Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury
Abstract
1. Cadmium: The Beginning of History in Humans
2. Nutraceuticals: The New Frontier of Nutrition
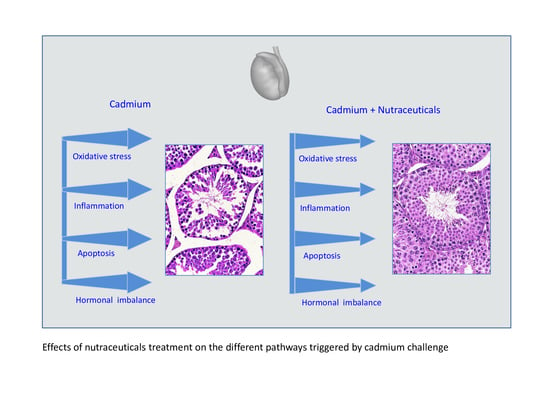
3. Cadmium and Toxicity of Testis: Potential Molecular Targets of Nutraceuticals
4. Cadmium-Induced Oxidative Stress Causes Testicular Injury and Represents an Attractive Molecular Target for Nutraceuticals
5. Cadmium Toxicity: Inflammation Is a Significant Molecular Pathway Targeted by Nutraceuticals
6. Nutraceuticals and Cadmium-Induced Apoptosis in Testis
7. Positive Effects of Nutraceuticals against Cadmium-Induced Testicular Injury on Blood–Testis Barrier Changes and Hormonal Imbalance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell. Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Cadmium as a testicular toxicant: A Review. J. Appl. Toxicol. 2021, 41, 105–117. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Manfo, F.P.; Moundipa, P.F.; Déchaud, H.; Tchana, A.N.; Nantia, E.A.; Zabot, M.T.; Pugeat, M. Effect of agropesticides use on male reproductive function: A study on farmers in Djutitsa (Cameroon). Environ. Toxicol. 2012, 27, 423–432. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Lentini, P.; Zanoli, L.; Granata, A.; Signorelli, S.S.; Castellino, P.; Dell’Aquila, R. Kidney and heavy metals—The role of environmental exposure (Review). Mol. Med. Rep. 2017, 15, 3413–3419. [Google Scholar] [CrossRef]
- Hariri, A.; Mohamad Noor, N.; Paiman, N.A.; Ahmad Zaidi, A.M.; Zainal Bakri, S.F. Heavy metals found in the breathing zone, toenails and lung function of welders working in an air-conditioned welding workplace. Int. J. Occup. Saf. Ergon. 2018, 24, 646–651. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]
- Cole, P.; Rodu, B. Epidemiologic studies of chrome and cancer mortality: A series of meta-analyses. Regul. Toxicol. Pharmacol. 2005, 43, 225–231. [Google Scholar] [CrossRef]
- Tapio, S.; Grosche, B. Arsenic in the aetiology of cancer. Mutat. Res. 2006, 612, 215–246. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Lee, J.Y.; Banno, H.; Imai, S.; Tokumoto, M.; Hasegawa, T.; Seko, Y.; Nagase, H.; Satoh, M. Cadmium induces iron deficiency anemia through the suppression of iron transport in the duodenum. Toxicol. Lett. 2020, 332, 130–139. [Google Scholar] [CrossRef]
- Piadé, J.; Jaccard, G.; Dolka, C.; Belushkin, M.; Wajrock, S. Differences in cadmium transfer from tobacco to cigarette smoke, compared to arsenic or lead. Toxicol. Rep. 2014, 2, 12–26. [Google Scholar] [CrossRef]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public Health 2017, 14, 1154. [Google Scholar] [CrossRef]
- Rinaldi, M.; Micali, A.; Marini, H.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K. Toxicology of cadmium and its damage to mammalian organs. Met. Ions Life Sci. 2013, 11, 415–490. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. The influence of calcium content in diet on cumulation and toxicity of cadmium in the organism. Arch. Toxicol. 1998, 72, 63–73. [Google Scholar] [CrossRef]
- Koch, W.; Karim, M.R.; Marzec, Z.; Miyataka, H.; Himeno, S.; Asakawa, Y. Dietary intake of metals by the young adult population of Eastern Poland: Results from a market basket study. J. Trace Elem. Med. Biol. 2016, 35, 36–42. [Google Scholar] [CrossRef]
