Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials
Highlights
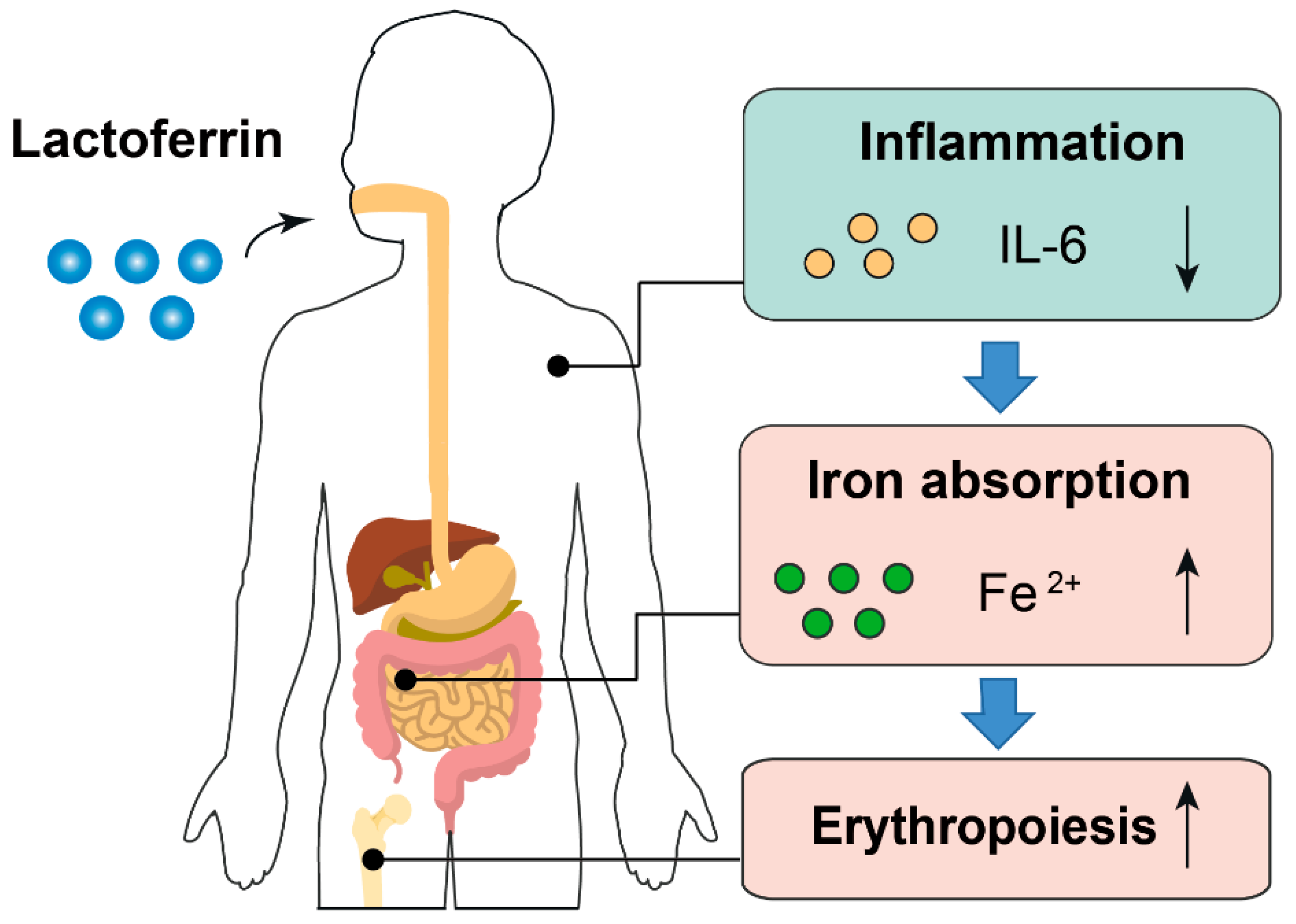
- Lactoferrin displays superior effects compared to ferrous sulfate in terms of improving iron status and erythropoiesis in clinical trials.
