Associations between Dietary Patterns and Cardiometabolic Risk Factors—A Longitudinal Analysis among High-Risk Individuals for Diabetes in Kerala, India
Abstract
1. Introduction
2. Materials and Methods
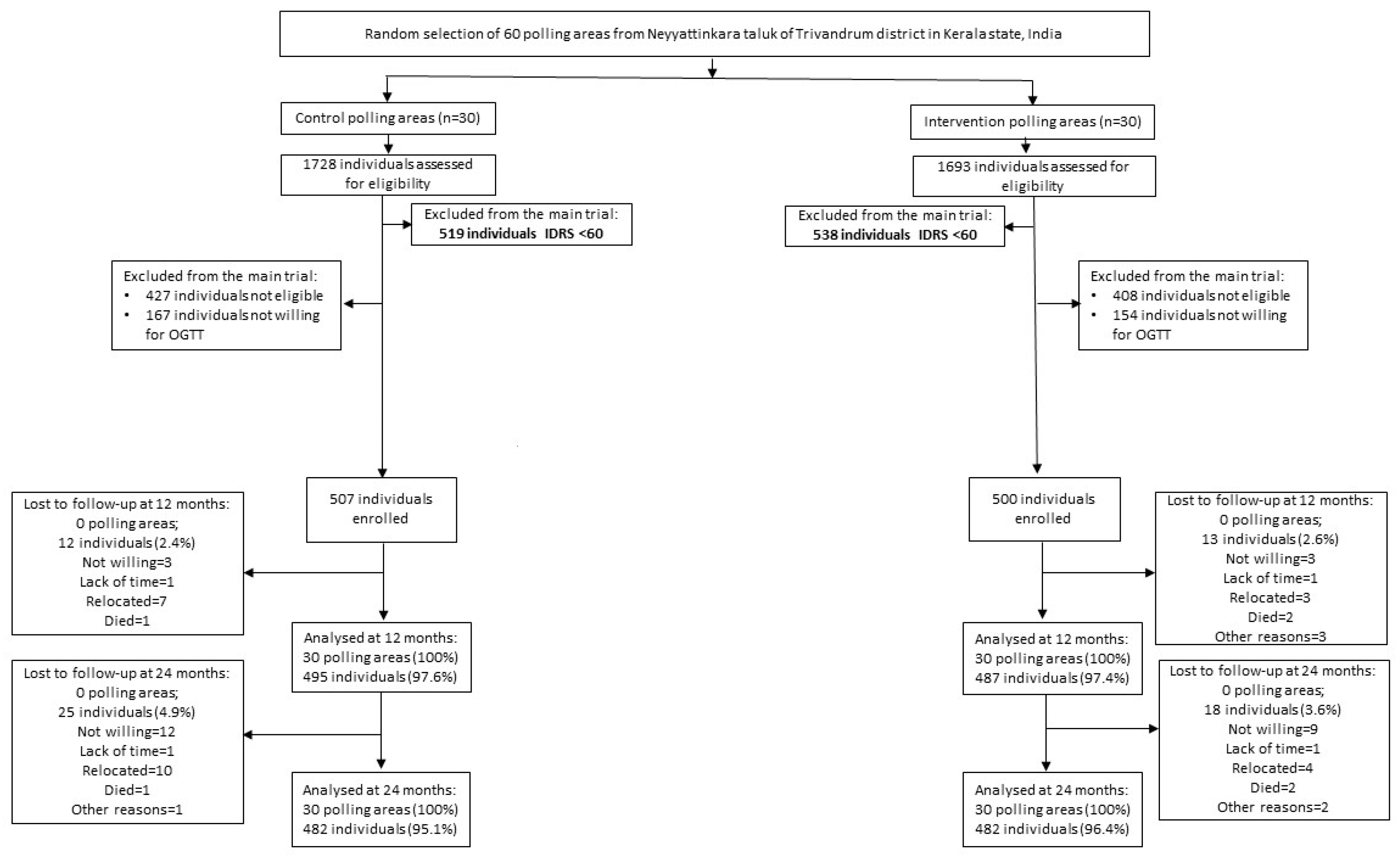
2.1. Study Participants
2.2. Cardiometabolic Factors
2.3. Dietary Measures and Dietary Patterns
2.4. Key Confounding Variables
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Dietary Patterns
3.3. Participants’ Characteristics by Dietary Patterns
3.4. Participants’ Cardiometabolic Risks by Dietary Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Tandon, N.; Anjana, R.M.; Mohan, V.; Kaur, T.; Afshin, A.; Ong, K.; Mukhopadhyay, S.; Thomas, N.; Bhatia, E.; Krishnan, A.; et al. The increasing burden of diabetes and variations among the states of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, e1352–e1362. [Google Scholar] [CrossRef]
- Ahirwar, R.; Mondal, P.R. Prevalence of obesity in India: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Volgman, A.S.; Palaniappan, L.S.; Aggarwal, N.T.; Gupta, M.; Khandelwal, A.; Krishnan, A.V.; Lichtman, J.H.; Mehta, L.S.; Patel, H.N.; Shah, K.S.; et al. Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Diet and Nutrition: Implications to Cardiometabolic Health. J. Cardiol. Cardiovasc. Sci. 2019, 3, 4–9. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Green, R.; Milner, J.; Joy, E.J.M.; Agrawal, S.; Dangour, A.D. Dietary patterns in India: A systematic review. Br. J. Nutr. 2016, 116, 142–148. [Google Scholar] [CrossRef]
- Johns, D.J.; Lindroos, A.; Jebb, S.A.; Sjöström, L.; Carlsson, L.M.S.; Ambrosini, G.L. Dietary patterns, cardiometabolic risk factors, and the incidence of cardiovascular disease in severe obesity. Obesity 2015, 23, 1063–1070. [Google Scholar] [CrossRef]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. Int. Rev. J. 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Daniel, C.R.; Prabhakaran, D.; Kapur, K.; Graubard, B.I.; Devasenapathy, N.; Ramakrishnan, L.; George, P.S.; Shetty, H.; Ferrucci, L.M.; Yurgalevitch, S.; et al. A cross-sectional investigation of regional patterns of diet and cardio-metabolic risk in India. Nutr. J. 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B.; Bowen, L.; Bharathi, A.V.; Vaz, M.; Prabhakaran, D.; Reddy, K.S.; Ben-Shlomo, Y.; Smith, G.D.; Kinra, S.; et al. Dietary patterns in India and their association with obesity and central obesity. Public Health Nutr. 2015, 18, 3031–3041. [Google Scholar] [CrossRef]
