Casein Oligochitosan-Glycation by Transglutaminase Enhances the Anti-Inflammatory Potential of Casein Hydrolysates to the Lipopolysaccharide-Stimulated IEC-6 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Assays of Protein and Glucosamine Contents
2.4. Cell Line and Cell Culture
2.5. Assays of Cell Viability
2.6. Assay of LPS Cytotoxicity
2.7. Assays of Cytokine Secretion
2.8. Assays of Protein Expression
2.9. Statistical Analysis
3. Results
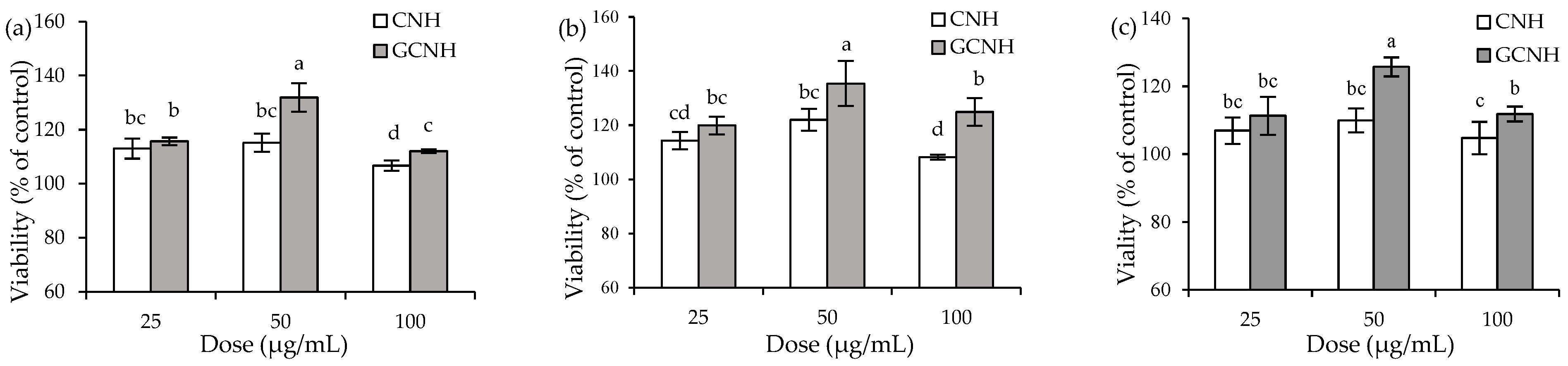
3.1. Effect of the Two Hydrolysates on Cell Growth
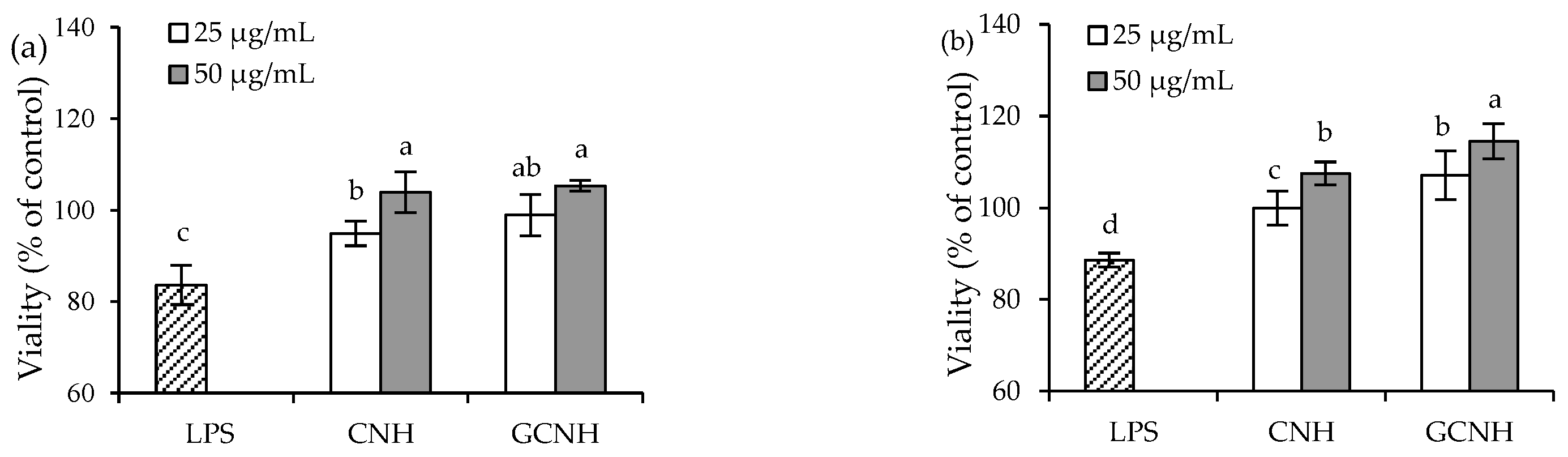
3.2. Effect of the Two Hydrolysates on the LPS-Induced Cellular Injury
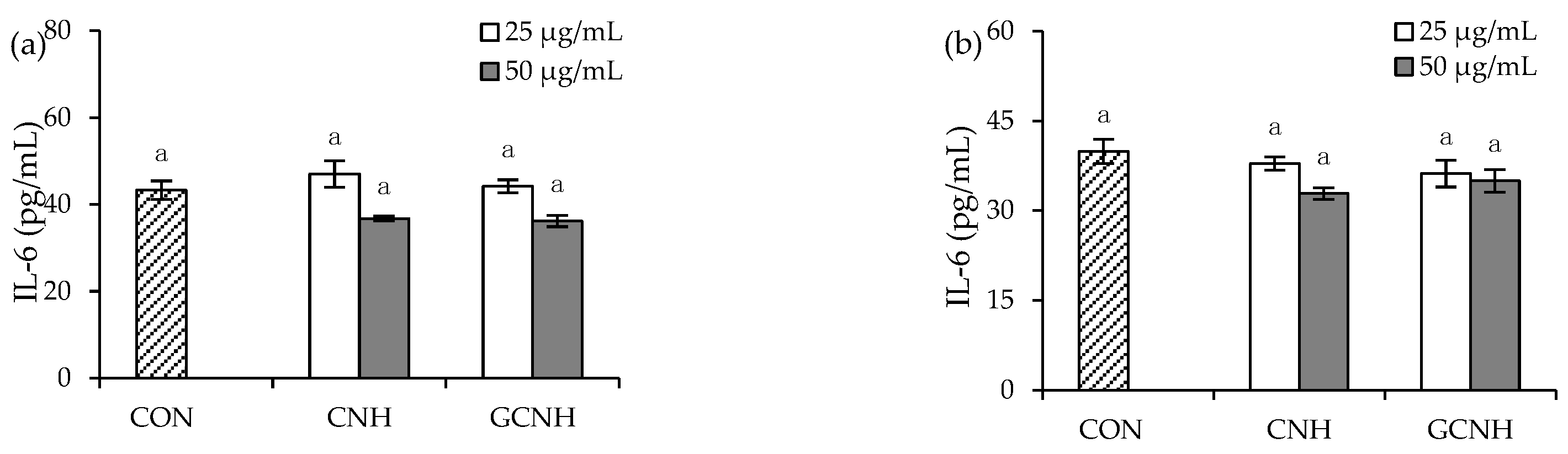
3.3. Effect of Two Hydrolysates on the Secretion of Inflammatory Mediators
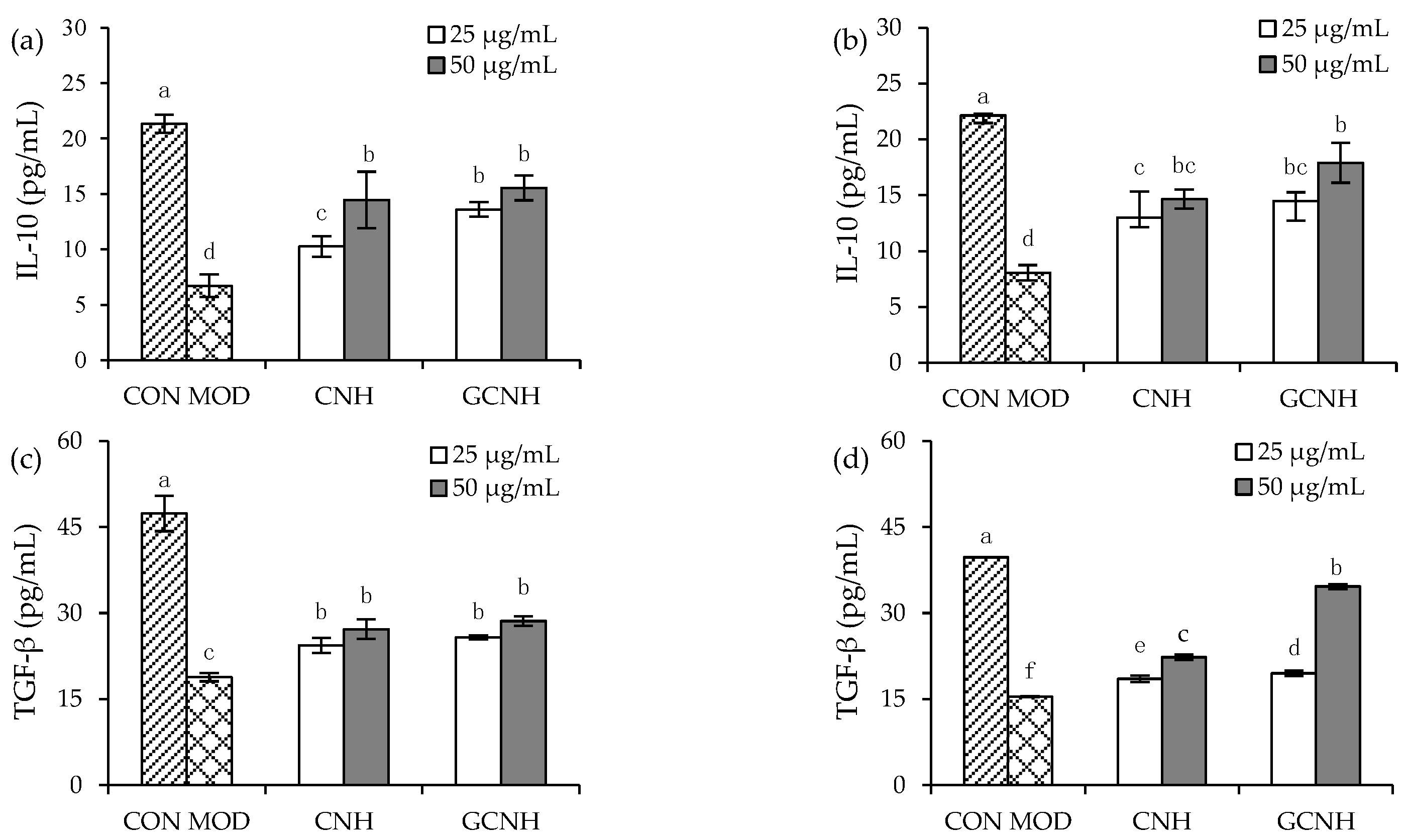
3.4. Effect of Two Hydrolysates on the Secretion of Anti-Inflammatory Mediators
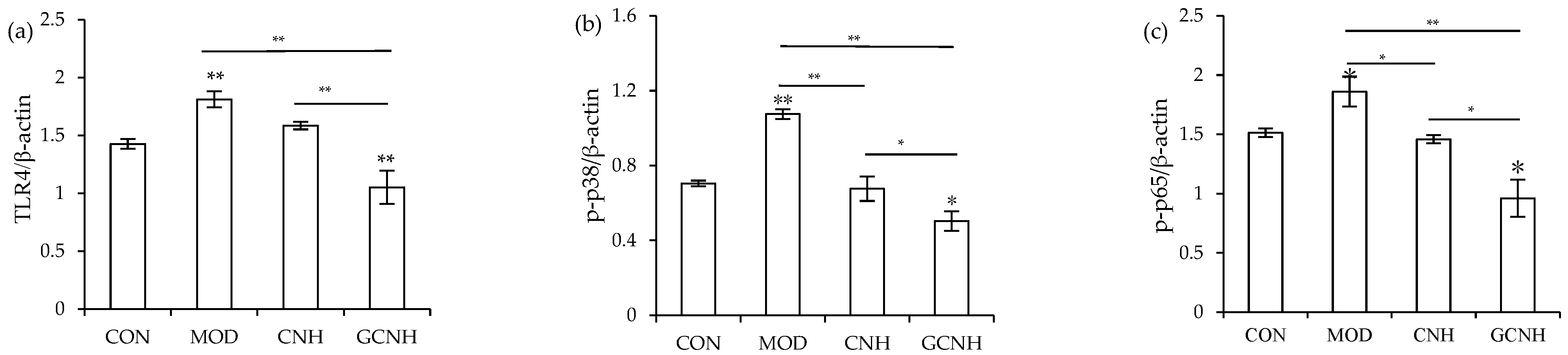
3.5. Expression Changes of the Signaling Pathway-Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, C.Y.; Wang, X.P.; Zhao, X.H. Property modification of caseinate responsible to transglutaminase-induced glycosylation and crosslinking in the presence of a degraded chitosan. Food Sci. Biotechnol. 2015, 24, 843–850. [Google Scholar] [CrossRef]
- Liu, X.L.; Song, C.L.; Chen, J.P.; Liu, X.; Ren, J.; Zheng, X.Q. Preparation and evaluation of new glycopeptides obtained by proteolysis from corn gluten meal followed by transglutaminase-induced glycosylation with glucosamine. Foods 2020, 9, e555. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, R.; Heinonen, M. Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022, 369, e130879. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.M.; Shao, Y.H.; Zhang, J.L.; Tu, Z.C. The mechanism of the reduction in allergenic reactivity of bovine alpha-lactalbumin induced by glycation, phosphorylation and acetylation. Food Chem. 2020, 310, e125853. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, Y.; Ren, L.; Li, M.; Lv, Y.; Guo, S.; Waqar, K. Physical-chemical properties and in vitro digestibility of phosphorylated and glycosylated soy protein isolate. Lwt- Food Sci. Technol. 2021, 152, e112380. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442. [Google Scholar] [CrossRef]
