The Impact of Preconception Gastric Bypass Surgery on Maternal Micronutrient Status before and during Pregnancy: A Retrospective Cohort Study in the Netherlands between 2009 and 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
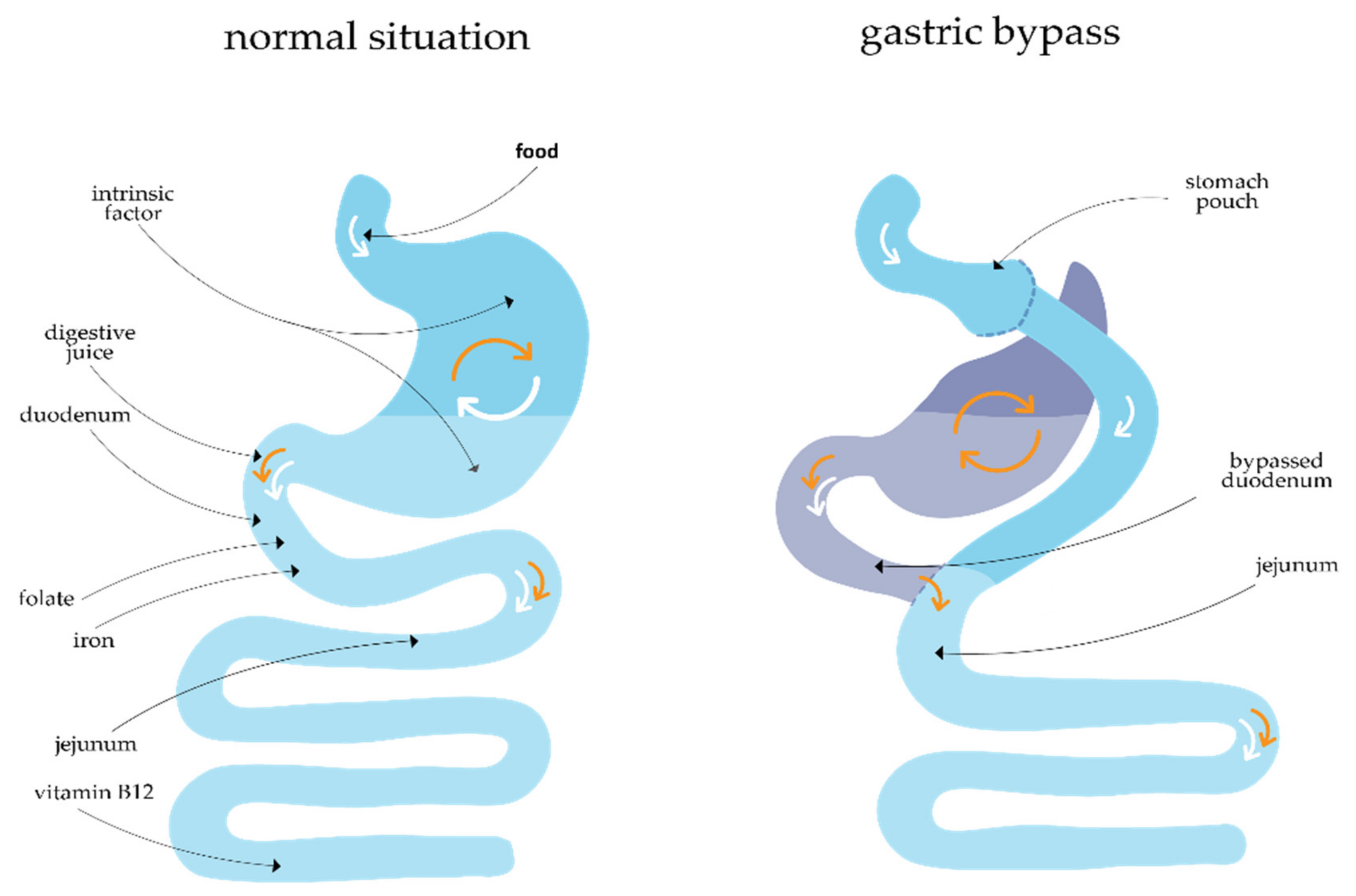
2.1.1. Gastric Bypass Surgery
2.1.2. Clinical Parameters
2.1.3. Multivitamin Supplement Use
2.1.4. Laboratory Blood Analysis
2.1.5. Statistical Analysis
3. Results
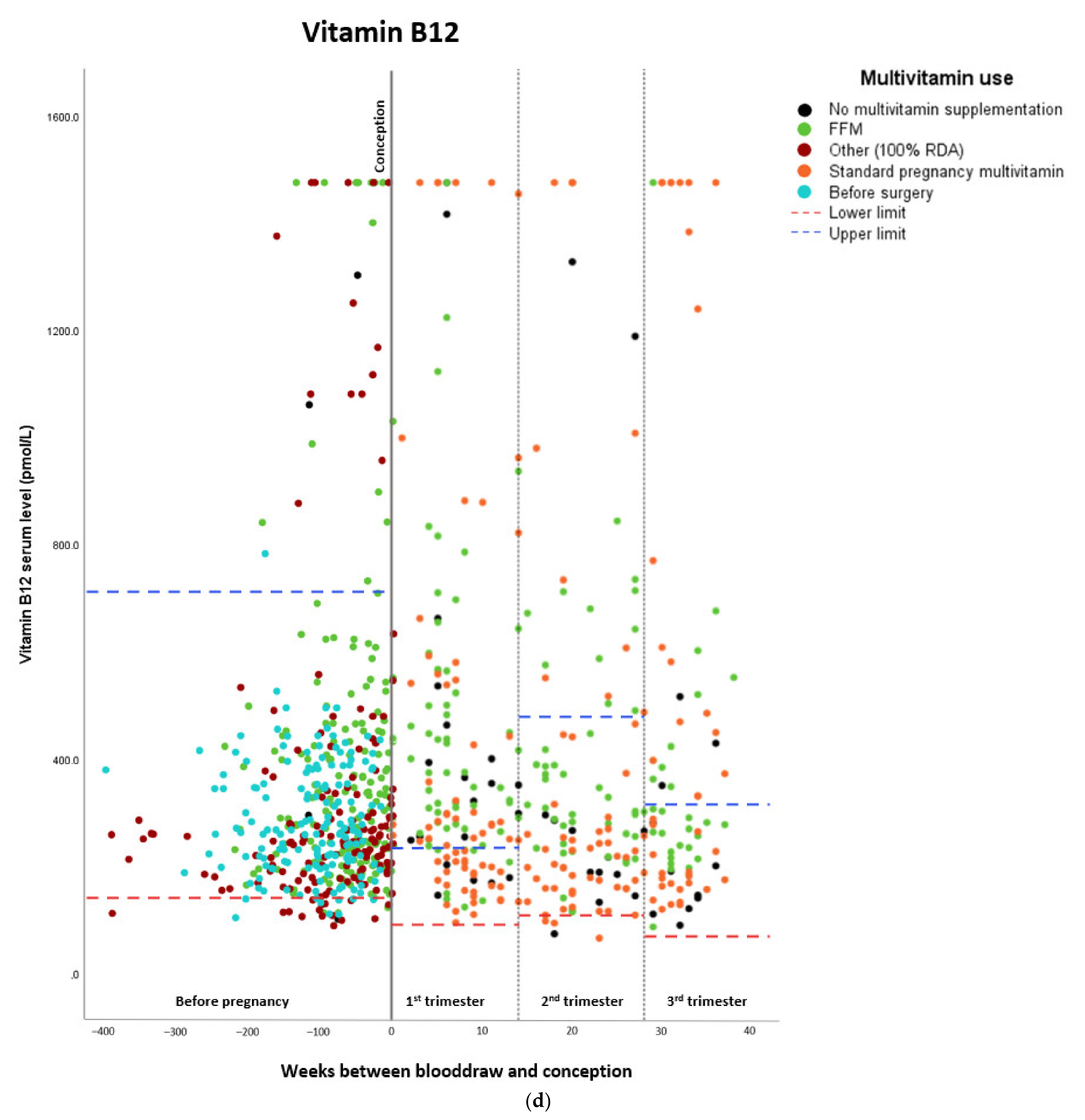
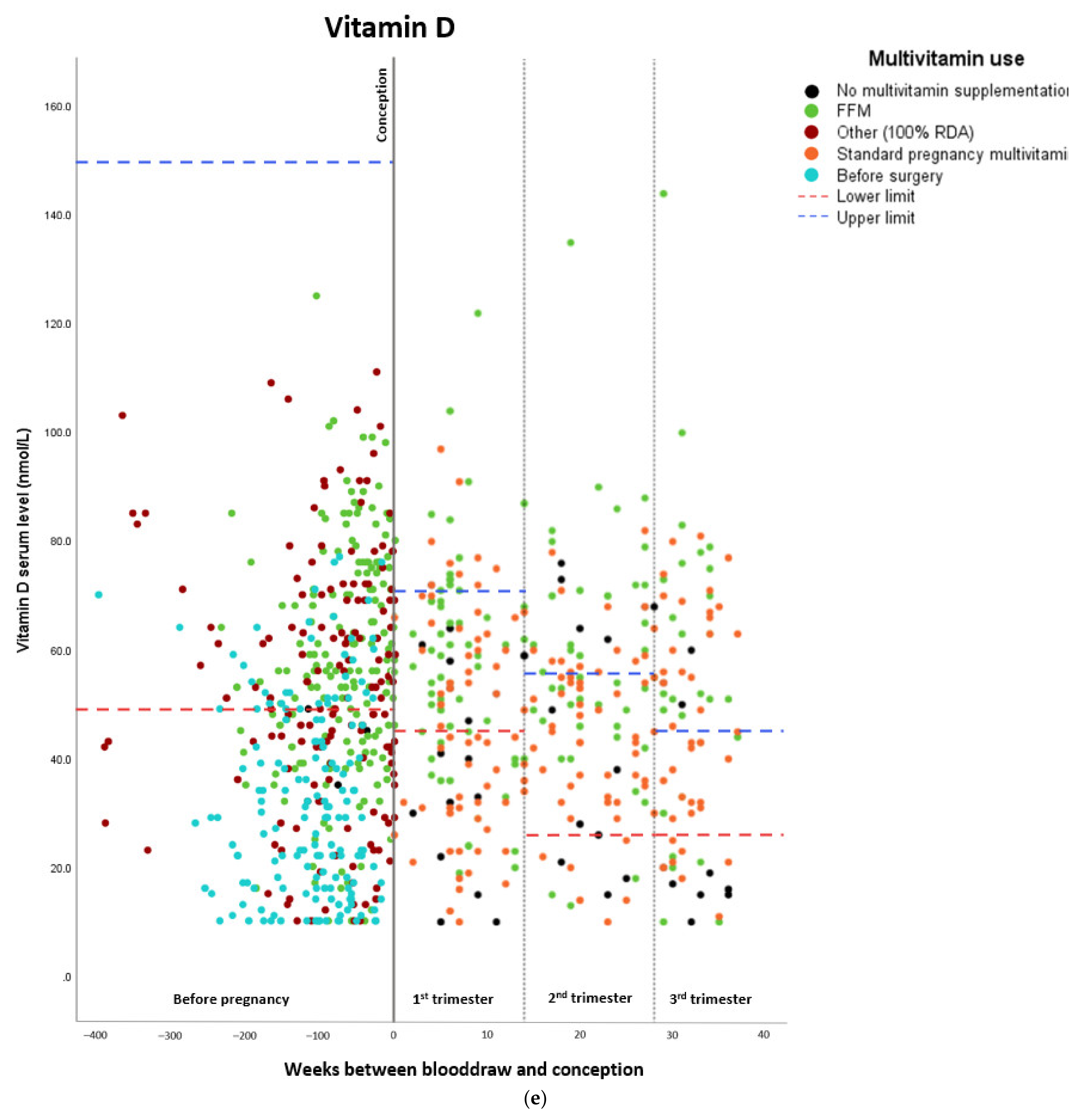
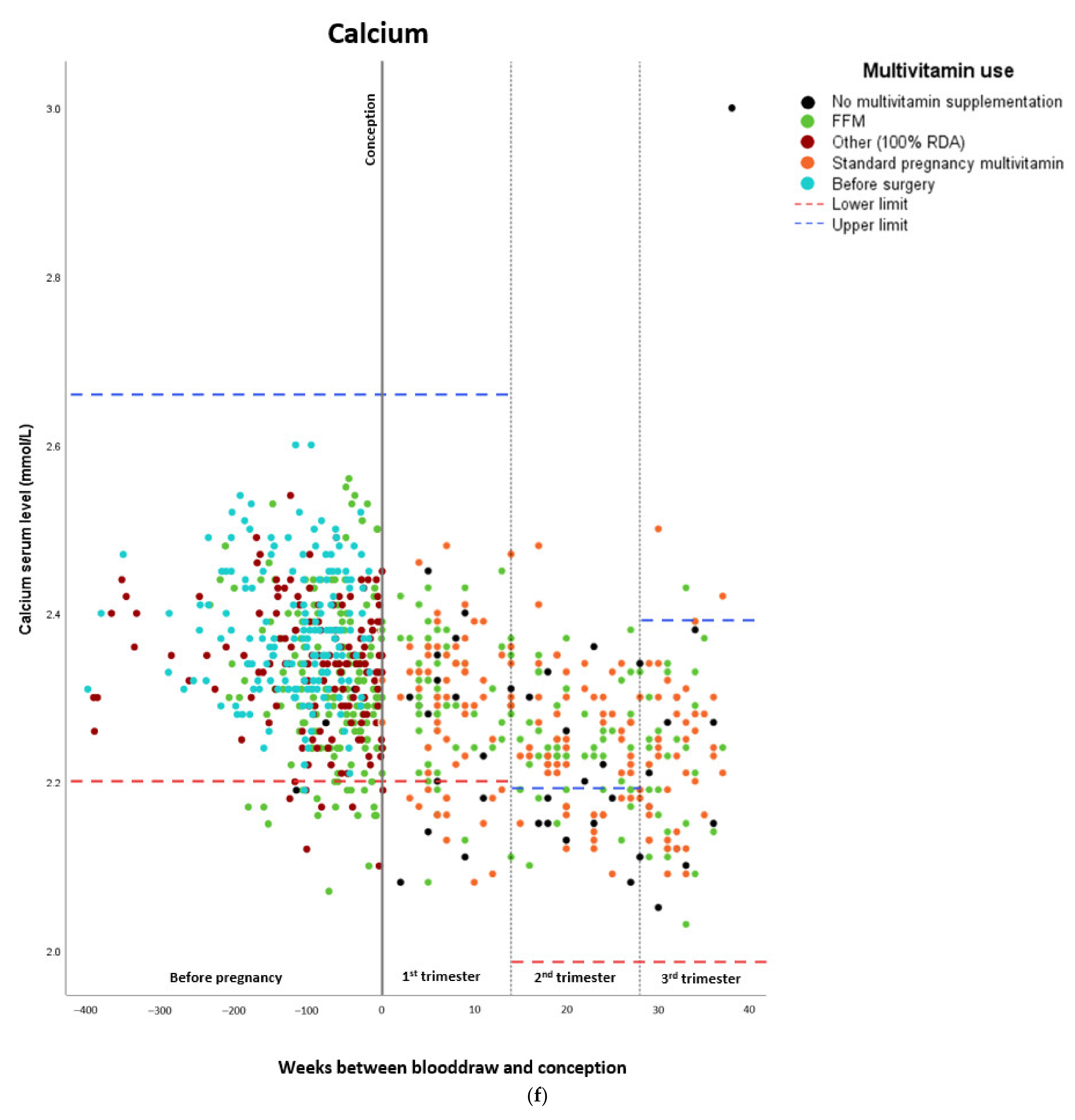
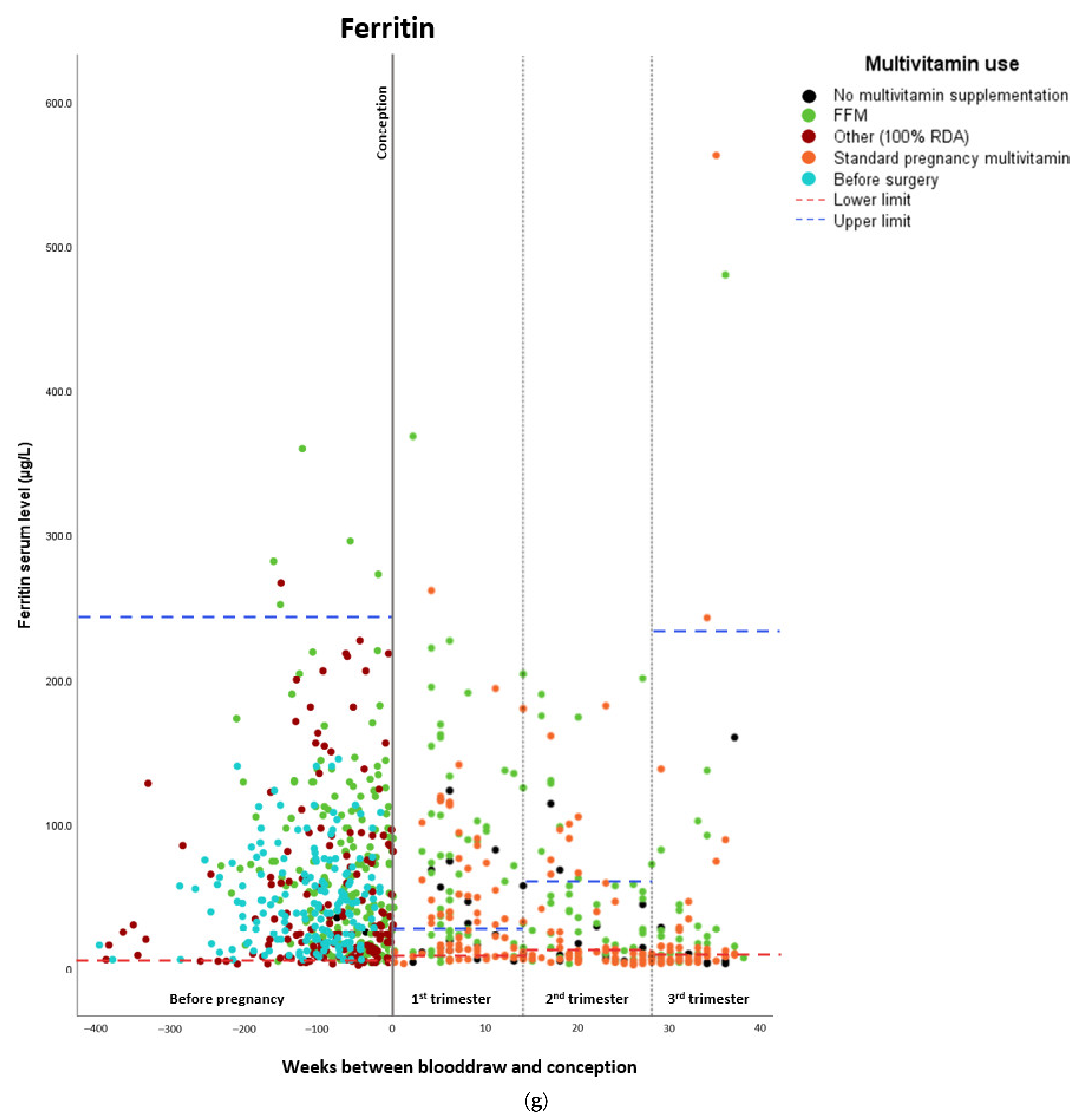
Micronutrient Blood Levels before Surgery, after Surgery, and during Pregnancy
4. Discussion
4.1. Postoperative Effects of Supplementation on Vitamin Status
4.2. Multivitamin Supplementation Adherence
4.3. Clinical Implications
4.4. Research Implications
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, M.; Lemstra, M.; Bird, Y.; Nwankwo, C.; Moraros, J. Weight-loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, M.; Hainer, V.; Basdevant, A.; Buchwald, H.; Deitel, M.; Finer, N.; Greve, J.W.M.; Horber, F.; Mathus-Vliegen, E.; Scopinaro, N.; et