- Compared with ferrous sulfate, lactoferrin supplementation reduces circulating inflammatory cytokines.
- The anti-inflammatory effect of lactoferrin may explain why it had better iron- and erythropoiesis-improving effects even with lower fractional iron absorption than ferrous sulfate.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Study Characteristic
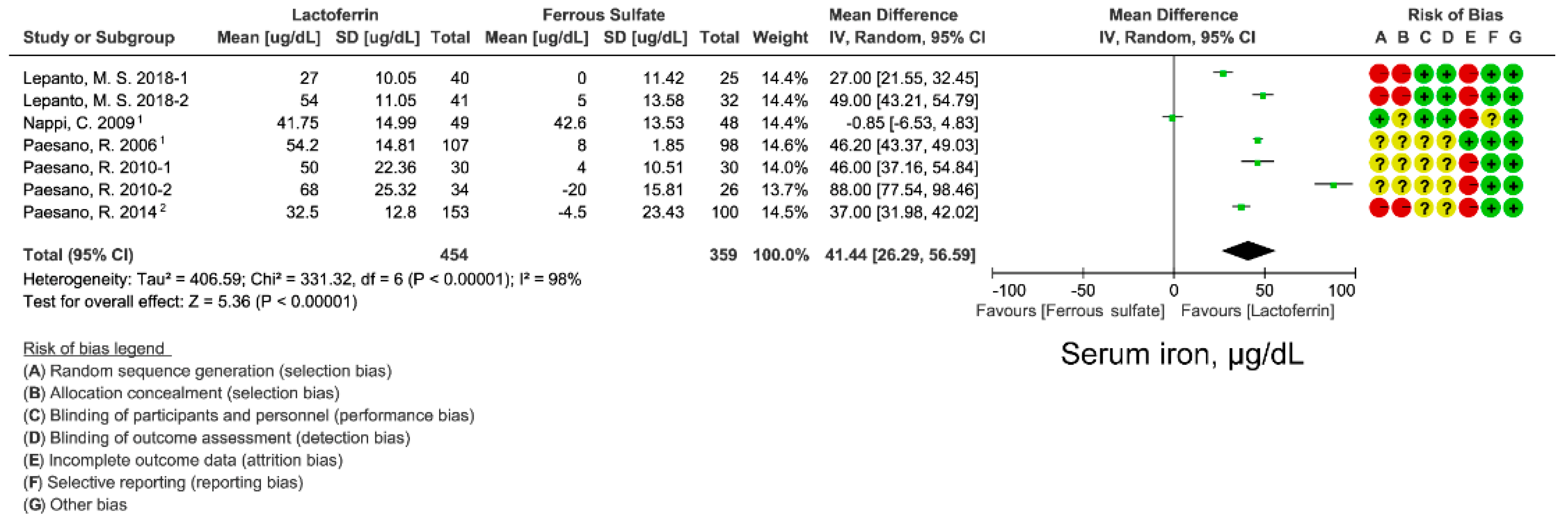
3.2. Effect of Lactoferrin and Ferrous Sulfate Supplementation on SI
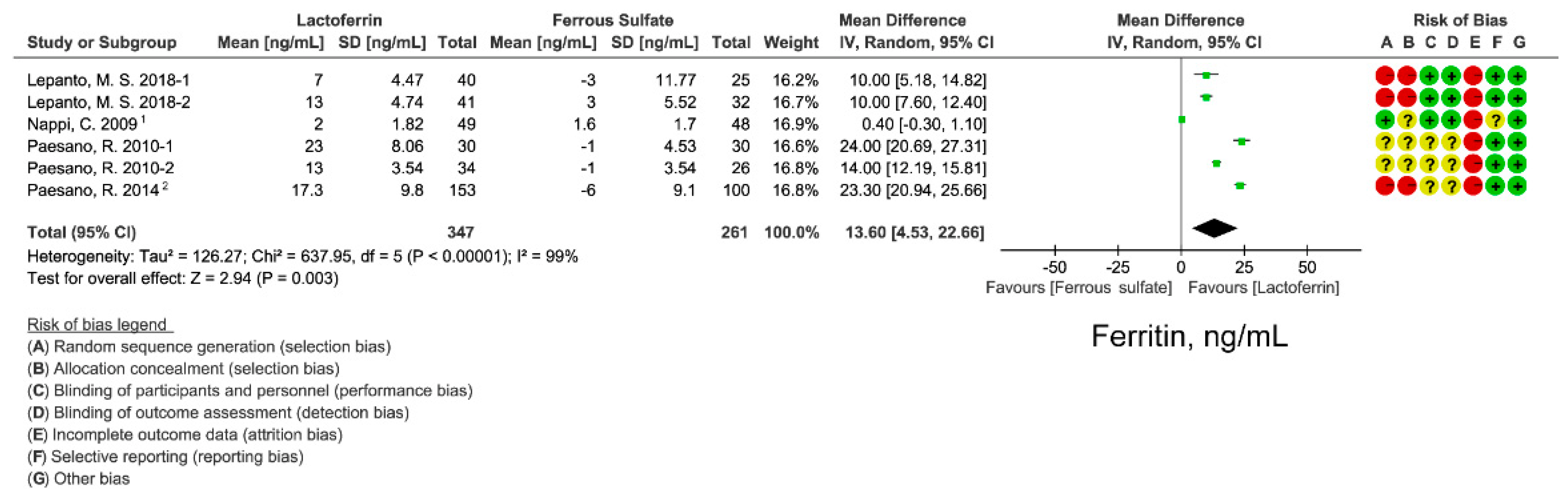
3.3. Effect of Lactoferrin and Ferrous Sulfate Supplementation on SF
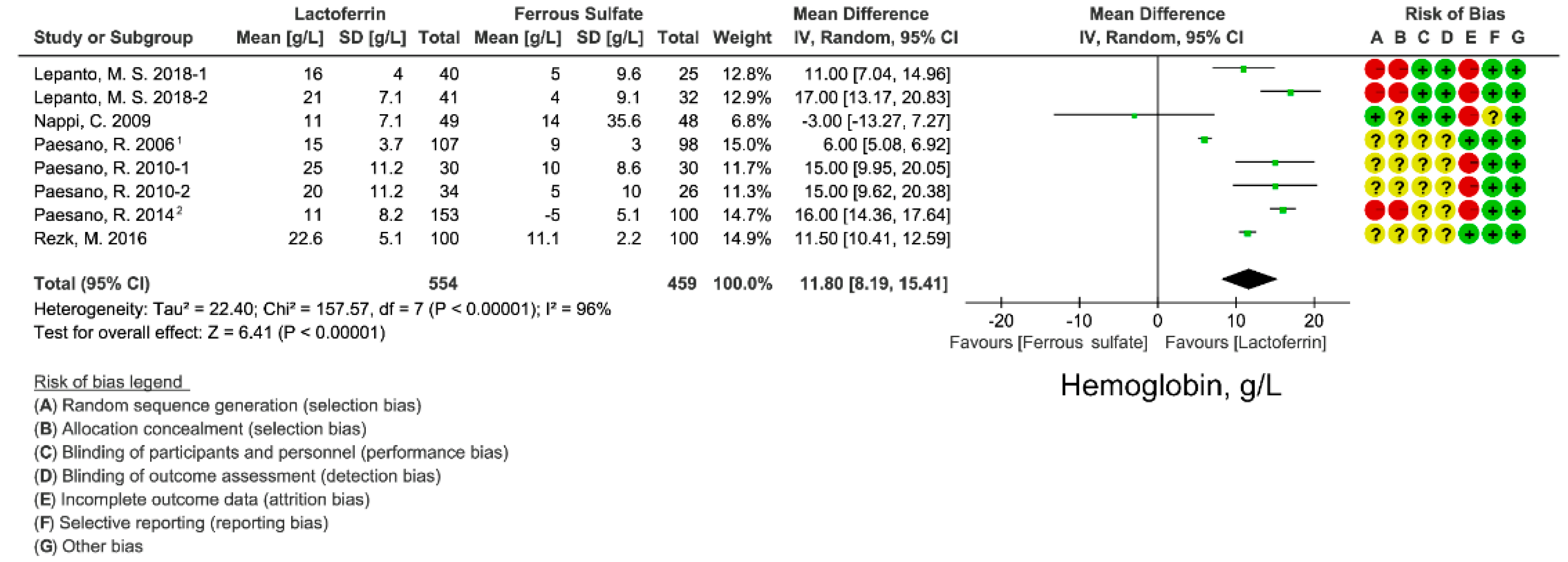
3.4. Effect of Lactoferrin and Ferrous Sulfate Supplementation on Hemoglobin Concentration
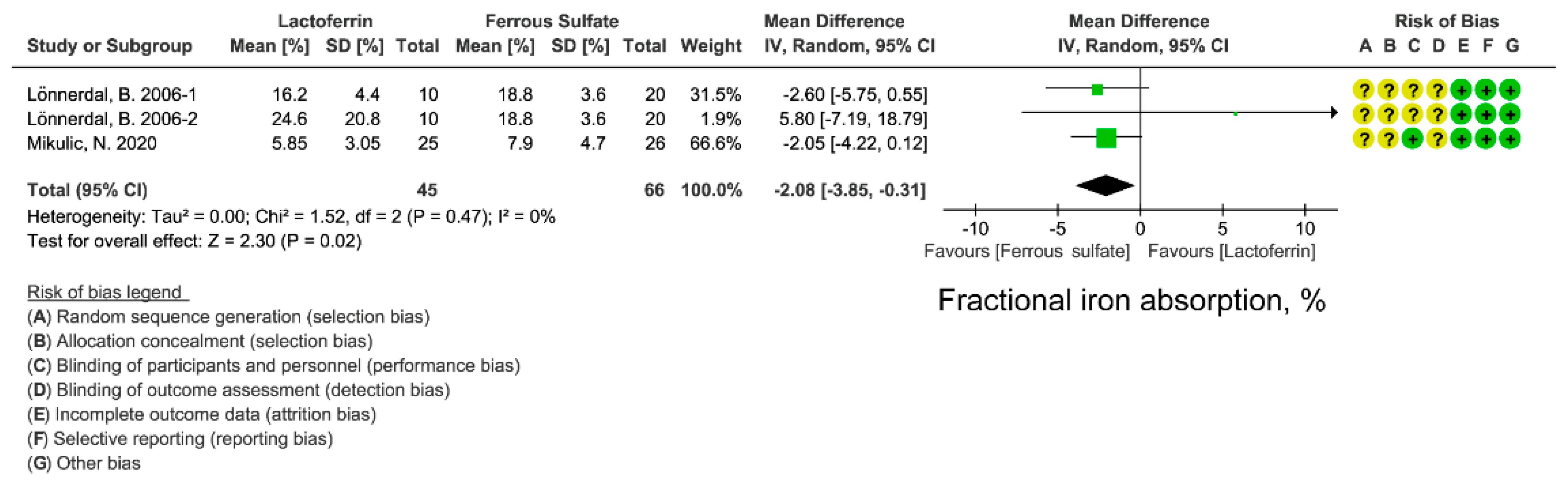
3.5. Effect of Lactoferrin and Ferrous Sulfate Supplementation on FIA
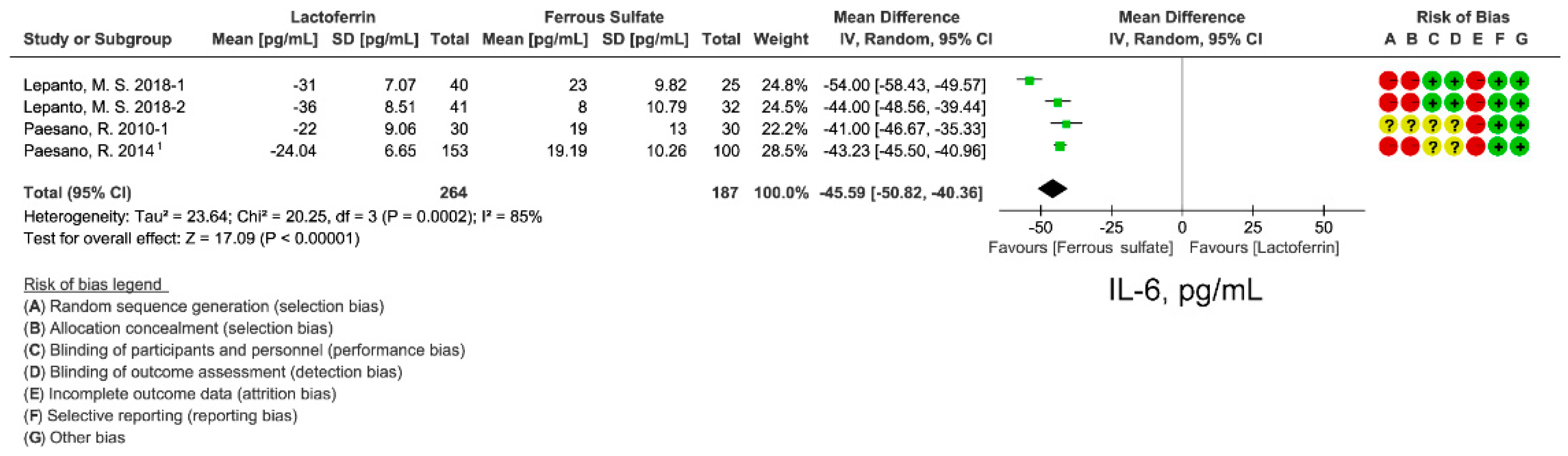
3.6. Effect of Lactoferrin and Ferrous Sulfate Supplementation on Inflammatory Cytokine IL-6
