A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates
Abstract
:1. Introduction
2. Methods
2.1. Trial Design
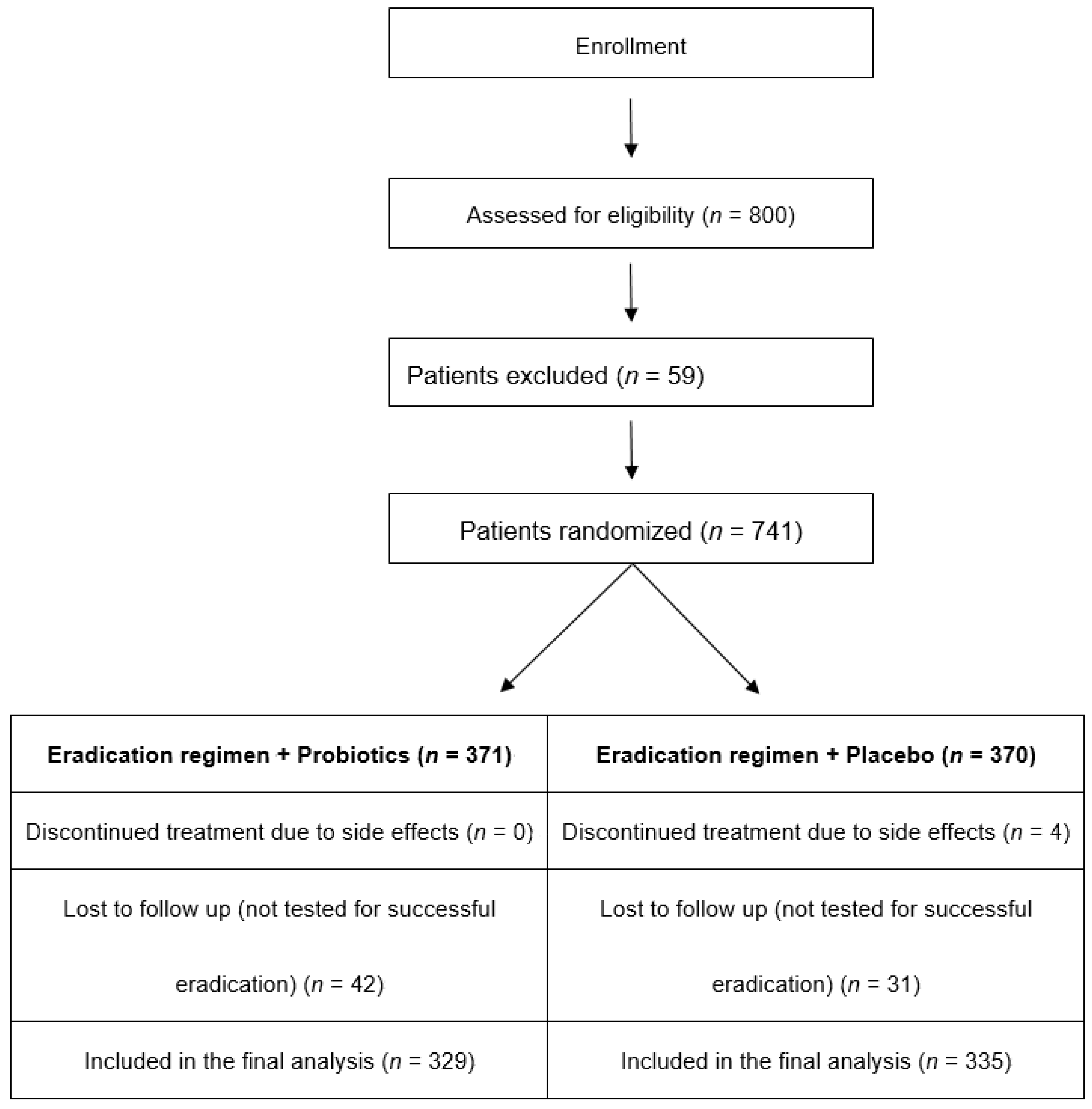
2.2. Patients
2.3. Diagnosis of H. pylori Infection
2.4. Therapeutic Regimens
The study was conducted in a randomized, double-blind method
2.5. Patients’ Follow Up
2.6. Statistical Analysis
3. Results
3.1. Adverse Events
3.2. Eradication Rates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, J.; Barry Marshall, M.D. H pylori 35 years later. JAMA 2017, 317, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, S.D.; Xirouchakis, E.; Martinez-Gonzalez, B.; Sgouras, D.N.; Spiliadi, C.; Mentis, A.F.; Laoudi, F. Clinical evaluation of a ten-day regimen with esomeprazole, metronidazole, amoxicillin, and clarithromycin for the eradication of Helicobacter pylori in a high clarithromycin resistance area. Helicobacter 2013, 18, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, S.D.; Michopoulos, S.; Rokkas, T.; Apostolopoulos, P.; Giamarellos, E.; Kamberoglou, D.; Mentis, A.; Triantafyllou, K. Hellenic consensus on helicobacter pylori infection. Ann. Gastroenterol. 2020, 33, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus document. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Johnson, J.L.; Kale-Pradhan, P.B. Treating bugs with bugs: The role of probiotics as adjunctive therapy for Helicobacter pylori. Ann. Pharm. 2011, 45, 960–966. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Ruggiero, P. Use of probiotics in the fight against Helicobacter pylori. World J. Gastrointest. Pathophysiol. 2014, 5, 384–391. [Google Scholar] [CrossRef]
- Szajewska, H.; Horvath, A.; Piwowarczyk, A. Meta-analysis: The effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment. Pharm. 2010, 32, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Sang, J.; He, H.; Wan, X.; Lin, Y.; Li, L.; Li, Y.; Yu, C. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: A meta-analysis. Sci. Rep. 2016, 6, 23522. [Google Scholar] [CrossRef]
- Navarro-Rodriguez, T.; Silva, F.M.; Barbuti, R.C.; Mattar, R.; Moraes-Filho, J.P.; de Oliveira, M.N.; Bogsan, C.S.; Chinzon, D.; Eisig, J.N. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: A prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.; Kuipers, E.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myllyluoma, E.; Veijola, L.; Ahlroos, T.; Tynkkynen, S.; Kankuri, E.; Vapaatalo, H.; Rautelin, H.; Korpela, R. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy. A placebo controlled, double-blind randomized pilot study. Aliment. Pharm. 2005, 21, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Pagkalos, E.; Thanopoulou, A.; Sampanis, C.; Bousboulas, S.; Melidonis, A.; Tentolouris, N.; Alexandrides, T.; Migdalis, I.; Karamousouli, E.; Papanas, N. The real-life effectiveness and care patterns of type 2 diabetes management in Greece. Exp. Clin. Endocrinol. Diabetes 2018, 126, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Montori, V.; Guyatt, G. Intention-to-treat principle. CMAJ 2001, 165, 1339–1341. [Google Scholar] [PubMed]