- Misra, A.; Singhal, N.; Sivakumar, B.; Bhagat, N.; Jaiswal, A.; Khurana, L. Nutrition transition in India: Secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J. Diabetes 2011, 3, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.H.; Rao, K.S.; Fryatt, R. Lumping and splitting: The health policy agenda in India. Health Policy Plan. 2003, 18, 249–260. [Google Scholar] [CrossRef]
- Sathish, T.; Williams, E.D.; Pasricha, N.; Absetz, P.; Lorgelly, P.; Wolfe, R.; Mathews, E.; Aziz, Z.; Thankappan, K.R.; Zimmet, P.; et al. Cluster randomised controlled trial of a peer-led lifestyle intervention program: Study protocol for the Kerala diabetes prevention program. BMC Public Health 2013, 13, 1035. [Google Scholar] [CrossRef]
- Sathish, T.; Aziz, Z.; Absetz, P.; Thankappan, K.R.; Tapp, R.; Balachandran, S.; Shetty, S.S.; Oldenburg, B. Participant recruitment into a community-based diabetes prevention trial in India: Learnings from the Kerala Diabetes Prevention Program. Contemp. Clin. Trials Commun. 2019, 15, 100382. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Shaw, J.E.; Tapp, R.J.; Wolfe, R.; Thankappan, K.R.; Balachandran, S.; Oldenburg, B. Targeted screening for prediabetes and undiagnosed diabetes in a community setting in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Kannan, S.; Sarma, S.P.; Thankappan, K.R. Screening Performance of Diabetes Risk Scores Among Asians and Whites in Rural Kerala, India. Prev. Chronic Dis. 2013, 10, E37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization Regional Office for South-East Asia. WHO STEPS Survey: Step Towards a Healthier World: Monitoring Noncommunicable Diseases and their Risk Factors; World Health Organization Regional Office for South-East Asia: New Delhi, India, 2018. [Google Scholar]
- National Accreditation Board for Testing and Calibration Laboratories. Available online: http://www.nabl-india.org (accessed on 19 December 2021).