- Oste, R.E.; Dahlqvist, A.; Sjoestroem, H.; Noren, O.; Miller, R. Effect of Maillard reaction products on protein digestion. In vitro studies. J. Agric. Food Chem. 1986, 34, 355–358. [Google Scholar] [CrossRef]
- Song, C.L.; Zhao, X.H. Rheological, gelling and emulsifying properties of a glycosylated and cross-linked caseinate generated by transglutaminase. Int. J. Food Sci. Technol. 2013, 48, 2595–2602. [Google Scholar] [CrossRef]
- Song, C.L.; Zhao, X.H. Structure and property modification of an oligochitosan-glycosylated and crosslinked soybean protein generated by microbial transglutaminase. Food Chem. 2014, 163, 114–119. [Google Scholar] [CrossRef]
- Fu, M.; Zhao, X.H. Structure and property changes of transglutaminase-induced modification of sodium caseinate in the presence of oligochitosan of 5 kDa. Int. J. Food Prop. 2016, 19, 2596–2607. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Zhao, X.H. Modified properties of a glycated and cross-linked soy protein isolate by transglutaminase and an oligochitosan of 5 kDa. J. Sci. Food Agr. 2017, 97, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, X.H. Chemical features of the oligochitosan-glycated caseinate digest and its enhanced protection on barrier function of the acrylamide-injured IEC-6 cells. Food Chem. 2019, 290, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Ma, C.M.; Zhao, X.H. Activity of the peptic-tryptic caseinate digest with caseinate oligochitosan-glycation in rat intestinal epithelial (IEC-6) cells via the Wnt/beta-catenin signaling pathway. Chem.-Biol. Interact. 2020, 328, e109201. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, iNOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer. 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Dun, B.; Wei, Z.; Liu, C.; Tian, J.; Ren, G.; Yao, Y. Peptides released from extruded adzuki bean protein through simulated gastrointestinal digestion exhibit anti-inflammatory activity. J. Agric. Food Chem. 2021, 69, 7028–7036. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Sundarajan, K.; Edwin, J.R.; Gururaja, G.M.; Mundkinajeddu, D.; Agarwal, A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacogn. Res. 2013, 5, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Li, C.W.; Shih, Y.T.; Chuang, L.T. Antioxidant and anti-inflammatory properties of lower-polymerized polyphenols in oolong tea. Int. J. Food Prop. 2013, 17, 752–764. [Google Scholar] [CrossRef]
- Diao, J.; Chi, Z.; Guo, Z.; Zhang, L. Mung bean protein hydrolysate modulates the immune response through NF-kappaB pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Food Sci. 2019, 84, 2652–2657. [Google Scholar] [CrossRef]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon protein-derived peptides exert anti-inflammatory effects in LPS-stimulated RAW 264.7 macrophages via the MAPK pathway. J. Funct. Foods. 2020, 72, e104044. [Google Scholar] [CrossRef]
- Jiang, F.; Guan, H.; Liu, D.; Wu, X.; Fan, M.; Han, J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages through the MAPK and NF-kappaB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, J.Y. Protective effects of strawberry and mulberry fruit polysaccharides on inflammation and apoptosis in murine primary splenocytes. J. Food Drug Anal. 2014, 22, 210–219. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, M.; Guo, J.; Feng, Z. Effects of bovine lactoferrin on rat intestinal epithelial cells. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wan, G.; Han, B.; Zhang, Z. Echinacoside alleviated LPS-induced cell apoptosis and inflammation in rat intestine epithelial cells by inhibiting the mTOR/STAT3 pathway. Biomed. Pharmacother. 2018, 104, 622–628. [Google Scholar] [CrossRef]
- Kidd, B.L.; Urban, L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW 264.7 murine macrophages. Food Biosci. 2020, 36, e100636. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.J.; Kim, S.K. Purification of a peptide from seahorse, that inhibits TPA-induced MMP, iNOS and COX-2 expression through MAPK and NF-kappaB activation, and induces human osteoblastic and chondrocytic differentiation. Chem.-Biol. Interact. 2010, 184, 413–422. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Lin, X.; Wu, Y.; Ouyang, W.; Tang, L.; Ye, S.; Wang, Y.; Li, W.; Zhang, X.; et al. 0.005% Preservative-free latanoprost induces dry eye-like ocular surface damage via promotion of inflammation in mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3375–3384. [Google Scholar] [CrossRef] [Green Version]