3.7. Subgroup Analyses of Ferrous Sulfate Supplementation on Iron Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donker, A.E.; van der Staaij, H. The critical roles of iron during the journey from fetus to adolescent: Developmental aspects of iron homeostasis. Blood Rev. 2021, 50, 100866. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. 1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Rabe, H. Prevention of iron deficiency anemia in infants and toddlers. Pediatr. Res. 2021, 89, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Anaemia Reduction Efforts among Women of Reproductive Age: Impact, Achievement of Targets and the Way forward for Optimizing Efforts; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-924-001-220-2.
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; on behalf of Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015.
- Daru, J.; Zamora, J.; Fernández-Félix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tunçalp, Ö.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef] [Green Version]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Global Nutrition Targets 2025: Anaemia Policy Brief; World Health Organization: Geneva, Switzerland, 2019.
- Azzopardi, P.S.; Hearps, S.J.; Francis, K.L.; Kennedy, E.C.; Mokdad, A.H.; Kassebaum, N.J.; Lim, S.; Irvine, C.M.S.; Vos, T.; Brown, A.D.; et al. Progress in adolescent health and wellbeing: Tracking 12 headline indicators for 195 countries and territories, 1990–2016. Lancet 2019, 393, 1101–1118. [Google Scholar] [CrossRef] [Green Version]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 2011, 64, 281–286. [Google Scholar]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef] [Green Version]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130.e116. [Google Scholar] [CrossRef]
- Griffin, I.J. The Effects of Different Forms of Lactoferrin on Iron Absorption. J. Nutr. 2020, 150, 3053–3054. [Google Scholar] [CrossRef]
- Ke, C.; Lan, Z.; Hua, L.; Ying, Z.; Humina, X.; Jia, S.; Weizheng, T.; Ping, Y.; Lingying, C.; Meng, M. Iron metabolism in infants: Influence of bovine lactoferrin from iron-fortified formula. Nutrition 2015, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C. Efficacy and Safety of Oral Lactoferrin Supplementation in Combination with rHuEPO-β for the Treatment of Anemia in Advanced Cancer Patients Undergoing Chemotherapy: Open-Label, Randomized Controlled Study. Oncologist 2010, 15, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of oral administration mode on the efficacy of commercial bovine Lactoferrin against iron and inflammatory homeostasis disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Perri, H.; Meehan, T. Evolution of duplications in the transferrin family of proteins. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 140, 11–25. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Olszewska, P.; Pazdrak, B.; Krupinska, A.M.; Actor, J.K. New insights into the systemic effects of oral lactoferrin: Transcriptome profiling. Biochem. Cell Biol. 2021, 99, 47–53. [Google Scholar] [CrossRef]
- Available online: http://vassarstats.net/ (accessed on 30 September 2021).