- Misra, A.; Chowbey, P.; Makkar, B.M.; Vikram, N.K.; Wasir, J.S.; Chadha, D.; Joshi, S.R.; Sadikot, S.; Gupta, R.; Gulati, S.; et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J. Assoc. Physicians India 2009, 57, 163–170. [Google Scholar]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; IDF: Brussels, Belgium, 2006. [Google Scholar]
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes. Diabetes Care 2018, 41 (Suppl. S1), S1–S2.
- Soman, C.R.; Shahulhameed, S.; Ramankutty, V.; Vijayakumar, K.; Kunjukrishnapillai, R.; Ajayan, K.; Sajikumar, S. Cohort Profile: The PROLIFE study in Kerala, India. Int. J. Epidemiol. 2009, 40, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.H.; Krishnaveni, G.V.; Veena, S.R.; Guntupalli, A.M.; Margetts, B.M.; Fall, C.H.; Robinson, S.M. Diet patterns are associated with demographic factors and nutritional status in South Indian children. Matern. Child Nutr. 2014, 10, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br. J. Nutr. 2003, 89, 409–418. [Google Scholar] [CrossRef]
- Lin, J.; Fung, T.T.; Hu, F.B.; Curhan, G.C. Association of Dietary Patterns with Albuminuria and Kidney Function Decline in Older White Women: A Subgroup Analysis From the Nurses’ Health Study. Am. J. Kidney Dis. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global Physical Activity Questionnaire (GPAQ): Nine Country Reliability and Validity Study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Lotfaliany, M.; Sathish, T.; Shaw, J.; Thomas, E.; Tapp, R.J.; Kapoor, N.; Thankappan, K.R.; Oldenburg, B. Effects of a lifestyle intervention on cardiovascular risk among high-risk individuals for diabetes in a low- and middle-income setting: Secondary analysis of the Kerala Diabetes Prevention Program. Prev. Med. 2020, 139, 106068. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, K.R.; Sathish, T.; Tapp, R.; Shaw, J.E.; Lotfaliany, M.; Wolfe, R.; Absetz, P.; Mathews, E.; Aziz, Z.; Williams, E.; et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLOS Med. 2018, 15, e1002575. [Google Scholar] [CrossRef]
- Daivadanam, M.; Wahlström, R.; Thankappan, K.R.; Ravindran, T.K.S. Balancing expectations amidst limitations: The dynamics of food decision-making in rural Kerala. BMC Public Health 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z. Dietary Pattern during 1991–2011 and Its Association with Cardio Metabolic Risks in Chinese Adults: The China Health and Nutrition Survey. Nutrients 2017, 9, 1218. [Google Scholar] [CrossRef]