- Yu, G.; Zheng, W.; Wang, W.; Dai, F.; Zhang, Z.; Yuan, Y.; Wang, Q. Health risk assessment of Chinese consumers to Cadmium via dietary intake. J. Trace Elem. Med. Biol. 2017, 44, 137–145. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 1, 2. [Google Scholar] [CrossRef]
- Interdonato, M.; Bitto, A.; Pizzino, G.; Irrera, N.; Pallio, G.; Mecchio, A.; Cuspilici, A.; Minutoli, L.; Altavilla, D.; Squadrito, F. Levels of heavy metals in adolescents living in the industrialised area of Milazzo-Valle del Mela (northern Sicily). J. Environ. Public. Health 2014, 2014, 326845. [Google Scholar] [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bull. Environ. Contam. Toxicol. 2012, 88, 78–83. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant--cadmium(II) with human serum albumin. Environ. Sci. Pollut. Res. Int. 2014, 21, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Singh, J.; Sinha, S. Classification, Regulatory Acts and Applications of Nutraceuticals for Health. Int. J. Pharma. Biosci. 2012, 2, 177–187. [Google Scholar]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Pizent, A.; Tariba, B.; Živković, T. Reproductive toxicity of metals in men. Arh. Hig. Rada. Toksikol. 2012, 63 (Suppl. 1), 35–46. [Google Scholar] [CrossRef]
- Benoff, S.; Hauser, R.; Marmar, J.L.; Hurley, I.R.; Napolitano, B.; Centola, G.M. Cadmium concentrations in blood and seminal plasma: Correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol. Med. 2009, 15, 248–262. [Google Scholar] [CrossRef]
- Telisman, S.; Cvitković, P.; Jurasović, J.; Pizent, A.; Gavella, M.; Rocić, B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ. Health Perspect. 2000, 108, 45–53. [Google Scholar] [CrossRef]
- Monsefi, M.; Alaee, S.; Moradshahi, A.; Rohani, L. Cadmium-induced infertility in male mice. Environ. Toxicol. 2010, 25, 94–102. [Google Scholar] [CrossRef]
- Yari, A.; Asadi, M.H.; Bahadoran, H.; Dashtnavard, H.; Imani, H.; Naghii, M.R. Cadmium toxicity in spermatogenesis and protective effects of L-carnitine in adult male rats. Biol. Trace Elem. Res. 2010, 137, 216–225. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Changes in the peritubular tissue of rat testis after cadmium treatment. Biol. Trace Elem. Res. 2010, 134, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Micali, A.; Pisani, A.; Puzzolo, D.; Bitto, A.; Rinaldi, M.; Pizzino, G.; Irrera, N.; Galfo, F.; Arena, S.; et al. Flavocoxid Protects Against Cadmium-Induced Disruption of the Blood–Testis Barrier and Improves Testicular Damage and Germ Cell Impairment in Mice. Toxicol. Sci. 2015, 148, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Micali, A.; Pallio, G.; Vita, R.; Malta, C.; Puzzolo, D.; Irrera, N.; Squadrito, F.; Altavilla, D.; Minutoli, L. Effects of Myo-inositol Alone and in Combination with Seleno-Lmethionine on Cadmium-Induced Testicular Damage in Mice. Curr. Mol. Pharmacol. 2019, 12, 311–323. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Micali, A.; Marini, H.R.; Freni, J.; Santoro, G.; Puzzolo, D.; Squadrito, F.; Pallio, G.; Navarra, M.; Cirmi, S.; et al. A Flavonoid-Rich Extract from Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, Shows Protective Effects in a Murine Model of Cadmium-Induced Testicular Injury. Pharmaceuticals 2021, 14, 386. [Google Scholar] [CrossRef]
- IARC. Cadmium and cadmium compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 119–237. [Google Scholar]
