Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study
Abstract
1. Introduction
2. Materials and Methods
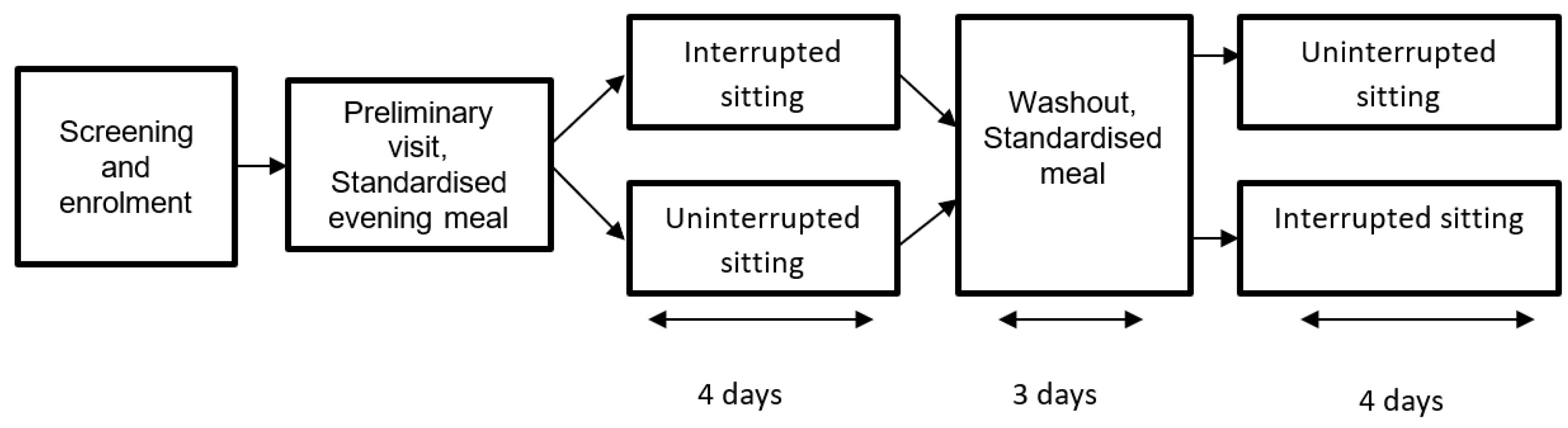
2.1. Study Design and Overview
2.2. Participants
2.3. Sample Size
2.4. Preliminary Visit
2.5. Experimental Design
2.5.1. Uninterrupted Sitting Regimen
2.5.2. Interrupted Sitting Regimen
2.5.3. Standardisation of Dietary Intake and Physical Activity
2.6. Measurements
2.6.1. Sitting, Standing and Stepping
2.6.2. Flash Glucose Monitoring
2.7. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Dietary Intake
3.3. Sitting, Standing and Stepping
3.4. Flash Glucose Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felber, J.-P.; Golay, A. Pathways from obesity to diabetes. Int. J. Obes. Relat. Metab. Disord. 2002, 26 (Suppl. S2), S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Brown, B.W.; Lamendola, C.; McLaughlin, T.; Reaven, G.M. Relationship between obesity, insulin resistance, and coronary heart disease risk. J. Am. Coll. Cardiol. 2002, 40, 937–943. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- de Vegt, F.; Dekker, J.M.; Jager, A.; Hienkens, E.; Kostense, P.J.; Stehouwer, C.D.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001, 285, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; 2019; Available online: https://diabetesatlas.org/en/ (accessed on 25 June 2020).