- Mohan, V.; Narasimhan, S.; Nagarajan, L.; Vaidya, R.; Gunasekaran, G.; Rajagopal, G.; Parthasarathy, V.; Unnikrishnan, R.; Anjana, R.M.; Sudha, V. Dietary fat intake and its association with risk of selected components of the metabolic syndrome among rural South Indians. Indian J. Endocrinol. Metab. 2016, 20, 47–54. [Google Scholar] [CrossRef]
- Mohan, V.; Radhika, G.; Vijayalakshmi, P.; Sudha, V. Can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess refined grain (rice) intake? Indian J. Med. Res. 2010, 131, 369–372. [Google Scholar] [PubMed]
- Kapoor, N. Thin Fat Obesity: The Tropical Phenotype of Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., De Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J., Hofland, D., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bosomworth, N.J. Normal-weight central obesity: Unique hazard of the toxic waist. Can. Fam. Physician 2019, 65, 399–408. [Google Scholar]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kong, W.; Zafar, M.I.; Chen, L.-L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Cardiovasc. Diabetol. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Snack-Fruit Pattern (Mean (SD)) | p-Value | Rice-Meat-Refined Wheat Pattern (Mean (SD)) | p-Value | |
|---|---|---|---|---|
| Age | 0.77 | 0.016 | ||
| ≤45 years (n = 463) | −0.0 (0.7) | 0.0 (0.8) | ||
| >45 years (n = 495) | −0.0 (0.7) | −0.1 (0.7) | ||
| Sex | <0.001 | <0.001 | ||
| Male (n = 491) | 0.1 (0.7) | 0.3 (0.8) | ||
| Female (n = 467) | −0.1 (0.6) | −0.4 (0.5) | ||
| Marital status | 0.22 | <0.001 | ||
| Married (n = 910) | −0.0 (0.7) | 0.0 (0.7) | ||
| Separated/divorced/widowed (n = 38) | 0.1 (0.9) | −0.5 (0.7) | ||
| Never married (n = 10) | 0.3 (0.8) | −0.5 (0.4) | ||
| Education | 0.045 | 0.022 | ||
| Up to primary school (n = 241) | −0.1 (0.7) | −0.0 (0.7) | ||
| Secondary school (n = 564) | 0.0 (0.7) | 0.0 (0.7) | ||
| Tertiary and above (n = 153) | 0.0 (0.8) | −0.2 (0.6) | ||
| Occupation | <0.001 | <0.001 | ||
| Skilled/unskilled (n = 683) | 0.1 (0.7) | 0.1 (0.8) | ||
| Homemaker/unemployed/retired (n = 275) | −0.2 (0.6) | −0.4 (0.5) | ||
| Leisure-time physical activity | <0.001 | 0.015 | ||
| Leisure inactive (n = 762) | −0.1 (0.7) | −0.0 (0.7) | ||
| Leisure active (n = 196) | 0.2 (0.7) | 0.1 (0.9) | ||
| Alcohol use | 0.001 | <0.001 | ||
| No (n = 762) | −0.0 (0.7) | −0.2 (0.6) | ||
| Yes (n = 196) | 0.1 (0.7) | 0.5 (0.9) | ||
| Tobacco use | <0.001 | <0.001 | ||
| No (n = 777) | −0.0 (0.7) | −0.1 (0.6) | ||
| Yes (n = 181) | 0.2 (0.8) | 0.4 (0.9) |
| Cardiometabolic Risk Factors | Snack-Fruit Pattern (Mean (SD)) | p-Value | Rice-Meat-Refined Wheat Pattern (Mean (SD)) | p-Value |