al. Interdisciplinary European Guidelines on Surgery of Severe Obesity. Obes. Facts 2008, 1, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs. Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef]
- IFSO. 4th IFSO Global Registry Report 2018; IFSO: Napoli, Italy, 2018. [Google Scholar]
- Bebber, F.E.; Rizzolli, J.; Casagrande, D.S.; Rodrigues, M.T.; Padoin, A.V.; Mottin, C.C.; Repetto, G. Pregnancy after Bariatric Surgery: 39 Pregnancies Follow-up in a Multidisciplinary Team. Obes. Surg. 2010, 21, 1546–1551. [Google Scholar] [CrossRef]
- Devlieger, R.; Guelinckx, I.; Jans, G.; Voets, W.; VanHolsbeke, C.; Vansant, G. Micronutrient Levels and Supplement Intake in Pregnancy after Bariatric Surgery: A Prospective Cohort Study. PLoS ONE 2014, 9, e114192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, N.C.; Sakkatos, P.; Sakellaropoulos, G.C.; Adonakis, G.L.; Alexandrides, T.K.; Kalfarentzos, F. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg. Obes. Relat. Dis. 2014, 10, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Sapre, N.; Munting, K.; Pandita, A.; Stubbs, R. Pregnancy following gastric bypass surgery: What is the expected course and outcome? N. Z. Med. J. 2009, 122, 33–42. [Google Scholar]
- Hazart, J.; Le Guennec, D.; Accoceberry, M.; Lemery, D.; Mulliez, A.; Farigon, N.; Lahaye, C.; Miolanne-Debouit, M.; Boirie, Y. Maternal Nutritional Deficiencies and Small-for-Gestational-Age Neonates at Birth of Women Who Have Undergone Bariatric Surgery. J. Pregnancy 2017, 2017, 4168541. [Google Scholar] [CrossRef]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.M.; Twigt, J.; Pestinger, V.; Sinclair, C.D. The periconceptional period, reproduction and long-term health of offspring: The im-portance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef] [Green Version]
- Ebisch, I.; Thomas, C.; Peters, W.; Braat, D.; Steegers-Theunissen, R. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Updat. 2007, 13, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hague, W.M. Homocysteine and pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 459–469. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.; Steegers, E. Nutrient-gene interactions in early pregnancy: A vascular hypothesis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 115–117. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B12 Deficiencies During Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29, S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Via, M.A.; Mechanick, J.I. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr. Obes. Rep. 2017, 6, 286–296. [Google Scholar] [CrossRef]
- Beard, C.M.; Panser, L.A.; Katusic, S.K. Is excess folic acid supplementation a risk factor for autism? Med. Hypotheses 2011, 77, 15–17. [Google Scholar] [CrossRef]
- Raghavan, R.; Fallin, M.D.; Wang, X. Maternal plasma folate, vitamin B12 levels and multivitamin supplementation during pregnancy and risk of Autism Spectrum Disorder in the Boston Birth Cohort. FASEB J. 2016, 30, 151.6. [Google Scholar]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef] [Green Version]
- Me, F.F. WLS Forte. 2020. Available online: https://www.fitforme.nl/wls-forte-21 (accessed on 18 May 2020).
