 6 mo | 45 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| # no eczema (scored 0–9.9) | 12 | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 4 |
| # Mild eczema (scored 10 to 28.9) | 10 | 4 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 4 |
| # Moderate eczema (scored 29.0 to 48.9) | 15 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 |
| # Severe eczema (scored 49.0 to 103) | 8 | 3 | 4 | 4 | 3 | 4 | 3 | 4 | 4 | 4 |
| # High risk * Family hx | 23 | 7 | 8 | 8 | 7 | 8 | 7 | 8 | 7 | 8 |
| Ethnicity | ||||||||||
| Hispanic | 4 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Afro American | 5 | 1 | 1 | 2 | 2 | 1 | 0 | 2 | 1 | 1 |
| Caucasian | 23 | 7 | 8 | 6 | 7 | 6 | 7 | 7 | 8 | 7 |
| Pacific Islander | 6 | 4 | 3 | 3 | 4 | 4 | 3 | 4 | 4 | 3 |
| Asian | 7 | 1 | 1 | 2 | 1 | 3 | 3 | 1 | 1 | 3 |
| Characteristics | Control N = 45 at Baseline | Control Arm Reactions During Study | Active N = 135 at Baseline | Active Arm Reactions During Study |
|---|---|---|---|---|
| No eczema | 8 (18%) | 4 (all mild rash) (9%) | 22 (16%) | 11 (all mild rash) (8%) |
| Mild eczema | 22 (49%) | 1 (2%) | 61 (45%) | 2 (1%) |
| Moderate eczema | 10 (22%) | none | 35 (26%) | 4 (3%) |
| Severe eczema | 5 (11%) | 1 (2%) | 17 (13%) | none |
| High risk * Family hx | N = 23 (51%) | 1 (2%) | N = 69 (51%) | 3 (2%) |
| Other adverse events within 2 h of serving | Control arm reactions during study | Active arm reactions during study | ||
| Vomiting | 0 | 0 | ||
| Diarrhea | 0 | 0 | ||
| Anaphylaxis (more than two organ systems involved) | 0 | 0 | ||
| Wheezing | 0 | 0 | ||
| Cough | 0 | 0 | ||
| Epinephrine use | 0 | 0 |
| Food Item Consumed by Participant at Start of Study | Participants Consuming Food Item at Start of and during the Study | Participants Consuming All 10 Food Items as Table Foods at End of 1st Year | ||
|---|---|---|---|---|
| N | % | N | % | |
| Milk | 15 | 100 | 6 | 40 |
| Egg | 15 | 100 | 5 | 33 |
| Peanut | 15 | 100 | 6 | 40 |
| Milk/egg | 15 | 100 | 7 | 47 |
| Egg/peanut | 15 | 100 | 7 | 47 |
| Milk/peanut | 15 | 100 | 8 | 53 |
| Milk/egg/peanut/cashew/almond/shrimp/walnut/wheat/fish/hazelnut (300 mg) | 15 | 100 | 15 | 100 |
| Milk/egg/peanut/cashew/almond/shrimp/walnut/wheat/fish/hazelnut (900 mg) | 15 | 100 | 15 | 100 |
| Milk/egg/peanut/cashew/almond/shrimp/walnut/wheat/fish/hazelnut (3000 mg) | 15 | 100 | 15 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quake, A.Z.; Liu, T.A.; D’Souza, R.; Jackson, K.G.; Woch, M.; Tetteh, A.; Sampath, V.; Nadeau, K.C.; Sindher, S.; Chinthrajah, R.S.; et al. Early Introduction of Multi-Allergen Mixture for Prevention of Food Allergy: Pilot Study. Nutrients 2022, 14, 737. https://doi.org/10.3390/nu14040737
Quake AZ, Liu TA, D’Souza R, Jackson KG, Woch M, Tetteh A, Sampath V, Nadeau KC, Sindher S, Chinthrajah RS, et al. Early Introduction of Multi-Allergen Mixture for Prevention of Food Allergy: Pilot Study. Nutrients. 2022; 14(4):737. https://doi.org/10.3390/nu14040737
Chicago/Turabian StyleQuake, Antonia Zoe, Taryn Audrey Liu, Rachel D’Souza, Katherine G. Jackson, Margaret Woch, Afua Tetteh, Vanitha Sampath, Kari C. Nadeau, Sayantani Sindher, R. Sharon Chinthrajah, and et al. 2022. "Early Introduction of Multi-Allergen Mixture for Prevention of Food Allergy: Pilot Study" Nutrients 14, no. 4: 737. https://doi.org/10.3390/nu14040737
APA StyleQuake, A. Z., Liu, T. A., D’Souza, R., Jackson, K. G., Woch, M., Tetteh, A., Sampath, V., Nadeau, K. C., Sindher, S., Chinthrajah, R. S., & Cao, S. (2022). Early Introduction of Multi-Allergen Mixture for Prevention of Food Allergy: Pilot Study. Nutrients, 14(4), 737. https://doi.org/10.3390/nu14040737