- Zhu, Q.; Li, X.; Ge, R.S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Orisakwe, O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health C Toxicol. Carcinog. 2020, 38, 204–244. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Lopes, T.; Almeida, T.; Pereira Mde, L.; Santos, C. Cadmium-induced genetic instability in mice testis. Hum. Exp. Toxicol. 2012, 31, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Amanpour, P.; Khodarahmi, P.; Salehipour, M. Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 349–358. [Google Scholar] [CrossRef]
- Yu, W.; Xu, Z.; Gao, Q.; Xu, Y.; Wang, B.; Dai, Y. Protective role of wogonin against cadmium induced testicular toxicity: Involvement of antioxidant, anti-inflammatory and anti-apoptotic pathways. Life Sci. 2020, 258, 118192. [Google Scholar] [CrossRef]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef]
- Interdonato, M.; Pizzino, G.; Bitto, A.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; De Luca, F.; Santamaria, A.; et al. Cadmium delays puberty onset and testis growth in adolescents. Clin. Endocrinol. (Oxf) 2015, 83, 357–362. [Google Scholar] [CrossRef]
- Mishra, R.K.; Jain, A.; Singh, S.K. Profertility effects of Shilajit on cadmium-induced infertility in male mice. Andrologia 2018, 50, e13064. [Google Scholar] [CrossRef]
- Fouad, A.A.; Qureshi, H.A.; Al-Sultan, A.I.; Yacoubi, M.T.; Ali, A.A. Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology 2009, 257, 153–160. [Google Scholar] [CrossRef]
- de Souza Predes, F.; Diamante, M.A.; Dolder, H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010, 91, 125–131. [Google Scholar] [CrossRef]
- Miura, N.; Yanagiba, Y.; Ohtani, K.; Mita, M.; Togawa, M.; Hasegawa, T. Diurnal variation of cadmium-induced mortality in mice. J. Toxicol. Sci. 2012, 37, 191–196. [Google Scholar] [CrossRef]
- Ohtani, K.; Yanagiba, Y.; Ashimori, A.; Takeuchi, A.; Takada, N.; Togawa, M.; Hasegawa, T.; Ikeda, M.; Miura, N. Influence of injection timing on severity of cadmium-induced testicular toxicity in mice. J. Toxicol. Sci. 2013, 38, 145–150. [Google Scholar] [CrossRef][Green Version]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019, 39, BSR20180515. [Google Scholar] [CrossRef]
- Aktas, C.; Kanter, M.; Erboga, M.; Ozturk, S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol. Ind. Health 2012, 28, 122–130. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod. Biol. Endocrinol. 2016, 14, 42. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, C.; Song, Z.; Chen, M.; Ding, L.; Liang, X.; Bi, X.; Li, Z.; Li, P.; Zheng, W. Heavy Metal in Rice and Vegetable and Human Exposure near a Large Pb/Zn Smelter in Central China. Int. J. Environ. Res. Public Health 2021, 18, 12631. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Efrat, M.; Stein, A.; Pinkas, H.; Unger, R.; Birk, R. Dietary patterns are positively associated with semen quality. Fertil. Steril. 2018, 109, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Oxidative stress and the etiology of male infertility. J. Assist. Reprod. Genet. 2016, 33, 1691–1692. [Google Scholar] [CrossRef]
- Torres-Arce, E.; Vizmanos, B.; Babio, N.; Marquez-Sandoval, F.; Salas-Huetos, A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology 2021, 10, 241. [Google Scholar] [CrossRef]
- Santoro, M.; Aquila, S.; Russo, G. Sperm performance in oligoasthenoteratozoospermic patients is induced by a nutraceuticals mix, containing mainly myo-inositol. Syst. Biol. Reprod. Med. 2021, 67, 50–63. [Google Scholar] [CrossRef]