- Daly, L.E. Folate levels and neural tube defects. Implications for prevention. JAMA 1995, 274, 1698–1702. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood J. Am. Soc. Hematol. 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, M.; Matos, A.C.; Pereira, S.E.; Saboya, C.; Ramalho, A. Vitamin D and its relation with ionic calcium, parathyroid hormone, maternal and neonatal characteristics in pregnancy after roux-en-Y gastric bypass. Arch. Gynecol. Obstet. 2015, 293, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef] [Green Version]
- Ribot, B.; Aranda, N.; Viteri, F.; Hernandez-Martinez, C.; Canals, J.; Arija, V. Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum. Reprod. 2012, 27, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, S.; Azami, M.; Badfar, G.; Parizad, N.; Sayehmiri, K. The relationship between maternal anemia during pregnancy with preterm birth: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2019, 33, 2679–2689. [Google Scholar] [CrossRef]
- Sunil, S.; Santiago, V.A.; Gougeon, L.; Warwick, K.; Okrainec, A.; Hawa, R.; Sockalingam, S. Predictors of Vitamin Adherence After Bariatric Surgery. Obes. Surg. 2017, 27, 416–423. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; De Gouvêa, H.R.; Amato, A.A. Supplementation Adherence and Outcomes Among Pregnant Women after Bariatric Surgery. Obes. Surg. 2018, 29, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Osland, E.; Powlesland, H.; Guthrie, T.; Lewis, C.-A.; Memon, M.A. Micronutrient management following bariatric surgery: The role of the dietitian in the postoperative period. Ann. Transl. Med. 2020, 8, S9. [Google Scholar] [CrossRef]
- Snoek, K.M.; Steegers-Theunissen, R.P.M.; Hazebroek, E.J.; Willemsen, S.P.; Galjaard, S.; Laven, J.S.E.; Schoenmakers, S. The effects of bariatric surgery on periconception maternal health: A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 1030–1055. [Google Scholar] [CrossRef]
- Paulen, M.E.; Zapata, L.B.; Cansino, C.; Curtis, K.M.; Jamieson, D.J. Contraceptive use among women with a history of bariatric surgery: A systematic review. Contraception 2010, 82, 86–94. [Google Scholar] [CrossRef]
| Characteristic | Study Population |
|---|---|
| Age at conception in years | |
| Median | 29.8 (26.6–33.4) |
| Missing | 0 |
| Parity | |
| Nulliparous | 78 (39.6) |
| Missing | 0 |
| Educational level | |
| Low | 33 (17.5) |
| Intermediate | 122 (58.7) |
| High | 45 (23.8) |
| Missing | 9 |
| Comorbidity | |
| Diabetes mellitus | 11 (5.6) |
| Hypertension | 13 (6.7) |
| Obstructive sleep apnea syndrome | 2 (1.0) |
| Asthma/COPD | 30 (15.4) |
| Hypothyroidism | 24 (12.3) |
| Missing | 2 |
| Period between surgery and conception in months | 18.7 (10.2–31.3) |
| Missing | 16 |
| BMI before surgery (kg/m2) * | |
