Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes
Abstract
1. Introduction
2. Materials and Methods
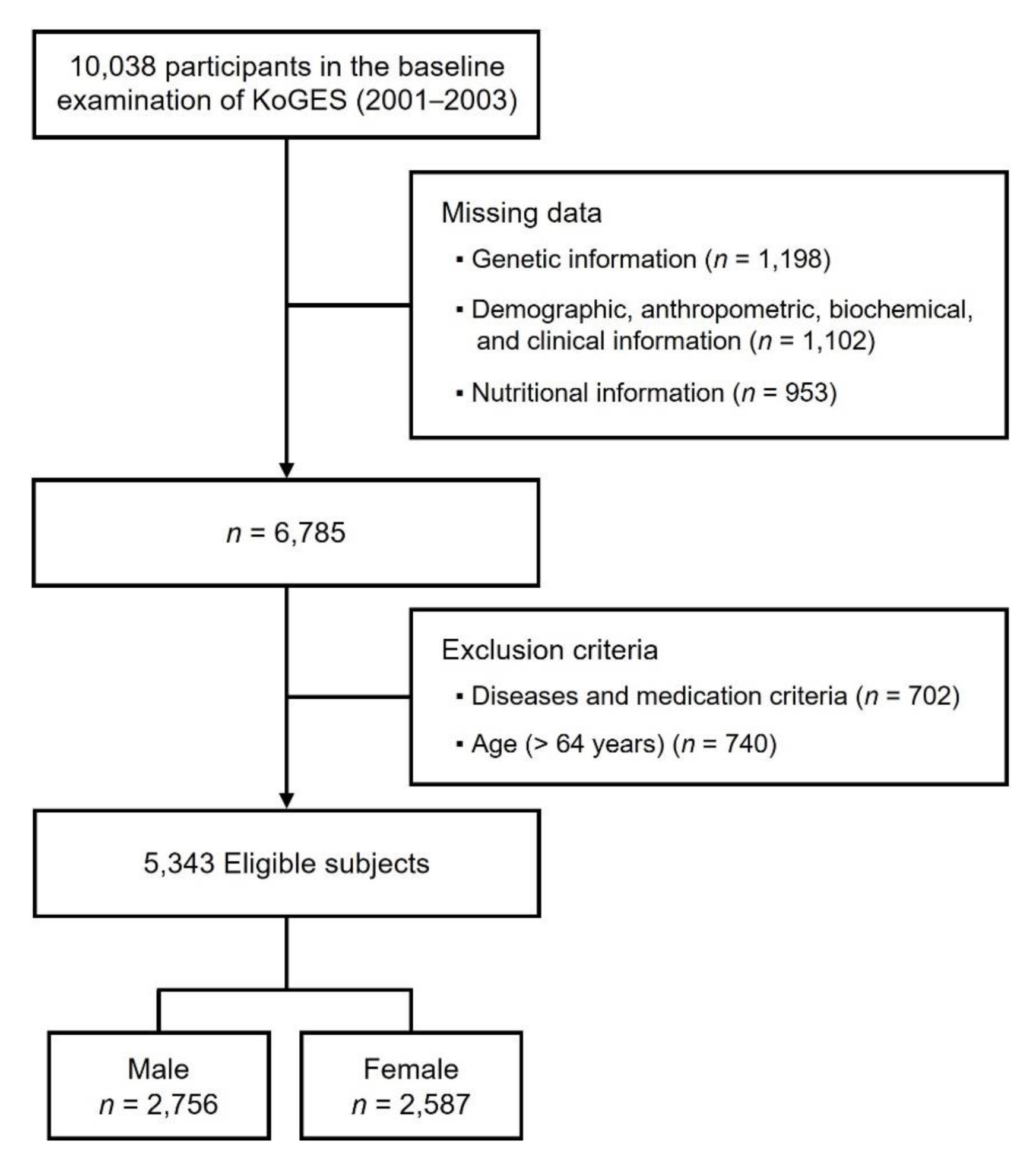
2.1. Study Data and Subjects
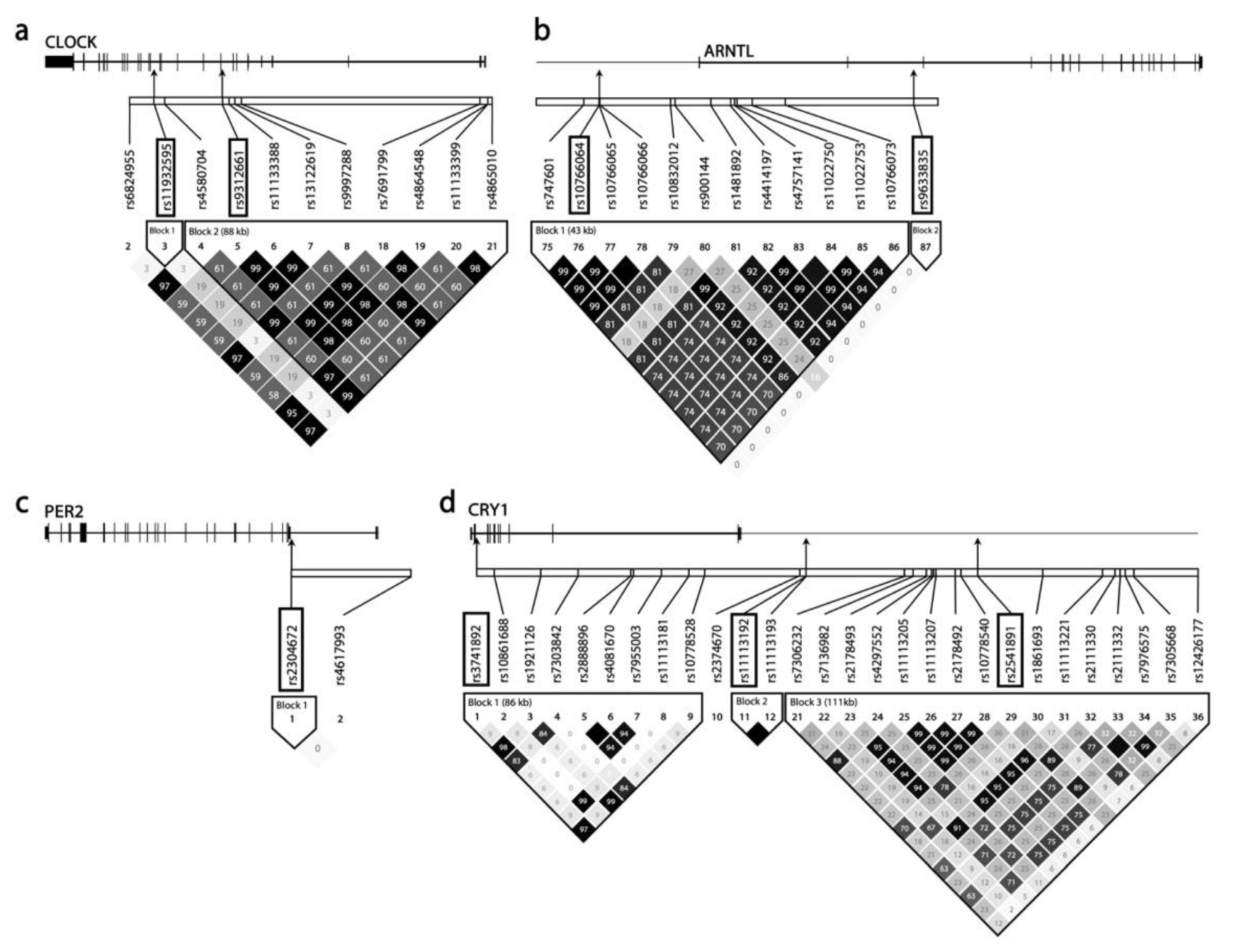
2.2. Selection and Analysis of SNPs
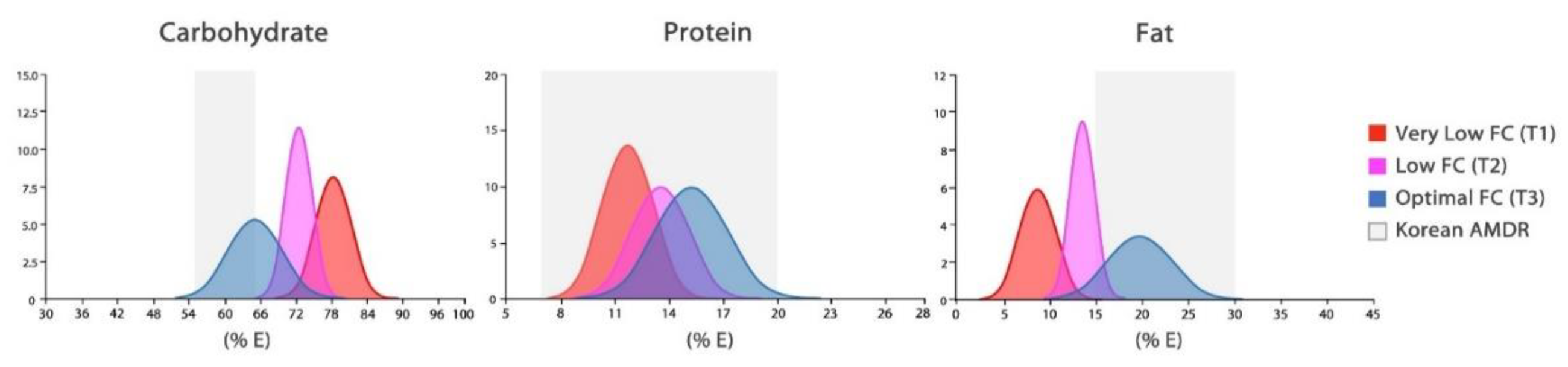
2.3. Macronutrient Patterns
2.4. Definitions of the Obesity and Abdominal Obesity
2.5. Statistical Analysis
3. Results
3.1. General Characteristics and Nutritional Intake
3.2. Risk of Obesity by Macronutrient Intake Patterns
3.3. Macronutrient Intake Patterns, Genetic Variants, and Risk of Obesity
3.4. Potential Links between Genetic Variants and Gene Regulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| SNP ID | Gene Symbol | Alleles | p-Value | Tissue |
|---|---|---|---|---|
| rs11932595 | CLOCK | A/G | 3 × 10−6 | Muscle–Skeletal |
| 5 × 10−7 | Cells–Cultured fibroblasts | |||
| rs9312661 | CLOCK | A/G | 2 × 10−25 | Thyroid |
| 2 × 10−17 | Skin–Sun Exposed (Lower leg) | |||
| 9 × 10−17 | Skin–Not Sun Exposed (Suprapubic) | |||
| 3 × 10−14 | Lung | |||
| 5 × 10−14 | Nerve–Tibial | |||
| 8 × 10−14 | Cells–Cultured fibroblasts | |||
| 7 × 10−13 | Spleen | |||
| 7 × 10−13 | Testis | |||
| 6 × 10−10 | Small Intestine–Terminal Ileum | |||
| 7 × 10−10 | Esophagus–Mucosa | |||
| 1 × 10−9 | Pancreas | |||
| 7 × 10−9 | Artery–Aorta | |||
| 2 × 10−7 | Colon–Transverse | |||
| 2 × 10−6 | Whole Blood | |||
| 1 × 10−5 | Breast–Mammary Tissue | |||
| 2 × 10−5 | Esophagus–Gastroesophageal Junction | |||
| 3 × 10−5 | Artery–Tibial | |||
| 1 × 10−4 | Adipose–Subcutaneous | |||
| rs10766065 | ARNTL | T/C | 1 × 10−12 | Whole Blood |
| rs9633835 | ARNTL | A/G | 1 × 10−16 | Whole Blood |
| rs2304672 | PER2 | G/C | 2 × 10−6 | Thyroid |
| rs3741892 | CRY1 | G/C | 1 × 10−35 | Testis |
| 4 × 10−10 | Muscle–Skeletal | |||
| rs11113192 | CRY1 | G/C | 1 × 10−6 | Testis |
| 6 × 10−5 | Esophagus–Gastroesophageal Junction | |||
| rs2541891 | CRY1 | C/G | 1 × 10−4 | Testis |
| rs7951225 | CRY2 | A/T | 3 × 10−12 | Whole Blood |
| 1 × 10−5 | Artery–Aorta | |||
| 1 × 10−5 | Artery–Tibial | |||
| 3 × 10−5 | Spleen |
Appendix B
| Variables | Total (n = 5343) | Male (n = 2756) | Female (n = 2587) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| General characteristics | |||||||||
| Age (year) | 49.4 ± 7.3 | 48.9 ± 7.0 | 49.9 ± 7.6 | ||||||
| BMI (kg/m2) | 24.7 ± 3.1 | 24.5 ± 2.9 | 24.8 ± 3.2 | ||||||
| Sleep duration (h) | 6.7 ± 1.3 | 6.7 ± 1.3 | 6.6 ± 1.4 | ||||||
| Nutritional intake | |||||||||