- Delbarba, A.; Arrighi, N.; Facondo, P.; Cappelli, C.; Ferlin, A. Positive effect of nutraceuticals on sperm DNA damage in selected infertile patients with idiopathic high sperm DNA fragmentation. Minerva Endocrinol. 2020, 45, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Ranganathan, P.; Rao, K.A.; Sudan, J.J.; Balasundaram, S. Cadmium effects on sperm morphology and semenogelin with relates to increased ROS in infertile smokers: An in vitro and in silico approach. Reprod. Biol. 2018, 18, 189–197. [Google Scholar] [CrossRef]
- Amara, S.; Abdelmelek, H.; Garrel, C.; Guiraud, P.; Douki, T.; Ravanat, J.L.; Favier, A.; Sakly, M.; Ben Rhouma, K. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J. Reprod. Dev. 2008, 54, 129–134. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Han, C.; Zhu, Y.; Yang, Z.; Fu, S.; Zhang, W.; Liu, C. Protective effect of Polygonatum sibiricum against cadmium-induced testicular injury in mice through inhibiting oxidative stress and mitochondria-mediated apoptosis. J. Ethnopharmacol. 2020, 261, 113060. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–223. [Google Scholar] [CrossRef]
- Papanas, N.; Ziegler, D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin. Pharmacother. 2014, 15, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, E.; Prahalathan, C.; Sudharsan, P.T.; Varalakshmi, P. Chemoprotective effect of lipoic acid against cyclophosphamide- induced changes in the rat sperm. Toxicology 2006, 217, 71–78. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Nassar, N.N. Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. J. Biochem. Mol. Toxicol. 2011, 25, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ameer, B.; Weintraub, R.A.; Johnson, J.V.; Yost, R.A.; Rouseff, R.L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmacol. Ther. 1996, 60, 34–40. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.J.; Choi, J.S.; Chung, H.Y. Hesperetin: A potent antioxidant against peroxynitrite. Free Radic. Res. 2004, 38, 761–769. [Google Scholar] [CrossRef]
- Shagirtha, K.; Pari, L. Hesperitin, a citrus flavonone, protects potentially cadmium induced oxidative testicular dysfunctions in rats. Ecotoxicol. Environ. Saf. 2011, 74, 2105–2111. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Egido, J.; Arevalo, M.A.; Lopeznovoa, J.M. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef]
- Altavilla, D.; Minutoli, L.; Polito, F.; Irrera, N.; Arena, S.; Magno, C.; Rinaldi, M.; Burnett, B.P.; Squadrito, F.; Bitto, A. Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br. J. Pharmacol. 2012, 167, 95–108. [Google Scholar] [CrossRef]
- Huang, Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y. Biological properties of baicalein in cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 177–184. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The protective effects of melatonin against oxidative stress and inflammation induced by acute cadmium exposure in mice testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Banni, M.; Saïd, L.; Saïd, K.; Kerkeni, A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem. Biol Interact. 2010, 188, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohé, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, F.G.; Tang, S.S.; Handy, D.E.; Loscalzo, J. Regulation of the extracellular antioxidant selenoprotein plasma glutathione peroxidase (GPx-3) in mammalian cells. Mol. Cell. Biochem. 2009, 327, 111–126. [Google Scholar] [CrossRef]