| Median | 43.3 (40.9–46.2) |
| Missing | 0 |
| BMI at conception (kg/m2) | |
| Median | 29.0 (26.6–32.5) |
| Missing | 44 |
| Geographic origin | |
| Caucasian | 122 (63.2) |
| African | 12 (6.2) |
| Asian | 1 (0.5) |
| Other/mixed | 58 (30.1) |
| Missing | 4 |
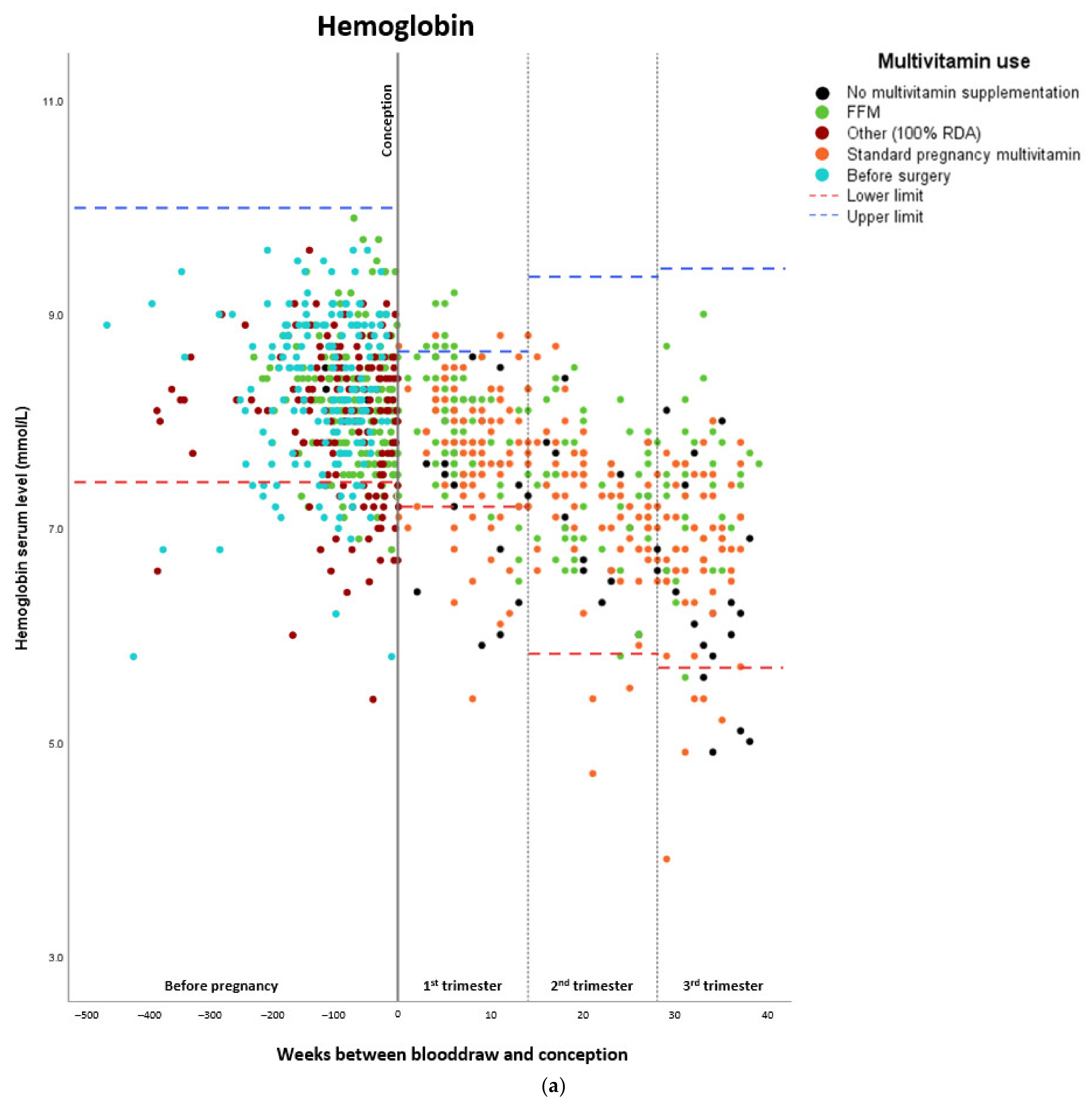
| Hemoglobin | ||||
| Estimated marginal means (mmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 8.30 | 0.059 | 8.184 | 8.416 |
| Preconceptionally after surgery | 8.10 | 0.062 | 7.979 | 8.221 |
| First trimester | 7.79 | 0.057 | 7.677 | 7.903 |
| Second trimester | 7.21 | 0.060 | 7.092 | 7.328 |
| Third trimester | 6.99 | 0.063 | 6.868 | 7.113 |
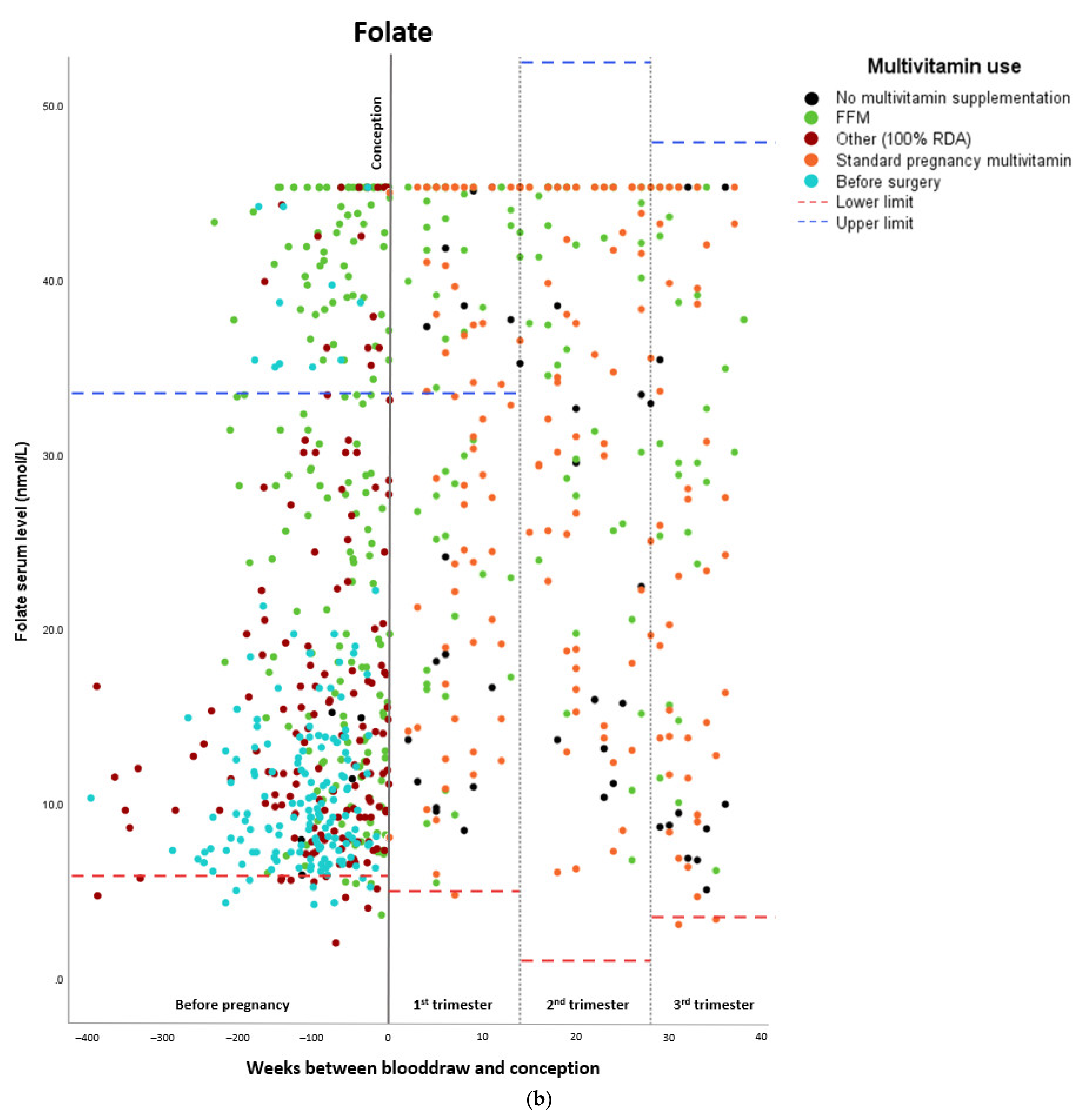
| Folate | ||||
| Estimated marginal means (nmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 12.0 | 1.13 | 9.785 | 14.215 |
| Preconceptionally after surgery | 22.0 | 1.14 | 19.766 | 24.234 |
| First trimester | 31.7 | 1.11 | 29.524 | 33.876 |
| Second trimester | 31.7 | 1.13 | 29.485 | 33.915 |
| Third trimester | 27.5 | 1.33 | 24.893 | 30.107 |
| Calcium | ||||
| Estimated marginal means (mmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 2.38 | 0.009 | 2.363 | 2.397 |
| Preconceptionally after surgery | 2.33 | 0.010 | 2.311 | 2.349 |
| First trimester | 2.30 | 0.010 | 2.281 | 2.319 |
| Second trimester | 2.24 | 0.009 | 2.222 | 2.258 |
| Third trimester | 2.23 | 0.013 | 2.205 | 2.255 |
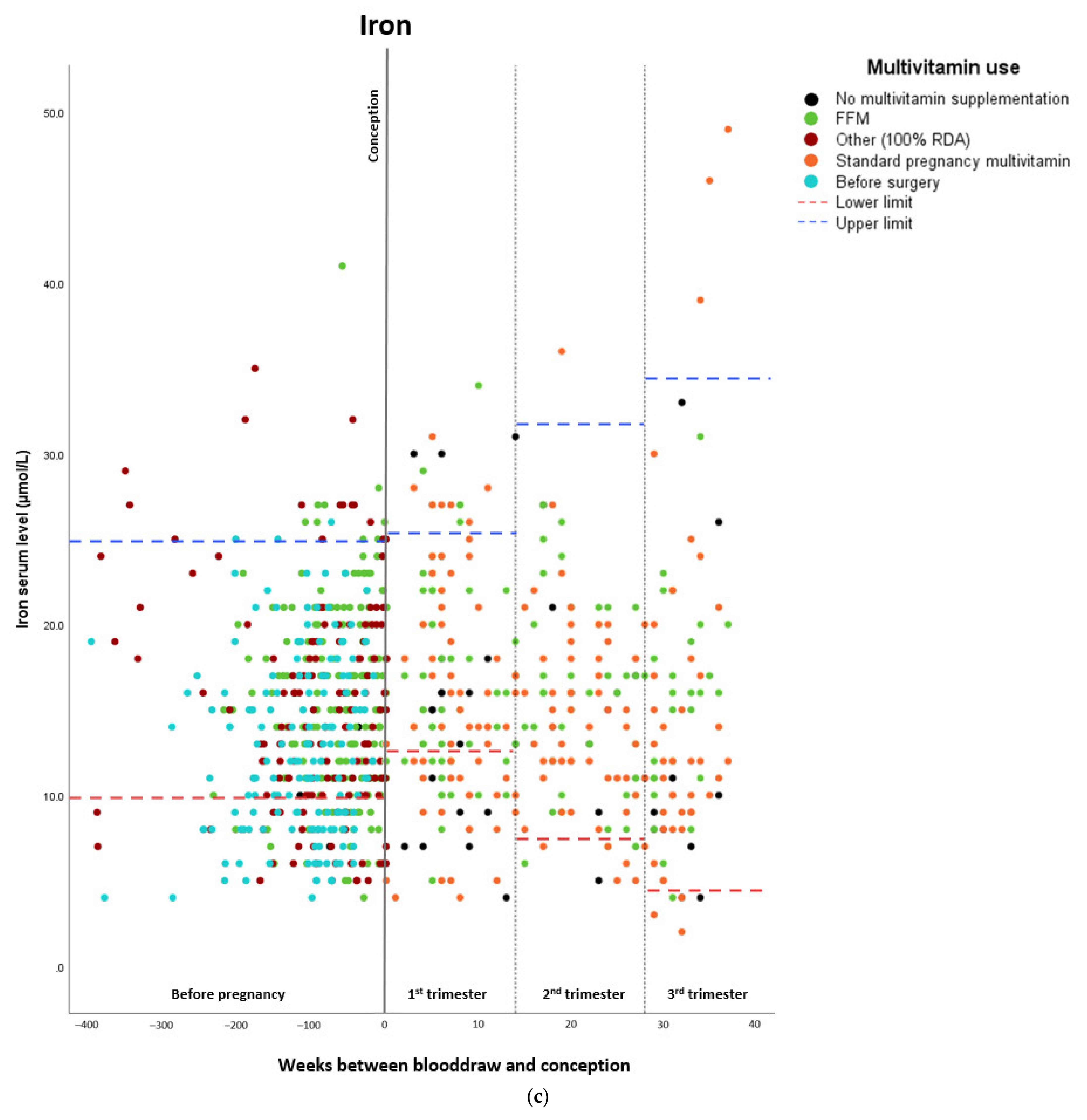
| Iron | ||||
| Estimated marginal means (µmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 12.6 | 0.588 | 11.448 | 13.752 |
| Preconceptionally after surgery | 14.9 | 0.629 | 13.667 | 16.133 |
| First trimester | 16.1 | 0.583 | 14.957 | 17.243 |
| Second trimester | 14.7 | 0.630 | 13.465 | 15.935 |
| Third trimester | 14.2 | 0.737 | 12.755 | 15.645 |
| Vitamin B12 * | ||||
| Estimated marginal means (pmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 257.24 | 1.056 | 230.442 | 287.149 |
| Preconceptionally after surgery | 357.81 | 1.065 | 316.494 | 404.518 |
| First trimester | 327.01 | 1.058 | 292.790 | 365.236 |
| Second trimester | 290.04 | 1.058 | 259.885 | 323.681 |
| Third trimester | 320.54 | 1.062 | 284.919 | 360.610 |
| Vitamin D | ||||
| Estimated marginal means (nmol/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 33.0 | 1.9 | 29.276 | 36.724 |
| Preconceptionally after surgery | 53.2 | 1.99 | 49.300 | 57.100 |
| First trimester | 49.3 | 1.86 | 45.654 | 52.946 |
| Second trimester | 48.4 | 1.96 | 44.558 | 52.242 |
| Third trimester | 48.4 | 2.15 | 44.186 | 52.614 |
| Ferritin | ||||
| Estimated marginal means (µg/L) | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Before surgery | 50.6 | 6.05 | 38.742 | 62.458 |
| Preconceptionally after surgery | 68.2 | 6.39 | 55.676 | 80.724 |