| Energy (kcal/day) | 1917.8 ± 550.6 | 1998.5 ± 527.5 | 1831.8 ± 561.8 | ||||||
| Carbohydrate (g/day) | 334.5 ± 92.2 | 342.7 ± 86.2 | 325.7 ± 97.3 | ||||||
| Protein (g/day) | 66.0 ± 23.9 | 69.6 ± 23.8 | 62.2 ± 23.4 | ||||||
| Fat (g/day) | 32.9 ± 17.2 | 36.4 ± 17.3 | 29.3 ± 16.3 | ||||||
| % Energy from each macronutrient | |||||||||
| Protein | 13.6 ± 2.2 | 13.8 ± 2.2 | 13.5 ± 2.2 | ||||||
| Carbohydrate | 70.3 ± 6.5 | 69.2 ± 6.2 | 71.6 ± 6.6 | ||||||
| Fat | 14.9 ± 5.0 | 15.9 ± 4.7 | 13.9 ± 5.1 | ||||||
| Number of regular meal | 2.8 ± 0.4 | 2.9 ± 0.3 | 2.8 ± 0.4 | ||||||
| Alcohol intake (g/day) | 10.8 ± 22.6 | 19.6 ± 28.4 | 1.5 ± 5.5 | ||||||
| Tobacco consumption (pack/year) | 9.2 ± 15.0 | 17.5 ± 16.9 | 0.3 ± 2.7 | ||||||
| Moderate physical activity (1) | 1956 (36.7) | 1028 (37.3) | 928 (35.87) | ||||||
Appendix C
References
- Takahashi, J.S.; Hong, H.-K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Kohsaka, A.; Bhawal, U.; Muragaki, Y. Potential roles of dec and bmal1 genes in interconnecting circadian clock and energy metabolism. Int. J. Mol. Sci. 2018, 19, 781. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Takahashi, J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000, 23, 713–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Etchegaray, J.-P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the circadian clock converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef]
- Rudic, R.D.; McNamara, P.; Curtis, A.-M.; Boston, R.C.; Panda, S.; Hogenesch, J.B.; FitzGerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Cheng, Y.; Kapoor, S.; Reilly, D.; Price, T.S.; FitzGerald, G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. USA 2007, 104, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Atsumi, G.-i.; Sugiyama, S.; Kodomari, I.; Kasamatsu, M.; Machida, K.; Ishida, N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006, 580, 127–130. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Carter, A.; Grant, P. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef]
- Woon, P.Y.; Kaisaki, P.J.; Bragança, J.; Bihoreau, M.-T.; Levy, J.C.; Farrall, M.; Gauguier, D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 14412–14417. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.A.; Rees, S.D.; Hydrie, M.Z.I.; Shera, A.S.; Bellary, S.; O’Hare, J.P.; Kumar, S.; Taheri, S.; Basit, A.; Barnett, A.H.; et al. Circadian gene variants and susceptibility to type 2 diabetes: A pilot study. PLoS ONE 2012, 7, e32670. [Google Scholar] [CrossRef] [PubMed]