- You, B.H.; Chae, H.S.; Song, J.; Ko, H.W.; Chin, Y.W.; Choi, Y.H. α-Mangostin ameliorates dextran sulfate sodium-induced colitis through inhibition of NF-kappaB and MAPK pathways. Int. Immunopharmacol. 2017, 49, 212–221. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pochec, E.; Zarawski, M. Anti-Inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int. J. Mol. Sci. 2017, 18, e701. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Hernandez-Chirlaque, C.; Gamez-Belmonte, R.; Drago, S.R.; Sanchez de Medina, F.; Martinez-Augustin, O. Molecular action mechanism of anti-inflammatory hydrolysates obtained from brewers’ spent grain. J. Sci. Food Agric. 2020, 100, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, X.H.; Fu, Y.; Lametsch, R. Transglutaminase-mediated caseinate oligochitosan glycation enhances the effect of caseinate hydrolysate to ameliorate the LPS-induced damage on the intestinal barrier function in IEC-6 cells. J. Agric. Food Chem. 2021, 69, 8787–8796. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elson, L.A.; Morgan, W.T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem. J. 1933, 27, 1824–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fu, Y.; Zhao, X.H.; Lametsch, R. Glycation sites and bioactivity of lactose-glycated caseinate hydrolysate in lipopolysaccharide-injured IEC-6 cells. J. Dairy Sci. 2021, 104, 1351–1363. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Verma, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, e109003. [Google Scholar] [CrossRef]
- Yang, R.; Zuo, P.; Zhang, M.; Meng, D.; Wang, B.; Zhen, T. Transglutaminase induced oligochitosan glycosylation of ferritin as a novel nanocarrier for food bioactive molecules. Food Hydrocolloids 2019, 94, 500–509. [Google Scholar] [CrossRef]