| First trimester | 55.7 | 5.85 | 44.234 | 67.166 |
| Second trimester | 37.0 | 6.06 | 25.122 | 48.878 |
| Third trimester | 34.3 | 7.29 | 20.012 | 48.588 |
| Hemoglobin | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.069 | 0.072 | –0.072 | 0.211 |
| First trimester | 0.171 | 0.088 | –0.001 | 0.342 |
| Second trimester | 0.701 | 0.095 | 0.515 | 0.887 |
| Third trimester | 0.906 | 0.101 | 0.709 | 1.103 |
| Folate | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | –9.087 | 1.501 | –12.029 | –6.145 |
| First trimester | –18.872 | 1.754 | –22.311 | –15.434 |
| Second trimester | –18.893 | 1.937 | –22.688 | –15.097 |
| Third trimester | –14.783 | 2.071 | –18.842 | –10.724 |
| Calcium | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.037 | 0.012 | 0.014 | 0.061 |
| First trimester | 0.057 | 0.013 | 0.030 | 0.083 |
| Second trimester | 0.110 | 0.016 | 0.079 | 0.141 |
| Third trimester | 0.122 | 0.017 | 0.088 | 0.156 |
| Iron | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | –2.027 | 0.867 | –3.727 | –0.327 |
| First trimester | –3.313 | 0.983 | –5.240 | –1.385 |
| Second trimester | –1.744 | 1.130 | –3.959 | 0.471 |
| Third trimester | –1.570 | 1.182 | –3.887 | 0.748 |
| Vitamin B12 * | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | –0.245 | 0.086 | –2.093 | –1.123 |
| First trimester | –0.072 | 0.104 | –0.748 | 0.356 |
| Second trimester | 0.087 | 0.107 | –0.334 | 0.807 |
| Third trimester | 0.009 | 0.111 | –0.568 | 0.753 |
| Vitamin D | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | –2.179 | 2.473 | –7.026 | 2.668 |
| First trimester | –2.118 | 3.002 | –8.001 | 3.766 |
| Second trimester | 2.046 | 3.275 | –4.372 | 8.464 |
| Third trimester | –2.108 | 3.458 | –8.884 | 4.669 |
| Ferritin | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | –25.684 | 8.546 | –42.434 | –8.935 |
| First trimester | –18.306 | 9.998 | –37.902 | 1.291 |
| Second trimester | –5.117 | 10.666 | –26.023 | 15.789 |
| Third trimester | –1.045 | 11.424 | –23.436 | 21.346 |
| Hemoglobin | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.106 | 0.103 | –0.096 | 0.309 |
| First trimester | 0.213 | 0.105 | 0.006 | 0.420 |
| Second trimester | 0.031 | 0.114 | –0.194 | 0.255 |
| Third trimester | 0.426 | 0.123 | 0.184 | 0.667 |
| Folate | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 6.953 | 2.117 | 2.803 | 11.103 |
| First trimester | 3.179 | 2.353 | –1.433 | 7.791 |
| Second trimester | 1.038 | 2.360 | –3.587 | 5.663 |
| Third trimester | 3.230 | 2.577 | –1.821 | 8.281 |
| Calcium | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.006 | 0.020 | –0.032 | 0.045 |
| First trimester | –0.002 | 0.021 | –0.043 | 0.038 |
| Second trimester | –0.007 | 0.023 | –0.052 | 0.038 |