- Pappa, K.I.; Gazouli, M.; Anastasiou, E.; Iliodromiti, Z.; Antsaklis, A.; Anagnou, N.P. The major circadian pacemaker ARNT-like protein-1 (BMAL1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Škrlec, I.; Milić, J.; Steiner, R. The impact of the circadian genes clock and arntl on myocardial infarction. J. Clin. Med. 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.-T.; Bandin, C.; Yang, H.-W.; Scheer, F.A.; Hu, K.; Garaulet, M. CLOCK 3111T/C genetic variant influences the daily rhythm of autonomic nervous function: Relevance to body weight control. Int. J. Obes. 2018, 42, 190. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Sánchez-Moreno, C.; Smith, C.E.; Lee, Y.-C.; Nicolás, F.; Ordovás, J.M. Ghrelin, sleep reduction and evening preference: Relationships to CLOCK 3111 T/C SNP and weight loss. PLoS ONE 2011, 6, e17435. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur. J. Hum. Genet. 2010, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Corbalán-Tutau, M.D.; Madrid, J.A.; Baraza, J.C.; Parnell, L.D.; Lee, Y.-C.; Ordovas, J.M. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J. Am. Diet. Assoc. 2010, 110, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, K.-W. CLOCK Gene Variation Is Associated with the Incidence of Metabolic Syndrome Modulated by Monounsaturated Fatty Acids. J. Pers. Med. 2021, 11, 412. [Google Scholar] [CrossRef]
- Garcia-Rios, A.; Gomez-Delgado, F.J.; Garaulet, M.; Alcala-Diaz, J.F.; Delgado-Lista, F.J.; Marin, C.; Rangel-Zuñiga, O.A.; Rodriguez-Cantalejo, F.; Gomez-Luna, P.; Ordovas, J.M. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int. 2014, 31, 401–408. [Google Scholar] [CrossRef]
- Loria-Kohen, V.; Espinosa-Salinas, I.; Marcos-Pasero, H.; Lourenço-Nogueira, T.; Herranz, J.; Molina, S.; Reglero, G.; de Molina, A.R. Polymorphism in the CLOCK gene may influence the effect of fat intake reduction on weight loss. Nutrition 2016, 32, 453–460. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef]
- Sahar, S.; Sassone-Corsi, P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer 2009, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.-i.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, clock genes, and chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Kuroda, H.; Tahara, Y.; Saito, K.; Ohnishi, N.; Kubo, Y.; Seo, Y.; Otsuka, M.; Fuse, Y.; Ohura, Y.; Hirao, A.; et al. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci. Rep. 2012, 2, 711. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Santos, R.B.; Fekry, B.; Tamim, Y.M.; Shivshankar, S.; Mohamed, A.M.; Baumgartner, C.; Kwok, C.; Gebhardt, C.; Rivera, A.; et al. Cellular and physiological circadian mechanisms drive diurnal cell proliferation and expansion of white adipose tissue. Nat. Commun. 2021, 12, 3482. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Hirao, A.; Tahara, Y.; Kimura, I.; Shibata, S. A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS ONE 2009, 4, e6909. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P.; Murakami, M.; Liu, Y.; Eckel-Mahan, K.L.; Newman, J.C.; Verdin, E.; Baldi, P.; Sassone-Corsi, P. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 2017, 26, 523–538.e5. [Google Scholar] [CrossRef]
- Oishi, K.; Uchida, D.; Itoh, N. Low-carbohydrate, high-protein diet affects rhythmic expression of gluconeogenic regulatory and circadian clock genes in mouse peripheral tissues. Chronobiol. Int. 2012, 29, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Sadeghirad, B.; Ball, G.D.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.; Desai, M.; King, A.C. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The DIETFITS randomized clinical trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Yang, Q.; Lang, X.; Li, W.; Liang, Y. The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 16–27. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Czeisler, C.A.; Phillips, A.J.; Keating, L.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Caloric and macronutrient intake differ with circadian phase and between lean and overweight young adults. Nutrients 2019, 11, 587. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; The KoGES Group. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Jang, S.-N.; Kawachi, I.; Chang, J.; Boo, K.; Shin, H.-G.; Lee, H.; Cho, S.-i. Marital status, gender, and depression: Analysis of the baseline survey of the Korean Longitudinal Study of Ageing (KLoSA). Soc. Sci. Med. 2009, 69, 1608–1615. [Google Scholar] [CrossRef]
- Consortium, G.P. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56. [Google Scholar] [CrossRef]
- Cho, Y.S.; Go, M.J.; Kim, Y.J.; Heo, J.Y.; Oh, J.H.; Ban, H.-J.; Yoon, D.; Lee, M.H.; Kim, D.-J.; Park, M.; et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009, 41, 527–534. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2004, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- The Genotype-Tissue Expression Project (GTEx). Available online: https://www.gtexportal.org (accessed on 20 April 2021).