- Hollis, S.; Campbell, F. What is meant by intention to treat analysis? Survey of published randomized controlled trials. BMJ 1999, 319, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Crowe, S.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Georgopoulos, S.; Papastergiou, V.; Xirouchakis, E.; Laudi, F.; Papantoniou, N.; Lisgos, P.; Spiliadi, C.; Fragou, P.; Skorda, L.; Karatapanis, S. Evaluation of a four-drug, three-antibiotic, non-bismuth containing “concomitant” therapy as first line Helicobacter pylori eradication regimen in Greece. Helicobacter 2012, 17, 49–53. [Google Scholar] [CrossRef]
- Georgopoulos, S.; Papastergiou, V.; Karatapanis, S. Current options for the treatment of Helicobacter pylori. Expert Opin. Pharm. 2013, 14, 211–223. [Google Scholar] [CrossRef]
- Georgopoulos, S.; Papastergiou, V. An update on current and advancing pharmacotherapy options for the treatment of H. pylori infection. Expert Opin. Pharm. 2021, 22, 729–741. [Google Scholar] [CrossRef]
- Georgopoulos, S.D.; Xirouchakis, E.; Martinez-Gonzales, B.; Zampeli, E.; Grivas, E.; Spiliadi, C.; Sotiropoulou, M.; Petraki, K.; Zografos, K.; Laoudi, F.; et al. Randomized clinical trial comparing ten-day concomitant and sequential therapies for Helicobacter pylori eradication in a high clarithromycin resistance area. Eur. J. Intern. Med. 2016, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter Pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyssen, O.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Zhou, L.; Liu, D.Y.; Yao, X.J.; Li, Y. What roles do probiotics play in the eradication of Helicobacter pylori? Current knowledge and ongoing research. Gastroenterol. Res. Pr. 2018, 2018, 9379480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyssen, O.; Perez-Aisa, A.; Tepes, B.; Castro-Fernandez, M.; Kupcinskas, J.; Jonaitis, L.; Bujanda, L.; Lucendo, A.; Jurecic, N.B.; Perez-Lasala, J.; et al. Adverse event profile during the treatment of Helicobacter Pylori: A real world experience of 22,000 patients from the European registry on H. pylori management (Hp-EuReg). Am. J. Gastroenterol. 2021, 116, 1220–1229. [Google Scholar] [CrossRef]
- Losurdo, G.; Cubisino, R.; Barone, M.; Principi, M.; Leandro, G.; Ieraldi, E.; DiLeo, A. Probiotic monotherapy and helicobacter pylori eradication: A systematic review with pooled-data analysis. World J. Gastroenterol. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterol. 2021, 21, 388. [Google Scholar] [CrossRef]
