Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Intervention and Materials
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
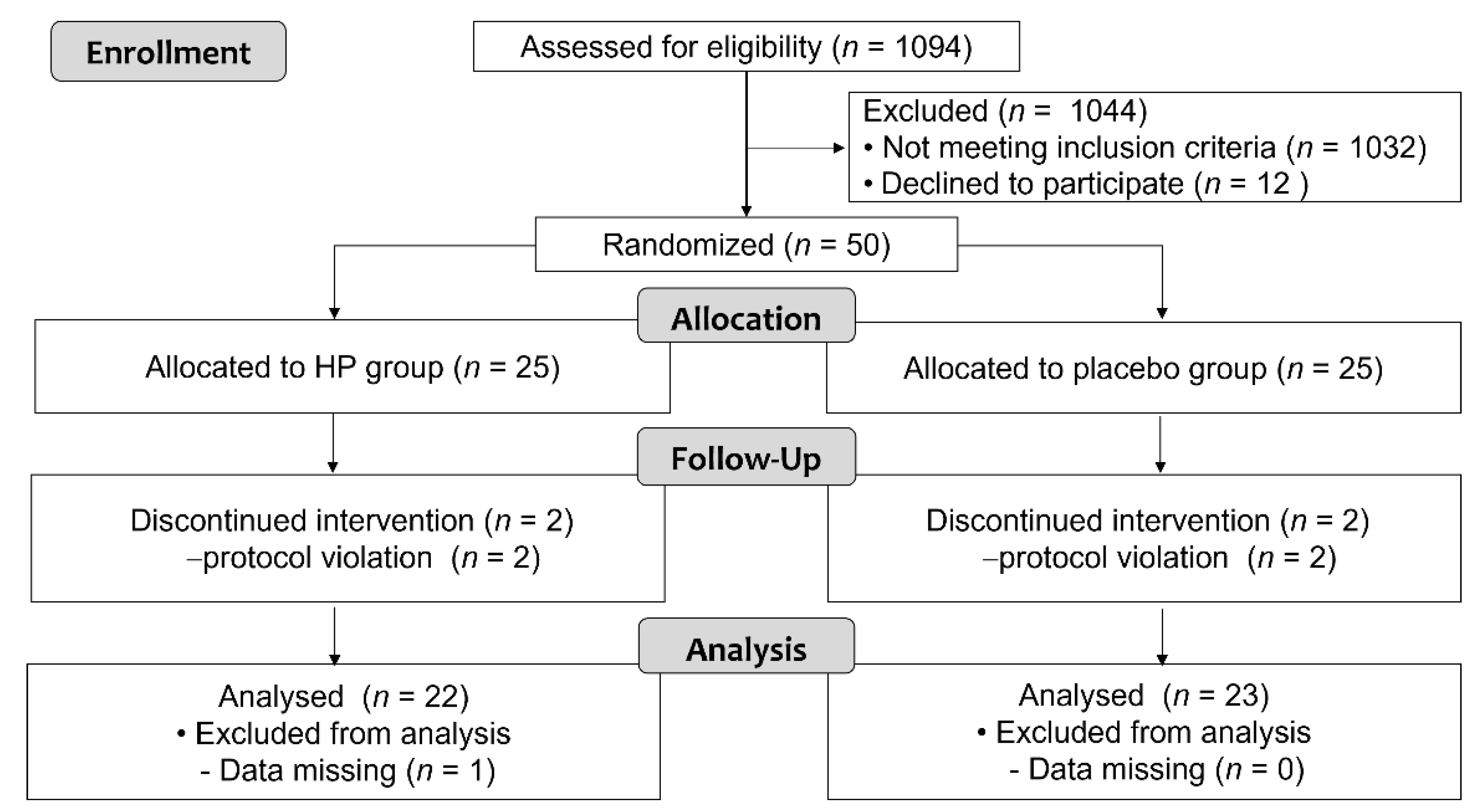
3.1. Study Participants
3.2. Primary Outcomes
3.3. Secondary Outcomes and Others
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Scherbakov, N.; Sandek, A.; Doehner, W. Stroke-related sarcopenia: Specific characteristics. J. Am. Med. Dir. Assoc. 2015, 16, 272–276. [Google Scholar] [CrossRef]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke induced Sarcopenia: Muscle wasting and disability after stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Dobrovolny, C.L.; Smith, G.V.; Silver, K.H.; Macko, R.F. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch. Phys. Med. Rehabil. 2002, 83, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [Green Version]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr. Gerontol. Int. 2019, 19, 429–437. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Elhakeem, A.; Hartley, A.; Luo, Y.; Goertzen, A.L.; Hannam, K.; Clark, E.M.; Leslie, W.D.; Tobias, J.H. Lean mass and lower limb muscle function in relation to hip strength, geometry and fracture risk indices in community-dwelling older women. Osteoporos. Int. 2019, 30, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Batsis, J.A.; Mackenzie, T.A.; Jones, J.D.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 National Health and Nutrition Examination Survey. Clin. Nutr. 2016, 35, 1472–1483. [Google Scholar] [CrossRef]
- Scherbakov, N.; Dirnagl, U.; Doehner, W. Body weight after stroke: Lessons from the obesity paradox. Stroke 2011, 42, 3646–3650. [Google Scholar] [CrossRef] [Green Version]
- Hama, S.; Kitaoka, T.; Shigenobu, M.; Watanabe, A.; Imura, I.; Seno, H.; Tominaga, A.; Arita, K.; Kurisu, K. Malnutrition and nonthyroidal illness syndrome after stroke. Metab. Clin. Exp. 2005, 54, 699–704. [Google Scholar] [CrossRef]
- Lieber, A.C.; Hong, E.; Putrino, D.; Nistal, D.A.; Pan, J.S.; Kellner, C.P. Nutrition, Energy Expenditure, Dysphagia, and Self-Efficacy in Stroke Rehabilitation: A Review of the Literature. Brain Sci. 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-D.; Tsauo, J.-Y.; Wu, Y.-T.; Cheng, C.-P.; Chen, H.-C.; Huang, Y.-C.; Chen, H.-C.; Liou, T.-H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Tokuda, Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr. Gerontol. Int. 2018, 18, 1398–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A review on its effects on muscle strength, the risk of fall, and frailty. BioMed Res. Int. 2015, 2015, 953241. [Google Scholar] [CrossRef] [Green Version]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; von Berens, Å.; Melin, M.; Cederholm, T.; Gustafsson, T.; et al. Nutritional Supplementation with Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 73, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, M.; Wakabayashi, H.; Sakata, K.; Domen, K. The Effect of Branched Chain Amino Acids-Enriched Nutritional Supplements on Activities of Daily Living and Muscle Mass in Inpatients with Gait Impairments: A Randomized Controlled Trial. J. Nutr. Health Aging 2019, 23, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometriv study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Granger, C.V.; Hamilton, B.B. The Uniform Data System for Medical Rehabilitation report of first admissions for 1992. Am. J. Phys. Med. Rehabil. 1994, 73, 51–55. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: “Walking speed: The sixth vital sign”. J. Geriatr. Phys. Ther. 2009, 32, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Hamarsland, H.; Nordengen, A.L.; Nyvik Aas, S.; Holte, K.; Garthe, I.; Paulsen, G.; Cotter, M.; Børsheim, E.; Benestad, H.B.; Raastad, T. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [Green Version]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone, Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight. Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Momosaki, R.; Abo, M.; Urashima, M. Vitamin D Supplementation and Post-Stroke Rehabilitation: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.E.; Alexander, D.D.; Perez, V. Effects of whey protein and resistance exercise on body composition: A meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2014, 33, 163–175. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, W.-C.; Chiu, C.-C.; Chang, Y.-K.; Huang, C.-C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauriainen, E.; Storvik, M.; Finckenberg, P.; Merasto, S.; Martonen, E.; Pilvi, T.K.; Korpela, R.; Mervaala, E.M. Skeletal muscle gene expression profile is modified by dietary protein source and calcium during energy restriction. J. Nutr. Nutr. 2011, 4, 49–62. [Google Scholar] [CrossRef]
- Chanet, A.; Salles, J.; Guillet, C.; Giraudet, C.; Berry, A.; Patrac, V.; Domingues-Faria, C.; Tagliaferri, C.; Bouton, K.; Bertrand-Michel, J.; et al. Vitamin D supplementation restores the blunted muscle protein synthesis response in deficient old rats through an impact on ectopic fat deposition. J. Nutr. Biochem. 2017, 46, 30–38. [Google Scholar] [CrossRef]
- Thomas, M.K.; Lloyd-Jones, D.M.; Thadhani, R.I.; Shaw, A.C.; Deraska, D.J.; Kitch, B.T.; Vamvakas, E.C.; Dick, I.M.; Prince, R.L.; Finkelstein, J.S. Hypovitaminosis D in Medical Inpatients. N. Engl. J. Med. 1998, 338, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Pan, A.; Hu, F.B.; Manson, J.E.; Rexrode, K.M. 25-Hydroxyvitamin D levels and the risk of stroke: A prospective study and meta-analysis. Stroke 2012, 43, 1470–1477. [Google Scholar] [CrossRef]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.R.; Houshiarrad, A.; Gharavi, A.; Kalayi, A.; Shariatzadeh, N.; Zahedirad, M.; Khalaji, N.; et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: A randomized double-blind clinical trial. BMC Med. 2011, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.S.; Buscemi, A.; Forrester, L.; Hafer-Macko, C.E.; Ivey, F.M. Atrophy and intramuscular fat in specific muscles of the thigh: Associated weakness and hyperinsulinemia in stroke survivors. Neurorehabilit. Neural Repair 2011, 25, 865–872. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Bodreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
| HP Group | Placebo Group | p Value | |
|---|---|---|---|
| Age (years) | 64.2 ± 8.9 | 61.3 ± 11.5 | 0.354 |
| Gender (Male/Female) | 15/7 | 15/8 | 0.833 |
| Bodyweight (kg) | 61.8 ± 10.3 | 58.9 ± 12.4 | 0.405 |
| BMI (kg/m2) | 22.4 ± 2.8 | 22.1 ± 3.0 | 0.732 |
| Types of stroke | |||
| (Ischemic/Hemorrhagic) | 9/16 | 9/16 | 1.000 |
| Time from onset to rehabilitation admission | |||
| (days) | 29.0 ± 11.7 | 33.5 ± 12.1 | 0.192 |
| Complications | |||
| (No. of hypertension) | 22 | 21 | 1.000 |
| (No. of diabetes mellitus) | 3 | 1 | 0.609 |
| (No. of hyperlipidemia) | 5 | 5 | 1.000 |
| HP Group | Placebo Group | p Value | |
|---|---|---|---|
| Dietary consumption rates | |||
| Supplemental jellies (%) | 96.3 ± 3.1 | 95.6 ± 4.1 | 0.751 |
| Hospital meal (point) | 9.0 ± 1.5 | 9.6 ± 0.6 | 0.089 |
| Physical therapy (min./day) | 70 ± 6 | 68 ± 8 | 0.694 |
| HP Group | Placebo Group | |||
|---|---|---|---|---|
| 0 Week | Endpoint | 0 Week | Endpoint | |
| SMI (kg/m2) | 4.70 ± 0.95 (22) | 4.48 ± 0.58 (22) | 4.65 ± 0.74 (23) | 4.54 ± 0.66 (23) |
| CSA (cm2) | ||||
| Total muscle area | ||||
| Paretic side | 87.0 ± 20.3 (22) | 88.5 ± 20.5 (22) | 90.3 ± 22.7 (23) | 94.1 ± 27.1 (22) |
| Non-paretic side | 99.8 ± 22.8 (22) | 103.9 ± 20.7 (22) | 104.8 ± 26.2 (23) | 111.3 ± 26.7 (22) |
| Normal muscle area | ||||
| Paretic side | 62.7 ± 16.0 (22) | 65.7 ± 16.7 (22) | 66.5 ± 17.2 (23) | 68.8 ± 20.5 (22) |
| Non-paretic side | 74.5 ± 17.6 (22) | 81.1 ± 16.6 (22) | 80.1 ± 19.4 (23) | 85.3 ± 19.7 (22) |
| Muscle area with fat infiltration | ||||
| Paretic side | 24.3 ± 7.0 (22) | 22.8 ± 6.6 (22) # | 23.8 ± 7.7 (23) | 25.3 ± 8.2 (22) # |
| Non-paretic side | 25.2 ± 7.7 (22) | 22.9 ± 6.8 (22) # | 24.7 ± 8.8 (23) | 26.0 ± 8.9 (22) # |
| HP Group | Placebo Group | |||
|---|---|---|---|---|
| 0 Week | Endpoint | 0 Week | Endpoint | |
| Muscle strength | ||||
| Hand grip (kgf) | ||||
| Paretic side | 4.7 ± 8.1 (22) | 5.5 ± 7.8 (21) | 5.4 ± 8.1 (22) | 8.9 ± 9.6 (20) |
| Non-paretic side | 27.8 ± 9.3 (22) | 28.1 ± 8.3 (20) | 26.2 ± 10.4 (22) | 28.4 ± 9.8 (20) |
| Isometric knee extensor strength (Nm) | ||||
| Paretic side | 28.2 ± 22.7 (22) | 45.6 ± 37.9 (21) | 29.3 ± 23.9 (20) | 49.3 ± 40.8 (19) |
| Non-paretic side | 49.3 ± 31.3 (22) | 65.5 ± 42.0 (21) | 53.0 ± 38.0 (20) | 72.5 ± 43.8 (19) |
| Leg extensor torque (N) | ||||
| Paretic side | 56.9 ± 63.5 (21) | 90.3 ± 63.1 (22) | 74.3 ± 72.3 (22) | 134.3 ± 92.6 (19) |