- Ren, X.M.; Wang, G.G.; Xu, D.Q.; Luo, K.; Liu, Y.X.; Zhong, Y.H.; Cai, Y.Q. The protection of selenium on cadmium-induced inhibition of spermatogenesis via activating testosterone synthesis in mice. Food Chem. Toxicol. 2012, 50, 3521–3529. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.H.; Tang, L.; Feng, L.; Zhou, X.Q. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat. Toxicol. 2011, 105, 543–551. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of Myo-Inositol and D-Chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef]
- Nayak, B.; Kondeti, V.K.; Xie, P.; Lin, S.; Viswakarma, N.; Raparia, K.; Kanwar, Y.S. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J. Biol. Chem. 2011, 286, 27594–27611. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Palomba, S.; Laganà, A.S.; Orio, F. Inositols in the Treatment of Insulin-Mediated Diseases. Int. J. Endocrinol. 2016, 2016, 3058393. [Google Scholar] [CrossRef] [PubMed]
- Duéñez-Guzmán, E.A.; Haig, D. The evolution of reproduction-related NLRP genes. J. Mol. Evol. 2014, 78, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, B. Negative regulation of NLRP3 inflammasome signaling. Protein Cell. 2013, 4, 251–258. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Burnett, B.P.; Levy, R.M. 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 2012, 29, 79–98. [Google Scholar] [CrossRef]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother. Res. 2017, 31, 27–39. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective Effect of Bergamot Juice in 6-OHDA-Induced SH-SY5Y Cell Death, an In Vitro Model of Parkinson’s Disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.D.; Cronstein, B.N.; Montesinos, M.C. Adenosine receptor agonists for promotion of dermal wound healing. Biochem. Pharmacol. 2009, 77, 1117–11124. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Desai-Merchant, A.; Cronstein, B.N. Promotion of Wound Healing by an Agonist of Adenosine A2A Receptor Is Dependent on Tissue Plasminogen Activator. Inflammation 2015, 38, 2036–20341. [Google Scholar] [CrossRef]
- Guerrero, A. A2A Adenosine Receptor Agonists and their Potential Therapeutic Applications. An Update. Curr. Med. Chem. 2018, 25, 3597–3612. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Di Stefano, V.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 554–560. [Google Scholar] [CrossRef]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef] [PubMed]
- Angenard, G.; Muczynski, V.; Coffigny, H.; Pairault, C.; Duquenne, C.; Frydman, R.; Habert, R.; Rouiller-Fabre, V.; Livera, G. Cadmium increases human fetal germ cell apoptosis. Environ. Health Perspect. 2010, 118, 331–337. [Google Scholar] [CrossRef]
- Bu, T.; Mi, Y.; Zeng, W.; Zhang, C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat. Rec. 2011, 294, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Findlay, J.K.; Hutt, K.J.; Kerr, J.B. Apoptosis in the germ line. Reproduction 2011, 141, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.L.; Wang, H.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.H.; Xu, D.X. N-acetylcysteine protects against cadmium-induced germ cell apoptosis by inhibiting endoplasmic reticulum stress in testes. Asian J. Androl. 2013, 15, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Uren, R.T.; Kluck, R.M. Probing BAK and BAX Activation and Pore Assembly with Cytochrome c Release, Limited Proteolysis, and Oxidant-Induced Linkage. Methods Mol. Biol. 2019, 1877, 201–216. [Google Scholar] [CrossRef]