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K.; Park, C.; Kim, D. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia Pty Limited: Balmain, NSW, Australia, 2000. [Google Scholar]
- Lee, S.; Park, H.S.; Kim, S.M.; Kwon, H.S.; Kim, D.Y.; Kim, D.J.; Cho, G.J.; Han, J.H.; Kim, S.R.; Park, C.Y.; et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J. Obes. 2006, 15, 1–9. [Google Scholar]
- The Korean Nutrition Society. Dietary Reference Intakes for Koreans; Ministry of Health and Welfare: Sejong, Korea, 2020; p. 9. [Google Scholar]
- Pivovarova, O.; Jürchott, K.; Rudovich, N.; Hornemann, S.; Ye, L.; Möckel, S.; Murahovschi, V.; Kessler, K.; Seltmann, A.-C.; Maser-Gluth, C.; et al. Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J. Clin. Endocrinol. Metab. 2015, 100, 2291–2302. [Google Scholar] [CrossRef]
- Stranger, B.E.; Forrest, M.S.; Dunning, M.; Ingle, C.E.; Beazley, C.; Thorne, N.; Redon, R.; Bird, C.P.; De Grassi, A.; Lee, C.; et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 2007, 315, 848–853. [Google Scholar] [CrossRef]
- Fadason, T.; Ekblad, C.; Ingram, J.R.; Schierding, W.S.; O’Sullivan, J.M. Physical interactions and expression quantitative traits loci identify regulatory connections for obesity and type 2 diabetes associated SNPs. Front. Genet. 2017, 8, 150. [Google Scholar] [CrossRef]
- Lavebratt, C.; Sjöholm, L.K.; Partonen, T.; Schalling, M.; Forsell, Y. PER2 variantion is associated with depression vulnerability. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 570–581. [Google Scholar] [CrossRef]
- Forbes, E.E.; Dahl, R.E.; Almeida, J.R.; Ferrell, R.E.; Nimgaonkar, V.L.; Mansour, H.; Sciarrillo, S.R.; Holm, S.M.; Rodriguez, E.E.; Phillips, M.L. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol. Psychiatry 2012, 71, 451–457. [Google Scholar] [CrossRef]
- Carpen, J.D.; Archer, S.N.; Skene, D.J.; Smits, M.; von Schantz, M. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. J. Sleep Res. 2005, 14, 293–297. [Google Scholar] [CrossRef]
- Satoh, K.; Mishima, K.; Inoue, Y.; Ebisawa, T.; Shimizu, T. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep 2003, 26, 416–417. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-J.; Kim, L.; Kang, S.-G.; Yoon, H.-K.; Choi, J.-E.; Park, Y.-M.; Kim, S.J.; Kripke, D.F. PER2 variation is associated with diurnal preference in a Korean young population. Behav. Genet. 2011, 41, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rios, A.; Perez-Martinez, P.; Delgado-Lista, J.; Phillips, C.M.; Gjelstad, I.M.; Wright, J.W.; Karlström, B.; Kie´c-Wilk, B.; van Hees, A.; Helal, O. A Period 2 genetic variant interacts with plasma SFA to modify plasma lipid concentrations in adults with metabolic syndrome. J. Nutr. 2012, 142, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef]
- Martinez, J.A.; Navas-Carretero, S.; Saris, W.H.; Astrup, A. Personalized weight loss strategies—The role of macronutrient distribution. Nat. Rev. Endocrinol. 2014, 10, 749–760. [Google Scholar] [CrossRef]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Hu, T.; Reynolds, K.; Yao, L.; Bunol, C.; Liu, Y.; Chen, C.-S.; Klag, M.J.; Whelton, P.K.; He, J. Effects of low-carbohydrate and low-fat diets: A randomized trial. Ann. Intern. Med. 2014, 161, 309–318. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Sharman, M.J.; Gómez, A.L.; Judelson, D.A.; Rubin, M.R.; Watson, G.; Sokmen, B.; Silvestre, R.; French, D.N.; Kraemer, W.J. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr. Metab. 