| Non-paretic side | 129.2 ± 59.6 (21) | 150.3 ± 70.7 (22) | 155.5 ± 81.3 (22) | 192.8 ± 75.2 (19) |
| ADL | ||||
| FIM-Motor | 49.2 ± 15.9 (22) | 73.1 ± 14.2 (22) | 49.8 ± 15.0 (23) | 74.4 ± 18.3 (23) |
| Physical function | ||||
| 10-m walk test (m/s) | ||||
| Comfortable | 0.31 ± 0.28 (22) | 0.60 ± 0.38 (21) | 0.30 ± 0.30 (22) | 0.69 ± 0.38 (21) |
| Maximum | 0.38 ± 0.44 (22) | 0.76 ± 0.53 (21) | 0.39 ± 0.42 (21) | 0.90 ± 0.55 (20) |
| Time Up and Go (s) | ||||
| Paretic side | 102.5 ± 128.7 (22) | 28.2 ± 27.0 (21) | 98.7 ± 125.4 (21) | 20.7 ± 15.2 (20) |
| Non-paretic side | 106.6 ± 129.7 (22) | 28.1 ± 26.6 (21) | 99.7 ± 126.5 (21) | 21.4 ± 16.8 (20) |
| 30-s Chair test (n) | 7.4 ± 4.4 (22) | 11.2 ± 7.6 (22) | 8.3 ± 4.2 (21) | 13.5 ± 5.8 (18) |
| Nutritional indicators and others | ||||
| Alb (g/dL) | 3.8 ± 0.3 (22) | 4.0 ± 0.3 (22) | 3.9 ± 0.3 (23) | 4.1 ± 0.4 (23) |
| pre-Alb (mg/dL) | 25.3 ± 6.6 (22) | 25.6 ± 5.5 (22) | 28.6 ± 7.3 (23) | 29.3 ± 7.9 (22) |
| IGF-1 (ng/dL) | 125.3 ± 44.5 (22) | 131.2 ± 50.2 (22) | 154.1 ± 39.8 (23) | 147.5 ± 41.4 (22) |
| 25(OH)D (ng/mL) | 15.2 ± 5.1 (22) | 25.2 ± 4.7 (22) # | 17.0 ± 7.0 (23) | 17.2 ± 5.9 (23) # |
| REE (kcal/kgBW/day) | 22.5 ± 3.4 (22) | 26.2 ± 4.6 (22) # | 23.5 ± 4.5 (23) | 23.1 ± 3.3 (22) # |
| HP Group | Placebo Group | |||
|---|---|---|---|---|
| 0 Week | Endpoint | 0 Week | Endpoint | |
| BMI (kg/m2) | 22.4 ± 2.8 (25) | 21.5 ± 2.3 (24) | 21.9 ± 3.1 (25) | 21.7 ± 3.0 (25) |
| Serum states | ||||
| TG (mg/dL) | 110.6 ± 35.8 (25) | 92.8 ± 28.0 (24) | 124.7 ± 54.8 (25) | 123.9 ± 85.9 (25) |
| HbA1c (%) | 5.7 ± 0.6 (25) | 5.6 ± 0.4 (24) # | 5.7 ± 0.4 (25) | 5.4 ± 0.3 (25) # |
| BUN (mg/dL) | 11.7 ± 2.9 (25) | 15.5 ± 3.9 (24) # | 11.1 ± 2.7 (25) | 11.6 ± 3.0 (25) # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honaga, K.; Mori, N.; Akimoto, T.; Tsujikawa, M.; Kawakami, M.; Okamoto, T.; Sakata, Y.; Hamano, H.; Takeda, Y.; Kondo, K. Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial. Nutrients 2022, 14, 685. https://doi.org/10.3390/nu14030685
Honaga K, Mori N, Akimoto T, Tsujikawa M, Kawakami M, Okamoto T, Sakata Y, Hamano H, Takeda Y, Kondo K. Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial. Nutrients. 2022; 14(3):685. https://doi.org/10.3390/nu14030685
Chicago/Turabian StyleHonaga, Kaoru, Naoki Mori, Tomonori Akimoto, Masahiro Tsujikawa, Michiyuki Kawakami, Tomoyuki Okamoto, Yasuyuki Sakata, Hirokazu Hamano, Yasuhiro Takeda, and Kunitsugu Kondo. 2022. "Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial" Nutrients 14, no. 3: 685. https://doi.org/10.3390/nu14030685
APA StyleHonaga, K., Mori, N., Akimoto, T., Tsujikawa, M., Kawakami, M., Okamoto, T., Sakata, Y., Hamano, H., Takeda, Y., & Kondo, K. (2022). Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial. Nutrients, 14(3), 685. https://doi.org/10.3390/nu14030685