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J.H. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Bailey, D.P.; Hewson, D.J.; Champion, R.B.; Sayegh, S.M. Sitting Time and Risk of Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.H.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef]
- Bellettiere, J.; Winkler, E.A.H.; Chastin, S.F.M.; Kerr, J.; Owen, N.; Dunstan, D.W.; Healy, G. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS ONE 2017, 12, e0180119. [Google Scholar] [CrossRef]
- Stephens, B.R.; Granados, K.; Zderic, T.W.; Hamilton, M.T.; Braun, B. Effects of 1 day of inactivity on insulin action in healthy men and women: Interaction with energy intake. Metabolism 2011, 60, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Davies, M.J.; Bodicoat, D.H.; Edwardson, C.L.; Gill, J.M.; Stensel, D.J.; Tolfrey, K.; Dunstan, D.W.; Khunti, K.; Yates, T. Breaking Up Prolonged Sitting With Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes Care 2016, 39, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.P.; Locke, C.D. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J. Sci. Med. Sport 2015, 18, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef]
- Bailey, D.; Broom, D.R.; Chrismas, B.; Taylor, L.; Flynn, E.; Hough, J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl. Physiol. Nutr. Metab. 2016, 41, 324–331. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Larsen, R.N.; Sethi, P.; Sacre, J.W.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Benefits for Type 2 Diabetes of Interrupting Prolonged Sitting With Brief Bouts of Light Walking or Simple Resistance Activities. Diabetes Care 2016, 39, 964–972. [Google Scholar] [CrossRef]
- Duvivier, B.M.F.M.; Schaper, N.; Hesselink, M.K.C.; Van Kan, L.; Stienen, N.; Winkens, B.; Koster, A.; Savelberg, H.H.C.M. Breaking sitting with light activities vs structured exercise: A randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 2017, 60, 490–498. [Google Scholar] [CrossRef]
- Blankenship, J.M.; Chipkin, S.R.; Freedson, P.S.; Staudenmayer, J.; Lyden, K.; Braun, B. Managing free-living hyperglycemia with exercise or interrupted sitting in type 2 diabetes. J. Appl. Physiol. 2019, 126, 616–625. [Google Scholar] [CrossRef]
- Duvivier, B.M.F.M.; Schaper, N.C.; Koster, A.; Van Kan, L.; Peters, H.P.F.; Adam, J.J.; Giesbrecht, T.; Kornips, E.; Hulsbosch, M.; Willems, P.; et al. Benefits of Substituting Sitting with Standing and Walking in Free-Living Conditions for Cardiometabolic Risk Markers, Cognition and Mood in Overweight Adults. Front. Physiol. 2017, 8, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Mikines, K.J.; Sonne, B.; Farrell, P.A.; Tronier, B.; Galbo, H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am. J. Physiol. Metab. 1988, 254, E248–E259. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use of and Interpretation of Anthropometry: Report of a WHO Expert Committee; World Health Organization Technical Report Series 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Lean, M.E.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2016, 40, 622–632. [Google Scholar] [CrossRef]
- Marshall, A.L.; Miller, Y.D.; Burton, N.W.; Brown, W.J. Measuring total and domain-specific sitting: A study of reliability and validity. Med. Sci. Sports Exerc. 2010, 42, 1094–1102. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, T.M.; Rotteveel, J.; Serné, E.H.; Chinapaw, M.J.M. Effects of Multiple Sedentary Days on Metabolic Risk Factors in Free-Living Conditions: Lessons Learned and Future Recommendations. Front. Physiol. 2016, 7, 616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ricciardi, R.; Talbot, L.A. Use of bioelectrical impedance analysis in the evaluation, treatment, and prevention of overweight and obesity. J. Am. Acad. Nurse Pract. 2007, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Duvivier, B.M.F.M.; Schaper, N.; Bremers, M.A.; Van Crombrugge, G.; Menheere, P.P.C.A.; Kars, M.; Savelberg, H.H.C.M. Minimal Intensity Physical Activity (Standing and Walking) of Longer Duration Improves Insulin Action and Plasma Lipids More than Shorter Periods of Moderate to Vigorous Exercise (Cycling) in Sedentary Subjects When Energy Expenditure Is Comparable. PLoS ONE 2013, 8, e55542. [Google Scholar] [CrossRef]
- Lyden, K.; Keadle, S.; Staudenmayer, J.W.; Freedson, P.S. Validity of Two Wearable Monitors to Estimate Breaks from Sedentary Time. Med. Sci. Sports Exerc. 2012, 44, 2243–2252. [Google Scholar] [CrossRef]
- Grant, P.M.; Ryan, C.G.; Tigbe, W.; Granat, M.H. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br. J. Sports Med. 2006, 40, 992–997. [Google Scholar] [CrossRef]