2004, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Mitchell, S.J.; Coogan, S.C.; Cogger, V.C.; Gokarn, R.; McMahon, A.C.; Raubenheimer, D.; de Cabo, R.; Simpson, S.J.; Le Couteur, D.G. Dietary protein to carbohydrate ratio and caloric restriction: Comparing metabolic outcomes in mice. Cell Rep. 2015, 11, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.; Trichopoulos, D. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okuda, N.; Okamura, T.; Kadota, A.; Miyagawa, N.; Hayakawa, T.; Kita, Y.; Fujiyoshi, A.; Nagai, M.; Takashima, N.; et al. Low-carbohydrate diets and cardiovascular and total mortality in Japanese: A 29-year follow-up of NIPPON DATA80. Br. J. Nutr. 2014, 112, 916–924. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]

| Gene | SNP ID | Chromosome | Location | Alleles | MAF | HWE |
|---|---|---|---|---|---|---|
| CLOCK | rs11932595 | 4 | 55457430 | A/G | 0.1065 | 0.6955 |
| rs9312661 | 4 | 55476159 | G/A | 0.3604 | 0.2992 | |
| ARNTL | rs10766065 | 11 | 13256414 | T/C | 0.4983 | 0.9491 |
| rs9633835 | 11 | 13324046 | A/G | 0.4665 | 0.8643 | |
| PER2 | rs2304672 | 2 | 238277948 | G/C | 0.0620 | 0.5825 |
| rs3741892 | 12 | 106993385 | G/C | 0.2321 | 0.4557 | |
| CRY1 | rs11113192 | 12 | 107119148 | G/C | 0.2528 | 0.1215 |
| rs2541891 | 12 | 107184503 | C/G | 0.4131 | 0.3236 | |
| CRY2 | rs7951225 | 11 | 45853841 | A/T | 0.3498 | 0.5747 |
| Variables | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VLFC (T1) (n = 918) | LFC (T2) (n = 919) | OFC (T3) (n = 919) | p | Post Hoc | VLFC (T1) (n = 862) | LFC (T2) (n = 863) | OFC (T3) (n = 862) | p | Post Hoc | |
| General characteristics | ||||||||||
| Age (year) | 50.8 ± 7.3 | 48.6 ± 6.7 | 47.3 ± 6.4 | <0.0001 | A-B-C | 53.2 ± 7.5 | 49.7 ± 7.5 | 46.9 ± 6.3 | <0.0001 | A-B-C |
| BMI (kg/m2) | 24.2 ± 2.9 | 24.7 ± 2.9 | 24.6 ±2.8 | 0.0030 | A-B-B | 25.3 ± 3.4 | 24.7 ± 3.1 | 24.6 ± 3.3 | <0.0001 | A-B-B |
| Residential area—Urban | 522 (56.9) | 728 (79.3) | 779 (84.8) | <0.0001 | - | 361 (41.9) | 590 (68.4) | 672 (78) | <0.0001 | - |
| Body composition (1) | ||||||||||
| Lean body mass (kg) | 52.7 ± 5.8 | 54.2 ±6.1 | 54.6 ± 6.0 | <0.0001 | A-B-B | 39.8 ± 4.6 | 40.2 ± 4.3 | 40.7 ± 4.3 | 0.0011 | A-B-B |
| Lean body mass (%) | 78.1 ± 5.0 | 77.8 ± 4.9 | 78.2 ± 4.6 | 0.2600 | A-B-B | 67.7 ± 5.5 | 68.5 ± 4.9 | 68.9 ± 5.3 | 0.0002 | A-B-B |
| Body fat (kg) | 15.1 ± 4.7 | 15.7 ± 4.9 | 15.5 ± 4.6 | 0.0354 | A-B-B | 19.4 ± 5.3 | 18.8 ± 4.9 | 18.8 ± 5.3 | 0.0375 | A-B-B |
| Body fat (%) | 21.9 ± 4.9 | 22.1 ± 4.9 | 21.8 ± 4.5 | 0.3834 | A-B-B | 32.4 ± 5.1 | 31.5 ± 4.9 | 31.1 ± 5.2 | <0.0001 | A-B-B |
| Waist to hip ratio | 0.90 ± 0.04 | 0.90 ± 0.04 | 0.90 ± 0.04 | 0.1779 | A-B-B | 0.91 ± 0.05 | 0.90 ± 0.05 | 0.89 ± 0.05 | <0.0001 | A-B-B |
| Nutritional intake | ||||||||||
| Energy (kcal/day) | 1766.0 ± 509.6 | 1979.1 ± 423.3 | 2250.2 ± 527.9 | <0.0001 | A-B-C | 1640.4 ± 522.7 | 1834.6 ± 473.7 | 2020.5 ± 614.5 | <0.0001 | A-B-C |
| Carbohydrate (g/day) | 333.4 ± 99.3 | 344.0 ± 74.4 | 350.8 ± 82.3 | 0.0002 | A-B-B | 320.8 ± 103.4 | 330.8 ± 87.8 | 325.5 ± 100.0 | 0.095 | A-A-A |
| Protein (g/day) | 53.5 ± 16.4 | 67.9 ± 16.4 | 87.3 ± 24.3 | <0.0001 | A-B-C | 47.8 ± 16.4 | 61.8 ± 16.9 | 76.9 ± 25.7 | <0.0001 | A-B-C |
| Fat (g/day) | 21.4 ± 7.7 | 34.6 ± 8.4 | 53.1 ± 16.2 | <0.0001 | A-B-C | 15.9 ± 6.6 | 27.7 ± 8.0 | 44.2 ± 16.9 | <0.0001 | A-B-C |
| % Energy from each macronutrient | ||||||||||
| Carbohydrate | 75.5 ± 3.1 | 69.5± 1.9 | 62.5 ± 4.1 | <0.0001 | A-B-C | 78.2 ± 3.0 | 72.1 ± 2.1 | 64.5 ± 4.5 | <0.0001 | A-B-C |