|---|---|---|---|---|
| Obesity 1 | 0.39 | 0.59 | ||
| No (n = 506) | 0.0 (0.7) | −0.0 (0.7) | ||
| Yes (n = 452) | −0.0 (0.7) | −0.0 (0.8) | ||
| Central obesity 2 | 0.31 | 0.52 | ||
| No (n = 289) | 0.0 (0.8) | 0.0 (0.7) | ||
| Yes (n = 667) | −0.0 (0.7) | −0.0 (0.7) | ||
| Hypertriglyceridemia 3 | 0.12 | <0.001 | ||
| No (n = 760) | −0.0 (0.7) | −0.1 (0.7) | ||
| Yes (n = 198) | 0.1 (0.7) | 0.2 (0.8) | ||
| Low HDL 4 | 0.16 | 0.055 | ||
| No (n = 630) | 0.0 (0.7) | 0.0 (0.7) | ||
| Yes (n = 328) | −0.1 (0.7) | −0.1 (0.7) | ||
| Elevated blood pressure 5 | 0.57 | 0.20 | ||
| No (n = 639) | 0.0 (0.7) | −0.0 (0.7) | ||
| Yes (n = 319) | −0.0 (0.7) | 0.0 (0.8) | ||
| Prediabetes 6 | 0.89 | 0.74 | ||
| No (n = 271) | −0.0 (0.7) | −0.0 (0.8) | ||
| Yes (n = 687) | −0.0 (0.7) | −0.0 (0.7) | ||
| Metabolic syndrome 7 | 0.89 | 0.76 | ||
| No (n = 602) | −0.0 (0.7) | −0.0 (0.7) | ||
| Yes (n = 356) | −0.0 (0.7) | −0.0 (0.7) |
| Snack-Fruit Pattern | Rice-Meat-Refined Wheat Pattern | |
|---|---|---|
| Triglycerides (mg/dL) | ||
| Model 1 | 7.44 (3.30, 11.58) | 1.23 (−3.11, 5.58) |
| Model 2 | 7.59 (3.41, 11.78) | 1.84 (−2.57, 6.25) |
| Model 3 | 6.76 (2.63, 10.89) | −1.34 (−5.75, 3.06) |
| HDL cholesterol (mg/dL) | ||
| Model 1 | −0.41 (−1.18, 0.36) | −0.09 (−0.90, 0.72) |
| Model 2 | −0.59 (−1.37, 0.18) | 0.08 (−0.74, 0.89) |
| Model 3 | −0.55 (−1.32, 0.22) | −0.37 (−1.19, 0.45) |
| Systolic blood pressure (mmHg) | ||
| Model 1 | −0.80 (−1.75, 0.14) | 0.08 (−0.92, 1.08) |
| Model 2 | −0.90 (−1.85, 0.05) | 0.34 (−0.67, 1.35) |
| Model 3 | −0.87 (−1.82, 0.07) | −0.05 (−1.07, 0.97) |
| Diastolic blood pressure (mmHg) | ||
| Model 1 | −0.45 (−1.10, 0.19) | −0.54 (−1.19, 0.11) |
| Model 2 | −0.33 (−1.02, 0.35) | −0.18 (−0.88, 0.51) |
| Fasting glucose (mmol/L) | ||
| Model 1 | 0.01 (−0.03, 0.05) | 0.03 (−0.02, 0.07) |
| Model 2 | −0.00 (−0.04, 0.04) | 0.04 (−0.01, 0.08) |
| Model 3 | 0.00 (−0.04, 0.04) | 0.04 (−0.01, 0.08) |
| Two-hour glucose (mmol/L) | ||
| Model 1 | −0.05 (−0.16, 0.06) | 0.02 (−0.09, 0.14) |
| Model 2 | −0.05 (−0.16, 0.06) | 0.04 (−0.08, 0.16) |
| Model 3 | −0.04 (−0.15, 0.07) | 0.04 (−0.08, 0.16) |
| Hb1Ac (%) | ||
| Model 1 | 0.01 (−0.02, 0.03) | 0.04 (0.01, 0.07) |
| Model 2 | 0.00 (−0.03, 0.03) | 0.05 (0.01, 0.08) |
| Model 3 | 0.00 (−0.03, 0.03) | 0.04 (0.01, 0.07) |
| Snack-Fruit Pattern | Rice-Meat-Refined Wheat Pattern | |
|---|---|---|
| Obesity | ||
| Model 1 | 0.97 (0.86, 1.10) | 1.01 (0.89, 1.15) |
| Model 2 | 0.98 (0.87, 1.12) | 1.02 (0.89, 1.16) |