- Edwardson, C.L.; Winkler, E.A.H.; Bodicoat, D.H.; Yates, T.; Davies, M.J.; Dunstan, D.W.; Healy, G.N. Considerations when using the activPAL monitor in field-based research with adult populations. J. Sport Health Sci. 2017, 6, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.H.; Bodicoat, D.H.; Healy, G.N.; Bakrania, K.; Yates, T.; Owen, N.; Dunstan, D.W.; Edwardson, C.L. Identifying adults’ valid waking wear time by automated estimation in activPAL data collected with a 24 h wear protocol. Physiol. Meas. 2016, 37, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Bluher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Bergouignan, A.; Latouche, C.; Heywood, S.; Grace, M.S.; Reddy-Luthmoodoo, M.; Natoli, A.K.; Owen, N.; Dunstan, D.W.; Kingwell, B.A. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: Ancillary analysis from randomized clinical trials. Sci. Rep. 2016, 6, 32044. [Google Scholar] [CrossRef]
- Latouche, C.; Jowett, J.B.M.; Carey, A.L.; Bertovic, D.A.; Owen, N.; Dunstan, D.W.; Kingwell, B.A. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J. Appl. Physiol. 2013, 114, 453–460. [Google Scholar] [CrossRef]
- Healy, G.N.; Winkler, E.A.H.; Eakin, E.G.; Owen, N.; Lamontagne, A.D.; Moodie, M.; Dunstan, D.W. A Cluster RCT to Reduce Workers’ Sitting Time: Impact on Cardiometabolic Biomarkers. Med. Sci. Sports Exerc. 2017, 49, 2032–2039. [Google Scholar] [CrossRef]

| Variables | |
|---|---|
| Female (n) | 67% (n = 8) |
| Age (years) | 48 ± 10 |
| Body weight | 93.9 ± 13.8 kg |
| Body mass index (kg/m2) | 33.3 ± 5.5 |
| Body fat% | 39.4 ± 8.2 |
| Waist circumference (cm) | 107.8 ± 9.3 |
| Fasting glucose (mmol/L) | 5.4 ± 0.9 |
| Uninterrupted Sitting | Interrupted Sitting | Main Effect of Condition (p Value) | |
|---|---|---|---|
| Daily sitting time (min/day) | 671.2 (584.9, 757.3) | 613.5 (527.2, 699.8) | <0.01 |
| Time in short 0–30 min sitting bouts (min/day) | 284.8 (229.7, 340.0) | 331.9 (276.5, 387.2) | 0.01 |
| Time in prolonged ≥ 30 min sitting bouts(min/day) | 385.9 (311.9, 459.9) | 286.6 (212.2, 360.8) | <0.01 |
| Time in prolonged ≥ 60 min sitting bouts(min/day) | 215.0 (156.7, 273.3) | 93.9 (34.9, 152.9) | <0.01 |
| Sit-upright transitions (n) | 50.2 (45.0, 55.3) | 52.1 (46.9, 57.3) | 0.35 |
| Short sitting bouts (n) | 43.6 (37.7, 49.5) | 46.1 (40.2, 52.0) | 0.25 |
| Prolonged ≥ 30 min sitting bouts (n) | 6.8 (5.3, 8.2) | 6.2 (4.8, 7.6) | 0.13 |
| Prolonged ≥ 60 min sitting bouts (n) | 2.6 (1.9, 3.2) | 1.0 (0.4, 1.7) | <0.01 |
| Standing time (min/day) | 236.0 (155.6, 316.5) | 254.0 (173.5, 334.6) | 0.21 |
| Stepping time (min/day) | 80.9 (65.5, 96.4) | 120.8 (105.3, 136.3) | <0.01 |
| Total steps (n) | 6491 (5529, 8354) | 10,987 (9562, 12,412) | <0.01 |
| Uninterrupted Sitting | Interrupted Sitting | Main Effect of Condition (p Value) | |
|---|---|---|---|
| 24 h period | |||
| Mean glucose concentration (mmol/L) | 5.6 (5.1, 6.1) | 5.6 (5.1, 6.1) | 0.42 |
| Glucose total AUC (mmol/L∙24 h) | 131.9 (125.1, 138.6) | 134.8 (128.0, 141.6) | 0.47 |
| Glucose iAUC (mmol/L∙24 h) | 5.9 (−1.4, 13.1) | 5.6 (−1.7, 12.8) | 0.85 |
| Coefficient of variation, % | 19.0 (16.7, 21.3) | 18.8 (16.5, 21.1) | 0.78 |
| Waking hours | |||
| Mean glucose concentration (mmol/L) | 5.9 (5.4, 6.4)) | 5.8 (5.3, 6.3) | 0.56 |
| Glucose total AUC (mmol/L∙waking hours) | 101.2 (93.1, 109.2) | 100.2 (92.2, 108.3) | 0.63 |
| Glucose iAUC (mmol/L∙waking hours) | 6.8 (1.2, 12.3) | 5.6 (0.0, 11.1) | 0.37 |
| Coefficient of variation, % | 19.4 (17.2, 21.6) | 19.6 (17.3, 21.9) | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, D.P.; Stringer, C.A.; Maylor, B.D.; Zakrzewski-Fruer, J.K. Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study. Nutrients 2022, 14, 605. https://doi.org/10.3390/nu14030605
Bailey DP, Stringer CA, Maylor BD, Zakrzewski-Fruer JK. Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study. Nutrients. 2022; 14(3):605. https://doi.org/10.3390/nu14030605
Chicago/Turabian StyleBailey, Daniel P., Charlotte A. Stringer, Benjamin D. Maylor, and Julia K. Zakrzewski-Fruer. 2022. "Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study" Nutrients 14, no. 3: 605. https://doi.org/10.3390/nu14030605
APA StyleBailey, D. P., Stringer, C. A., Maylor, B. D., & Zakrzewski-Fruer, J. K. (2022). Lower Amounts of Daily and Prolonged Sitting Do Not Lower Free-Living Continuously Monitored Glucose Concentrations in Overweight and Obese Adults: A Randomised Crossover Study. Nutrients, 14(3), 605. https://doi.org/10.3390/nu14030605