| Protein | 12.1 ± 1.5 | 13.7 ± 1.4 | 15.5 ± 2.0 | <0.0001 | A-B-C | 11.7± 1.4 | 13.5 ± 1.6 | 15.3 ± 2.0 | <0.0001 | A-B-C |
| Fat | 10.8 ± 2.2 | 15.7 ± 1.2 | 21.1 ± 2.9 | <0.0001 | A-B-C | 8.6 ± 2.0 | 13.5 ± 1.3 | 19.6 ± 3.5 | <0.0001 | A-B-C |
| FC ratio | 0.14 ± 0.03 | 0.23 ± 0.02 | 0.34 ± 0.08 | <0.0001 | A-B-C | 0.11 ± 0.03 | 0.19 ± 0.02 | 0.31 ± 0.09 | <0.0001 | A-B-C |
| Number of regular meal (meal/day) | 2.9 ± 0.3 | 2.9 ± 0.3 | 2.8 ± 0.4 | <0.0001 | A-B-C | 2.9 ± 0.3 | 2.8 ± 0.4 | 2.7 ± 0.5 | <0.0001 | A-B-C |
| Alcohol intake (g/day) | 16.0 ± 24.6 | 18.0 ± 26.5 | 24.8 ± 32.7 | <0.0001 | A-A-B | 1.0 ± 4.0 | 1.2 ± 4.2 | 2.4 ± 7.6 | <0.0001 | A-A-B |
| Tobacco consumption (pack/year) | 17.9 ± 17.2 | 16.4 ± 16.2 | 18.2 ± 17.3 | 0.0585 | A-A-A | 0.3 ± 2.7 | 0.3 ± 2.9 | 0.4 ± 2.4 | 0.9189 | A-A-A |
| Sleep duration (h) | 6.9 ± 1.2 | 6.7 ± 1.3 | 6.6 ± 1.3 | 0.0003 | A-B-B | 6.8 ± 1.4 | 6.6 ± 1.4 | 6.4 ± 1.4 | <0.0001 | A-B-C |
| Moderate physical activity (2) | 314 (34.2) | 322 (35.0) | 392 (42.7) | 0.0002 | - | 244 (28.3) | 337 (39.0) | 347 (40.3) | <0.0001 | - |
| Male | p | Female | p | |
|---|---|---|---|---|
| Obesity (1) | ||||
| VLFC (T1) | 1.15 (0.93–1.42) | 0.205 | 1.50 (1.20–1.86) | 0.000 |
| LFC (T2) | 1.29 (1.07–1.57) | 0.010 | 1.12 (0.91–1.37) | 0.281 |
| OFC (T3) | 1 | 1 | ||
| Abdominal obesity (2) | ||||
| VLFC (T1) | 0.92 (0.64–1.33) | 0.670 | 1.84 (1.36–2.48) | <0.0001 |
| LFC (T2) | 0.87 (0.54–1.40) | 0.449 | 0.90 (0.67–1.20) | 0.462 |
| OFC (T3) | 1 | 1 |
| Gene | SNP | Obesity (1) | Abdominal Obesity (2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VLFC (T1) | LFC (T2) | OFC (T3) | p- Interaction | VLFC (T1) | LFC (T2) | OFC (T3) | p- Interaction | |||
| CLOCK | rs11932595 | AA | 1.14 (0.90–1.44) | 1.31 (1.06–1.63) | 1 | 0.892 | 0.95 (0.63–1.43) | 0.96 (0.66–1.39) | 1 | 0.604 |
| GA/GG | 1.41 (1.00–1.98) | 1.46 (1.04–2.04) | 1.21 (0.86–1.69) | 1.18 (0.67–2.07) | 0.93 (0.52–1.66) | 1.45 (0.84–2.51) | ||||
| rs9312661 | AA | 1.13 (0.83–1.54) | 1.34 (0.99–1.80) | 1 | 0.906 | 1.03 (0.59–1.77) | 1.11 (0.67–1.84) | 1 | 0.501 | |
| GA/GG | 1.35 (1.01–1.81) | 1.47 (1.11–1.94) | 1.16 (0.88–1.51) | 1.11 (0.66–1.86) | 0.95 (0.58–1.56) | 1.27 (0.80–2.03) | ||||
| ARNTL | rs10766065 | TT | 1.16 (0.79–1.71) | 1.38 (0.96–2.00) | 1 | 0.910 | 1.38 (0.71–2.68) | 0.77 (0.40–1.47) | 1 | 0.145 |
| CT/CC | 1.21 (0.88–1.66) | 1.34 (0.98–1.81) | 1.06 (0.78–1.43) | 1.08 (0.61–1.90) | 1.24 (0.72–2.13) | 1.33 (0.79–2.26) | ||||
| rs9633835 | AA | 1.24 (0.86–1.79) | 1.40 (0.97–2.02) | 1 | 0.839 | 0.74 (0.39–1.38) | 0.79 (0.42–1.46) | 1 | 0.700 | |
| GA/GG | 1.24 (0.90–1.70) | 1.40 (1.03–1.89) | 1.11 (0.83–1.50) | 0.71 (0.41–1.22) | 0.65 (0.39–1.08) | 0.71 (0.43–1.17) | ||||
| PER2 | rs2304672 | GG | 1.18 (0.94–1.47) | 1.33 (1.09–1.64) | 1 | 0.665 | 1.01 (0.69–1.49) | 0.89 (0.62–1.28) | 1 | 0.281 |
| CG/CC | 1.04 (0.69–1.59) | 1.15 (0.78–1.70) | 1.11 (0.72–1.70) | 0.57 (0.27–1.21) | 0.96 (0.50–1.86) | 1.21 (0.60–2.44) | ||||
| CRY1 | rs3741892 | GG | 1.01 (0.77–1.31) | 1.14 (0.89–1.47) | 1 | 0.180 | 0.75 (0.48–1.19) | 0.74 (0.48–1.13) | 1 | 0.262 |
| CG/CC | 1.12 (0.84–1.50) | 1.24 (0.95–1.63) | 0.81 (0.62–1.05) | 0.76 (0.46–1.25) | 0.69 (0.44–1.10) | 0.60 (0.38–0.96) | ||||
| rs11113192 | GG | 1.50 (1.14–1.97) | 1.52 (1.17–1.96) | 1 | 0.009 | 1.10 (0.68–1.77) | 1.02 (0.65–1.60) | 1 | 0.542 | |
| CG/CC | 1.06 (0.80–1.41) | 1.37 (1.04–1.80) | 1.28 (0.98–1.67) | 1.17 (0.70–1.95) | 1.14 (0.71–1.83) | 1.50 (0.95–2.36) | ||||
| rs2541891 | CC | 1.31 (0.94–1.84) | 1.47 (1.06–2.03) | 1 | 0.522 | 0.77 (0.43–1.38) | 1.01 (0.58–1.75) | 1 | 0.385 | |