- Hrynets, Y.; Ndagijimana, M.; Betti, M. Transglutaminase-catalyzed glycosylation of natural actomyosin (NAM) using glucosamine as amine donor: Functionality and gel microstructure. Food Hydrocolloids 2014, 36, 26–36. [Google Scholar] [CrossRef]
- Zhan, J.; Li, G.; Tan, B. Optimization of hemoglobin chitosan glycosylation conditions and structural characteristics and functions of glycosylated hemoglobin after an in vitro digestion. J. Aquat. Food Prod. Technol. 2021, 30, 794–805. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Ruminant Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Rahimi, M.; Ghaffari, S.M.; Salami, M.; Mousavy, S.J.; Niasari-Naslaji, A.; Jahanbani, R.; Yousefinejad, S.; Khalesi, M.; Moosavi-Movahedi, A.A. ACE-inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Sci. Technol. 2016, 96, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.H.; Kim, J.Y.; Kang, Y.I.; Woo, H.J.; Lee, H.J. Anticancer activity of hydrophobic peptides from soy proteins. BioFactors 2000, 12, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigon, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef]
- Ndiaye, F.; Vuong, T.; Duarte, J.; Aluko, R.E.; Matar, C. Anti-oxidant, anti-inflammatory and immunomodulating properties of an enzymatic protein hydrolysate from yellow field pea seeds. Eur. J. Nutr. 2012, 51, 29–37. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Je, J.-Y.; Cho, Y.-S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, D.; Chen, B.; Mao, X. Endotoxin-binding peptides derived from casein glycomacropeptide inhibit lipopolysaccharide-stimulated inflammatory responses via blockade of NF-kappaB activation in macrophages. Nutrients 2015, 7, 3119–3137. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, M.M.; Dauletbaev, N.; Kubow, S.; Mawji, N.; Lands, L.C. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. Br. J. Nutr. 2013, 110, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhao, X.H. Effect of caseinate glycation with oligochitosan and transglutaminase on the intestinal barrier function of the tryptic caseinate digest in IEC-6 cells. Food Funct. 