- Available online: https://methods.cochrane.org/bias/news/rob-2-tool/ (accessed on 10 October 2021).
- Rezk, M.; Dawood, R.; Abo-Elnasr, M.; Al Halaby, A.; Marawan, H. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: A randomized clinical trial. J. Matern.-Fetal Neonatal Med. 2016, 29, 1387–1390. [Google Scholar] [CrossRef]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstet. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Bryant, A. Absorption of iron from recombinant human lactoferrin in young US women. Am. J. Clin. Nutr. 2006, 83, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron Absorption is Greater from Apo-Lactoferrin and is Similar Between Holo-Lactoferrin and Ferrous Sulfate: Stable Iron Isotope Studies in Kenyan Infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Jaśkiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Mendoza-Meneses, M.; Cunningham, G.A.; Conneely, O.M. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell Biol. 2003, 23, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, C.N.; Mak, H.H.; Akpan, I.; Losyev, G.; Zurakowski, D.; Andrews, N.C. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood 2007, 109, 4038–4044. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, G.; Bennoun, M.; Porteu, A.; Mativet, S.; Beaumont, C.; Grandchamp, B.; Sirito, M.; Sawadogo, M.; Kahn, A.; Vaulont, S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 2002, 99, 4596–4601. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.Y.; Silver, M.; Parnes, A.; Nikiforow, S.; Berliner, N.; Vanasse, G.J. Resveratrol ameliorates TNFα-mediated suppression of erythropoiesis in human CD34+ cells via modulation of NF-κB signalling. Br. J. Haematol. 2011, 155, 93–101. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [Green Version]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [Green Version]
- Paganini, D.; Uyoga, M.A.; Cercamondi, C.I.; Moretti, D.; Mwasi, E.; Schwab, C.; Bechtler, S.; Mutuku, F.M.; Galetti, V.; Lacroix, C.; et al. Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: A stable-isotope study in Kenyan infants. Am. J. Clin. Nutr. 2017, 106, 1020–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Main Elements of Proposed Model. Available online: https://www.freepik.com/ (accessed on 20 January 2022).
- Main Elements of Proposed Model. Available online: http://smart.servier.com/ (accessed on 20 January 2022).
- Muñoz, M.; García-Erce, J.A.; Remacha, F. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, X.; Xu, T.; Luo, J.; Luo, Y.; An, P. Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials. Nutrients 2022, 14, 543. https://doi.org/10.3390/nu14030543
Zhao X, Zhang X, Xu T, Luo J, Luo Y, An P. Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials. Nutrients. 2022; 14(3):543. https://doi.org/10.3390/nu14030543
Chicago/Turabian StyleZhao, Xiya, Xu Zhang, Teng Xu, Junjie Luo, Yongting Luo, and Peng An. 2022. "Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials" Nutrients 14, no. 3: 543. https://doi.org/10.3390/nu14030543
APA StyleZhao, X., Zhang, X., Xu, T., Luo, J., Luo, Y., & An, P. (2022). Comparative Effects between Oral Lactoferrin and Ferrous Sulfate Supplementation on Iron-Deficiency Anemia: A Comprehensive Review and Meta-Analysis of Clinical Trials. Nutrients, 14(3), 543. https://doi.org/10.3390/nu14030543