- Cekin, A.H.; Sahinturk, Y.; AkbayHarmandar, F.; Uyar, S.; OguzYolcular, B.; Cekin, Y. Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: Impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turk. J. Gastroenterol. 2017, 28, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Kafshdooz, T.; Akbarzadeh, A.; MajdiSeghinsara, A.; Pourhassan, M.; Nasrabadi, H.T.; Milani, M. Role of probiotics in managing of Helicobacter pylori infection: A review. Drug Res. 2017, 67, 88–93. [Google Scholar] [CrossRef]
- McFarland, L.V.; Huang, Y.; Wang, L.; Malfertheiner, P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur. Gastroenterol. J. 2016, 4, 546–561. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, R.; Lionetti, E.; Castellaneta, S.P.; Magistà, A.M.; Maurogiovanni, G.; Bucci, N.; De Canio, A.; Indrio, F.; Cavallo, L.; Ierardi, E.; et al. Inhibition of helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: A pilot study. Helicobacter 2008, 13, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Jayaraman, T.; Daly, P.; Canchaya, C.; Curran, S.; Fang, F.; Quigley, E.; O’Toole, P.; O’Toole, P. Isolation of lactobacilli with probiotic properties from the human stomach. Lett. Appl. Microbiol. 2008, 47, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, J.; Matsumoto, A.; Tanaka, H.; Matsumura, I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett. 2018, 414, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Asasaka, T.; Sato, E.; Mori, K.; Matsumoto, M.; Ohori, H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 2002, 32, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panpetch, W.; Spinler, J.K.; Versalovic, J.; Tumwasorn, S. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori stimulated gastric epithelial cells. BMC Microbiol. 2016, 16, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilika, M.; Thoma, G.; Aidoni, Z.; Tsaousi, G.; Fotiadis, K.; Stavrou, G.; Malliou, P.; Chorti, A.; Massa, H.; Antypa, E.; et al. A four-probiotic preparation for ventilator associated pneumonia in multi-trauma patients: Results of a randomized clinical trial. Int. J. Antimicrob. Agents 2021, 59, 106471. [Google Scholar] [CrossRef]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Effect of a preparation of four probiotics on symptoms of patients with irritable bowel syndrome: Association with intestinal bacterial overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A four-probiotics regimen reduces post-operative complication after colorectal surgery. A randomized, double-blind, placebo-controlled study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Mo, L.; Shi, J.; Qin, M.; Huang, X. Efficacy and safety of probiotics in eradicating Helicobacter pylori. A network meta-analysis. Medicine 2019, 98, e15180. [Google Scholar] [CrossRef]