| GC/GG | 1.09 (0.81–1.47) | 1.23 (0.93–1.64) | 1.02 (0.77–1.35) | 1.18 (0.71–1.96) | 0.95 (0.58–1.55) | 1.16 (0.72–1.88) | ||||
| CRY2 | rs7951225 | AA | 1.17 (0.86–1.60) | 1.44 (1.07–1.92) | 1 | 0.617 | 0.94 (0.55–1.61) | 1.20 (0.73–1.96) | 1 | 0.159 |
| TA/TT | 1.35 (1.01–1.80) | 1.44 (1.09–1.89) | 1.20 (0.91–1.56) | 0.92 (0.56–1.50) | 0.68 (0.42–1.11) | 1.01 (0.64–1.60) | ||||
| Gene | SNP | Obesity (1) | Abdominal Obesity (2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VLFC (T1) | LFC (T2) | OFC (T3) | p- Interaction | VLFC (T1) | LFC (T2) | OFC (T3) | p- Interaction | |||
| CLOCK | rs11932595 | AA | 1.35 (1.06–1.71) | 1.04 (0.83–1.30) | 1 | 0.093 | 1.84 (1.32–2.56) | 0.84 (0.60–1.17) | 1 | 0.572 |
| GA/GG | 1.74 (1.23–2.46) | 1.14 (0.81–1.61) | 0.76 (0.53–1.08) | 2.05 (1.30–3.22) | 1.29 (0.79–2.09) | 1.14 (0.68–1.93) | ||||
| rs9312661 | AA | 1.17 (0.85–1.61) | 0.95 (0.69–1.30) | 1 | 0.123 | 2.26 (1.43–3.56) | 0.93 (0.58–1.48) | 1 | 0.404 | |
| GA/GG | 1.54 (1.14–2.07) | 1.09 (0.82–1.45) | 0.88 (0.66–1.16) | 2.11 (1.38–3.23) | 1.15 (0.75–1.77) | 1.32 (0.86–2.02) | ||||
| ARNTL | rs10766065 | TT | 1.66 (1.11–2.47) | 0.99 (0.67–1.47) | 0.434 | 2.46 (1.39–4.35) | 0.72 (0.39–1.34) | 1 | 0.113 | |
| CT/CC | 1.56 (1.12–2.17) | 1.25 (0.91–1.73) | 1.08 (0.79–1.49) | 2.15 (1.32–3.51) | 1.22 (0.75–1.99) | 1.30 (0.79–2.11) | ||||
| rs9633835 | AA | 1.48 (0.99–2.13) | 1.14 (0.79–1.65) | 1 | 0.953 | 1.11 (0.66–1.87) | 0.68 (0.39–1.16) | 1 | 0.070 | |
| GA/GG | 1.64 (1.19–2.27) | 1.20 (0.88–1.68) | 1.09 (0.80–1.48) | 1.56 (0.99–2.48) | 0.70 (0.44–1.10) | 0.7 (0.44–1.12) | ||||
| PER2 | rs2304672 | GG | 1.49 (1.18–1.87) | 1.14 (0.92–1.42) | 1 | 0.670 | 1.85 (1.35–2.54) | 0.87 (0.64–1.19) | 1 | 0.827 |
| CG/CC | 1.59 (1.04–2.43) | 0.94 (0.60–1.47) | 1.02 (0.66–1.56) | 1.28 (0.74–2.22) | 0.76 (0.39–1.49) | 0.68 (0.34–1.34) | ||||
| CRY1 | rs3741892 | GG | 1.60 (1.22–2.10) | 1.16 (0.90–1.51) | 1 | 0.728 | 1.90 (1.30–2.76) | 0.86 (0.59–1.26) | 1 | 0.793 |
| CG/CC | 1.76 (1.30–2.38) | 1.38 (1.03–1.83) | 1.29 (0.98–1.71) | 1.70 (1.13–2.56) | 0.93 (0.61–1.41) | 0.98 (0.64–1.49) | ||||
| rs11113192 | GG | 1.48 (1.12–1.96) | 1.14 (0.87–1.49) | 1 | 0.960 | 1.84 (1.25–2.70) | 0.91 (0.61–1.34) | 1 | 0.995 | |
| CG/CC | 1.37 (1.02–1.84) | 1.00 (0.75–1.32) | 0.91 (0.69–1.20) | 1.89 (1.26–2.82) | 0.91 (0.60–1.37) | 1.03 (0.68–1.56) | ||||
| rs2541891 | CC | 1.36 (0.96–1.94) | 0.91 (0.64–1.28) | 1 | 0.341 | 1.96 (1.20–3.20) | 0.80 (0.48–1.32) | 1 | 0.621 | |
| GC/GG | 1.55 (1.13–2.11) | 1.23 (0.91–1.65) | 0.99 (0.74–1.33) | 1.96 (1.26–3.06) | 1.05 (0.67–1.64) | 1.11 (0.71–1.72) | ||||
| CRY2 | rs7951225 | AA | 1.52 (1.10–2.08) | 1.10 (0.80–1.51) | 1 | 0.981 | 2.07 (1.32–3.24) | 0.93 (0.58–1.49) | 1 | 0.768 |
| TA/TT | 1.63 (1.20–2.23) | 1.23 (0.92–1.64) | 1.09 (0.82–1.45) | 1.96 (1.26–3.02) | 1.01 (0.66–1.56) | 1.15 (0.75–1.77) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shon, J.; Han, Y.; Park, Y.J. Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients 2022, 14, 478. https://doi.org/10.3390/nu14030478
Shon J, Han Y, Park YJ. Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients. 2022; 14(3):478. https://doi.org/10.3390/nu14030478
Chicago/Turabian StyleShon, Jinyoung, Yerim Han, and Yoon Jung Park. 2022. "Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes" Nutrients 14, no. 3: 478. https://doi.org/10.3390/nu14030478
APA StyleShon, J., Han, Y., & Park, Y. J. (2022). Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients, 14(3), 478. https://doi.org/10.3390/nu14030478