2019, 10, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, Q.; Zhao, X.H.; Wang, L. The impact of caseinate oligochitosan-glycation by transglutaminase on amino acid compositions and immune-promoting activity in BALB/c mice of the tryptic caseinate hydrolysate. Food Chem. 2021, 350, e129302. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chang, L.; Chang, H.M.; Chang, F. Intestinal and extraintestinal cancers associated with inflammatory bowel disease. Clin. Colorectal Canc. 2018, 17, e29–e37. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-kappaB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef]
- Soleimani, A.; Rahmani, F.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of the NF-kappaB signaling pathway in the pathogenesis of colorectal cancer. Gene 2020, 726, e144132. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Kimatu, B.M.; Yang, W.; Pei, F.; Zhao, L.; Du, H.; Su, A.; Hu, Q.; Xiao, H. Preparation of newly identified polysaccharide from Pleurotus eryngii and its anti-inflammation activities potential. J. Food Sci. 2020, 85, 2822–2831. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Wang, L.; Zhang, Q.; Zhao, X.-H.; Shi, J. Casein Oligochitosan-Glycation by Transglutaminase Enhances the Anti-Inflammatory Potential of Casein Hydrolysates to the Lipopolysaccharide-Stimulated IEC-6 Cells. Nutrients 2022, 14, 686. https://doi.org/10.3390/nu14030686
Chen N, Wang L, Zhang Q, Zhao X-H, Shi J. Casein Oligochitosan-Glycation by Transglutaminase Enhances the Anti-Inflammatory Potential of Casein Hydrolysates to the Lipopolysaccharide-Stimulated IEC-6 Cells. Nutrients. 2022; 14(3):686. https://doi.org/10.3390/nu14030686
Chicago/Turabian StyleChen, Na, Li Wang, Qiang Zhang, Xin-Huai Zhao, and Jia Shi. 2022. "Casein Oligochitosan-Glycation by Transglutaminase Enhances the Anti-Inflammatory Potential of Casein Hydrolysates to the Lipopolysaccharide-Stimulated IEC-6 Cells" Nutrients 14, no. 3: 686. https://doi.org/10.3390/nu14030686
APA StyleChen, N., Wang, L., Zhang, Q., Zhao, X.-H., & Shi, J. (2022). Casein Oligochitosan-Glycation by Transglutaminase Enhances the Anti-Inflammatory Potential of Casein Hydrolysates to the Lipopolysaccharide-Stimulated IEC-6 Cells. Nutrients, 14(3), 686. https://doi.org/10.3390/nu14030686