- Wang, D.H.; Hu, J.R.; Wang, L.Y.; Hu, Y.J.; Tan, F.Q.; Zhou, H.; Shao, J.Z.; Yang, W.X. The apoptotic function analysis of p53, Apaf1, Caspase3 and Caspase7 during the spermatogenesis of the Chinese fire-bellied newt Cynops orientalis. PLoS ONE 2012, 7, e39920. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Tascioglu, S. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol. Ind. Health 2016, 32, 1486–1494. [Google Scholar] [CrossRef]
- Mitra, S.; Bhattacharyya, S.; Ray, S.; Saha, R.; Ghosh, P.; Rauth, S.; Mandal, S.; Banerjee, S.; Murmu, N. Resveratrol Alleviates Cadmium-Induced Damage and Overexpression of Epidermal Growth Factor Receptor and its Downstream Signaling Proteins in the Reproductive System of Male Swiss Albino Mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 73–90. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Chung, N.P.; Cheng, C.Y. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 2001, 142, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. Ecotoxicol. Environ. Saf. 2019, 182, 109398. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, A. The hypothalamic-pituitary-gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem. Toxicol. 2013, 59, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 109, 1498–1507. [Google Scholar] [CrossRef]
- Russo, G.L.; Siani, A.; Fogliano, V.; Geleijnse, J.M.; Giacco, R.; Giampaoli, S.; Iacoviello, L.; Kromhout, D.; Lionetti, L.; Naska, A.; et al. The Mediterranean diet from past to future: Key concepts from the second "Ancel Keys" International Seminar. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a Lifestyle Change Intervention on Semen Quality in Healthy Young Men Living in Highly Polluted Areas in Italy: The FASt Randomized Controlled Trial. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
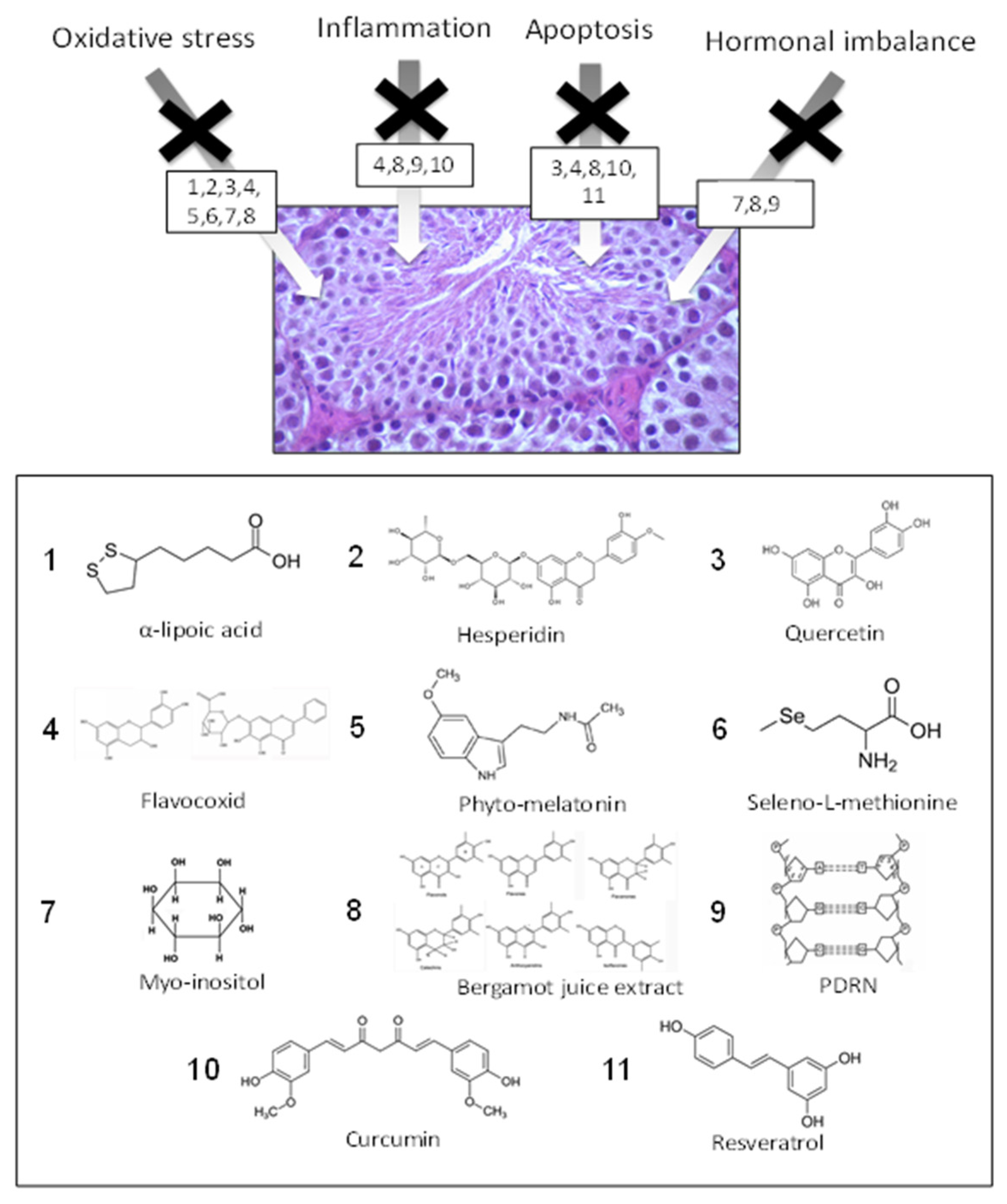
| Compound | Food Source | Mechanism of Action | References |
|---|---|---|---|
| α-lipoic acid | Spinach, broccoli | AO | [81,82,83] |
| Hesperidin | Citrus | AO–AI | [84,86] |
| Quercetin | Red apple, red onion, tomato | AO–AI | [123] |
| Flavocoxid | Cathechin (green tea, dark chocolate) Baicalin (onions) | AO–AI–AA–ABTBD | [44] |
| Phyto-melatonine | Coffe beans, apple, cherry, tomato | AO–AI | [91] |
| Seleno-L-methyonine | Brazilian nuts, potato, fish | AO–AHI | [93,94,96] |
| Myo-inositol | Cereals, citrus, dried plums, cantaloupe melon | AO | [46] |
| Bergamot juice | Citrus bergamia Risso et Poiteau (bergamot) fruits | AO–AI–AA | [47,113] |
| PDRN | Trout | AO–AI–AA–ABTBD | [45] |
| Curcumin | Curcuma longa | AO–AA | [47,63] |
| Resveratrol | Red wine, grape, peanuts, dried fruit | AO–AI–AA–ABTBD | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. https://doi.org/10.3390/nu14030663
Marini HR, Micali A, Squadrito G, Puzzolo D, Freni J, Antonuccio P, Minutoli L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients. 2022; 14(3):663. https://doi.org/10.3390/nu14030663
Chicago/Turabian StyleMarini, Herbert Ryan, Antonio Micali, Giovanni Squadrito, Domenico Puzzolo, José Freni, Pietro Antonuccio, and Letteria Minutoli. 2022. "Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury" Nutrients 14, no. 3: 663. https://doi.org/10.3390/nu14030663
APA StyleMarini, H. R., Micali, A., Squadrito, G., Puzzolo, D., Freni, J., Antonuccio, P., & Minutoli, L. (2022). Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients, 14(3), 663. https://doi.org/10.3390/nu14030663