- Fallone, C.; Chiba, N.; van Zanten, S.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef] [Green Version]
| Variable | Group A | Group B | p-Value |
|---|---|---|---|
| Age, years (SD) | 51.6 (15.9) | 49.9 (16.3) | NS |
| Sex, male/female | 183/188 | 178/192 | NS |
| Smoking habits | 129 (34.7%) | 141 (38.1%) | NS |
| Alcohol intake | 22 (5.9%) | 19 (5.1%) | NS |
| Family history of gastric cancer | 2 (0.5%) | 1 (0.3%) | NS |
| BMI (Kg/m2) | 28.6 (5.3) | 27.9 (6.9) | NS |
| Variable | Group A | Group B | p-Value |
|---|---|---|---|
| Peptic ulcer | n = 45(12.1%) | n = 37 (10.0%) | NS |
| Gastritis without gastric atrophy | n = 148 (39.9%) | n = 154 (41.6%) | NS |
| Gastritis with gastric atrophy | n = 122 (32.9%) | n = 130 (35.1%) | NS |
| Gastritis with intestinal metaplasia | n = 56 (15.1%) | n = 49 (13.2%) | NS |
| Variable | Group A | Group B | p-Value |
|---|---|---|---|
| Epigastric pain | 10 | 22 | 0.03 |
| Flatulence | 2 | 19 | 0.0001 |
| Early satiety | 1 | 9 | 0.01 |
| Bitter taste | 20 | 36 | 0.03 |
| Anorexia | 0 | 1 | - |
| Nausea | 3 | 22 | 0.0001 |
| Vomiting | 1 | 7 | 0.03 |
| Retrosternal burning | 7 | 19 | 0.01 |
| Skin rash | 0 | 0 | - |
| Diarrhea | 12 | 35 | 0.0006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viazis, N.; Argyriou, K.; Kotzampassi, K.; Christodoulou, D.K.; Apostolopoulos, P.; Georgopoulos, S.D.; Liatsos, C.; Giouleme, O.; Koustenis, K.; Veretanos, C.; et al. A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients 2022, 14, 632. https://doi.org/10.3390/nu14030632
Viazis N, Argyriou K, Kotzampassi K, Christodoulou DK, Apostolopoulos P, Georgopoulos SD, Liatsos C, Giouleme O, Koustenis K, Veretanos C, et al. A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients. 2022; 14(3):632. https://doi.org/10.3390/nu14030632
Chicago/Turabian StyleViazis, Nikos, Konstantinos Argyriou, Katerina Kotzampassi, Dimitrios K. Christodoulou, Periklis Apostolopoulos, Sotirios D. Georgopoulos, Christos Liatsos, Olga Giouleme, Kanellos Koustenis, Christos Veretanos, and et al. 2022. "A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates" Nutrients 14, no. 3: 632. https://doi.org/10.3390/nu14030632
APA StyleViazis, N., Argyriou, K., Kotzampassi, K., Christodoulou, D. K., Apostolopoulos, P., Georgopoulos, S. D., Liatsos, C., Giouleme, O., Koustenis, K., Veretanos, C., Stogiannou, D., Moutzoukis, M., Poutakidis, C., Mylonas, I. I., Tseti, I., & Mantzaris, G. J. (2022). A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients, 14(3), 632. https://doi.org/10.3390/nu14030632