| Third trimester | –0.023 | 0.024 | –0.070 | 0.025 |
| Iron | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.674 | 1.243 | –1.763 | 3.112 |
| First trimester | 1.839 | 1.215 | –0.543 | 4.221 |
| Second trimester | 1.597 | 1.306 | –0.963 | 4.157 |
| Third trimester | 1.557 | 1.613 | –1.605 | 4.719 |
| Vitamin B12 * | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 0.125 | 0.329 | 0.781 | –0.518 |
| First trimester | 1.074 | 0.343 | 1.743 | 0.404 |
| Second trimester | 0.919 | 0.343 | 1.591 | 0.246 |
| Third trimester | 0.424 | 0.437 | 1.281 | –0.432 |
| Vitamin D | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 7.941 | 3.657 | 0.774 | 15.108 |
| First trimester | 6.519 | 3.501 | –0.343 | 13.381 |
| Second trimester | 8.659 | 3.719 | 1.369 | 15.948 |
| Third trimester | 11.302 | 4.116 | 3.234 | 19.369 |
| Ferritin | ||||
| Beta | Standard error | Lower confidence interval (2.5%) | Upper confidence interval (97.5%) | |
| Preconceptionally after surgery | 15.952 | 11.640 | –6.862 | 38.766 |
| First trimester | 29.430 | 12.726 | 4.488 | 54.373 |
| Second trimester | 23.572 | 12.776 | –1.470 | 48.614 |
| Third trimester | 7.846 | 13.825 | –19.250 | 34.943 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snoek, K.; van de Woestijne, N.; Willemsen, S.; Klaassen, R.; Galjaard, S.; Laven, J.; Steegers-Theunissen, R.; Schoenmakers, S. The Impact of Preconception Gastric Bypass Surgery on Maternal Micronutrient Status before and during Pregnancy: A Retrospective Cohort Study in the Netherlands between 2009 and 2019. Nutrients 2022, 14, 736. https://doi.org/10.3390/nu14040736
Snoek K, van de Woestijne N, Willemsen S, Klaassen R, Galjaard S, Laven J, Steegers-Theunissen R, Schoenmakers S. The Impact of Preconception Gastric Bypass Surgery on Maternal Micronutrient Status before and during Pregnancy: A Retrospective Cohort Study in the Netherlands between 2009 and 2019. Nutrients. 2022; 14(4):736. https://doi.org/10.3390/nu14040736
Chicago/Turabian StyleSnoek, Katinka, Nadia van de Woestijne, Sten Willemsen, René Klaassen, Sander Galjaard, Joop Laven, Régine Steegers-Theunissen, and Sam Schoenmakers. 2022. "The Impact of Preconception Gastric Bypass Surgery on Maternal Micronutrient Status before and during Pregnancy: A Retrospective Cohort Study in the Netherlands between 2009 and 2019" Nutrients 14, no. 4: 736. https://doi.org/10.3390/nu14040736
APA StyleSnoek, K., van de Woestijne, N., Willemsen, S., Klaassen, R., Galjaard, S., Laven, J., Steegers-Theunissen, R., & Schoenmakers, S. (2022). The Impact of Preconception Gastric Bypass Surgery on Maternal Micronutrient Status before and during Pregnancy: A Retrospective Cohort Study in the Netherlands between 2009 and 2019. Nutrients, 14(4), 736. https://doi.org/10.3390/nu14040736