| Model 3 | 0.97 (0.85, 1.10) | 1.00 (0.87, 1.14) |
| Central obesity | ||
| Model 1 | 1.03 (0.90, 1.17) | 1.18 (1.03, 1.35) |
| Model 2 | 1.03 (0.90, 1.17) | 1.19 (1.03, 1.36) |
| Model 3 | 1.02 (0.90, 1.16) | 1.16 (1.01, 1.34) |
| Hypertriglyceridemia | ||
| Model 1 | 1.08 (0.94, 1.24) | 1.00 (0.87, 1.16) |
| Model 2 | 1.08 (0.94, 1.25) | 1.03 (0.89, 1.18) |
| Model 3 | 1.05 (0.91, 1.22) | 0.96 (0.82, 1.11) |
| Low HDL | ||
| Model 1 | 0.96 (0.85, 1.09) | 0.95 (0.84, 1.08) |
| Model 2 | 0.98 (0.87, 1.11) | 0.94 (0.82, 1.07) |
| Model 3 | 0.97 (0.86, 1.10) | 0.95 (0.83, 1.09) |
| Raised blood pressure | ||
| Model 1 | 0.89 (0.78, 1.01) | 1.05 (0.92, 1.20) |
| Model 2 | 0.90 (0.79, 1.02) | 1.07 (0.94, 1.22) |
| Model 3 | 0.90 (0.79, 1.03) | 1.03 (0.89, 1.18) |
| Diabetes | ||
| Model 1 | 1.04 (0.80, 1.35) | 1.20 (0.93, 1.56) |
| Model 2 | 0.99 (0.76, 1.29) | 1.28 (0.98, 1.66) |
| Model 3 | 0.99 (0.76, 1.29) | 1.28 (0.98, 1.67) |
| Metabolic syndrome | ||
| Model 1 | 0.99 (0.87, 1.13) | 1.14 (0.99, 1.30) |
| Model 2 | 1.00 (0.87, 1.13) | 1.14 (1.00, 1.31) |
| Model 3 | 0.99 (0.87, 1.13) | 1.11 (0.97, 1.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Huynh, Q.; Kapoor, N.; Jeemon, P.; Mello, G.T.d.; Oldenburg, B.; Thankappan, K.R.; Sathish, T. Associations between Dietary Patterns and Cardiometabolic Risk Factors—A Longitudinal Analysis among High-Risk Individuals for Diabetes in Kerala, India. Nutrients 2022, 14, 662. https://doi.org/10.3390/nu14030662
Cao Y, Huynh Q, Kapoor N, Jeemon P, Mello GTd, Oldenburg B, Thankappan KR, Sathish T. Associations between Dietary Patterns and Cardiometabolic Risk Factors—A Longitudinal Analysis among High-Risk Individuals for Diabetes in Kerala, India. Nutrients. 2022; 14(3):662. https://doi.org/10.3390/nu14030662
Chicago/Turabian StyleCao, Yingting, Quan Huynh, Nitin Kapoor, Panniyammakal Jeemon, Gabrielli Thais de Mello, Brian Oldenburg, Kavumpurathu Raman Thankappan, and Thirunavukkarasu Sathish. 2022. "Associations between Dietary Patterns and Cardiometabolic Risk Factors—A Longitudinal Analysis among High-Risk Individuals for Diabetes in Kerala, India" Nutrients 14, no. 3: 662. https://doi.org/10.3390/nu14030662
APA StyleCao, Y., Huynh, Q., Kapoor, N., Jeemon, P., Mello, G. T. d., Oldenburg, B., Thankappan, K. R., & Sathish, T. (2022). Associations between Dietary Patterns and Cardiometabolic Risk Factors—A Longitudinal Analysis among High-Risk Individuals for Diabetes in Kerala, India. Nutrients, 14(3), 662. https://doi.org/10.3390/nu14030662









