Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of Biochemical Parameters
2.3. Morphological Analysis of Pancreas and Kidney Tissues
2.4. Protein Extraction and Antioxidant Enzymes Activities
2.5. Acid Extraction, H2O2 Content, Lipid Peroxidation, and Thiol Content
2.6. Determination of Carbonylated Protein Content
2.7. Statistical Analysis
3. Results
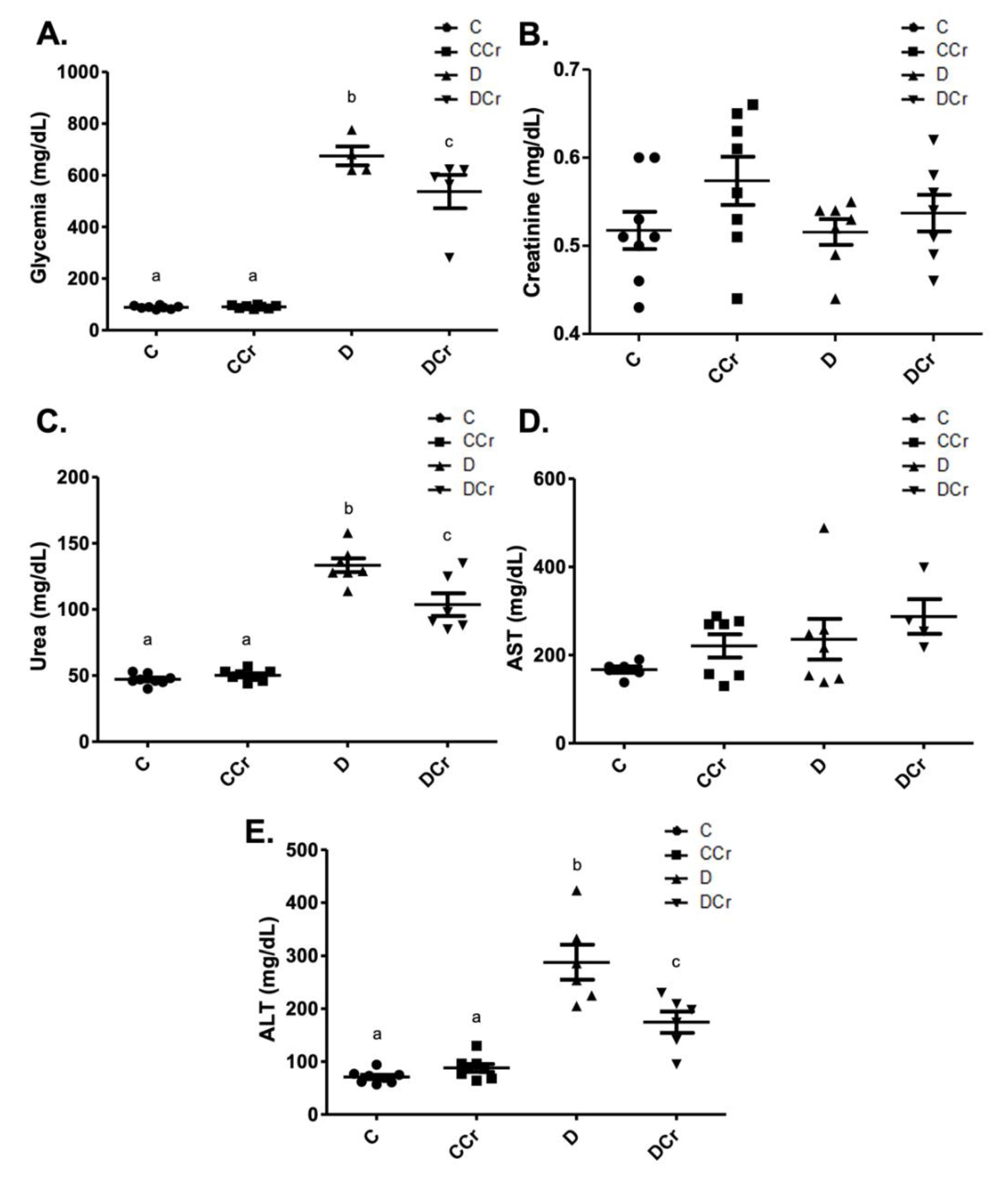
3.1. Evaluation of Physiologic and Clinical Parameters
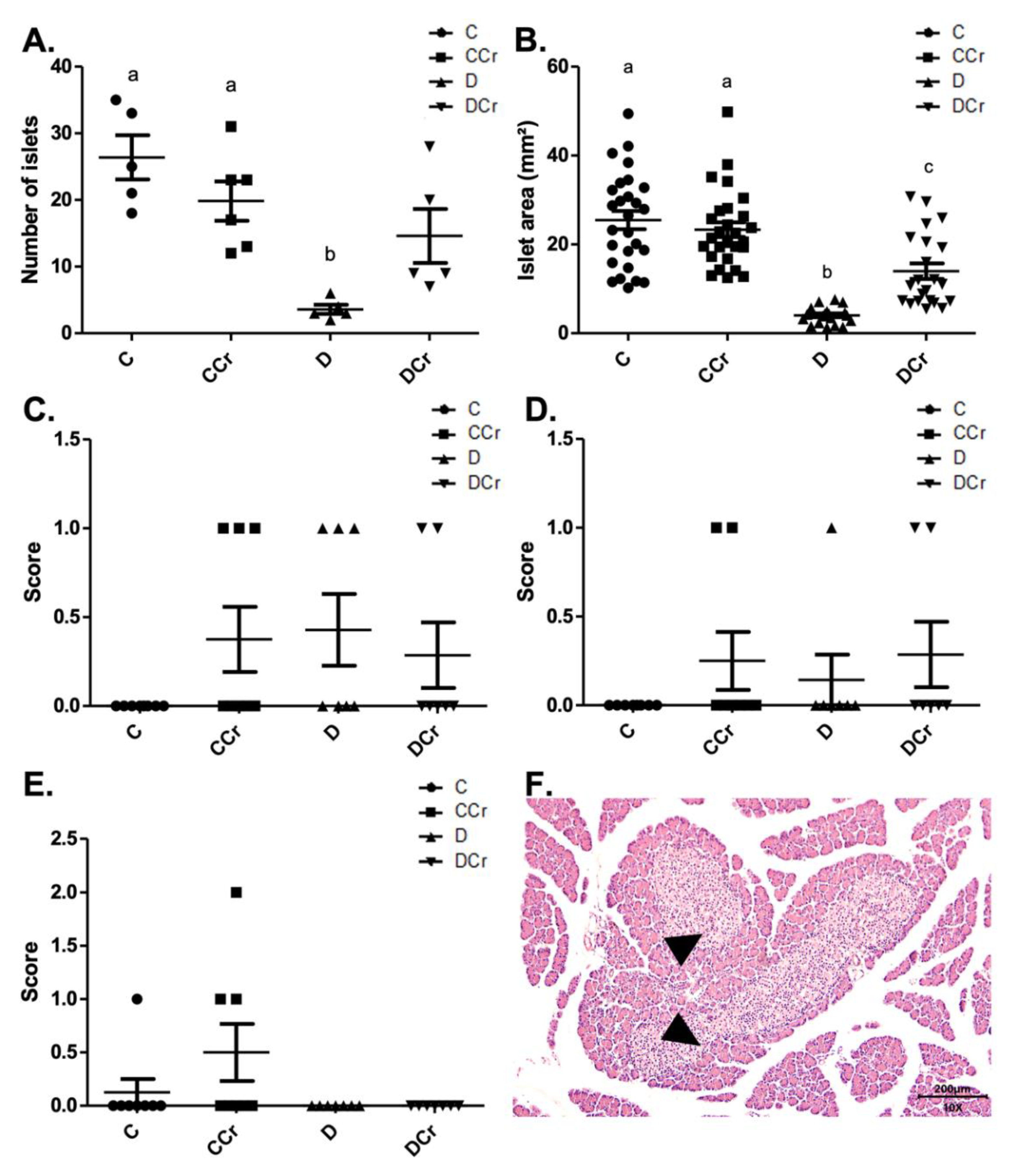
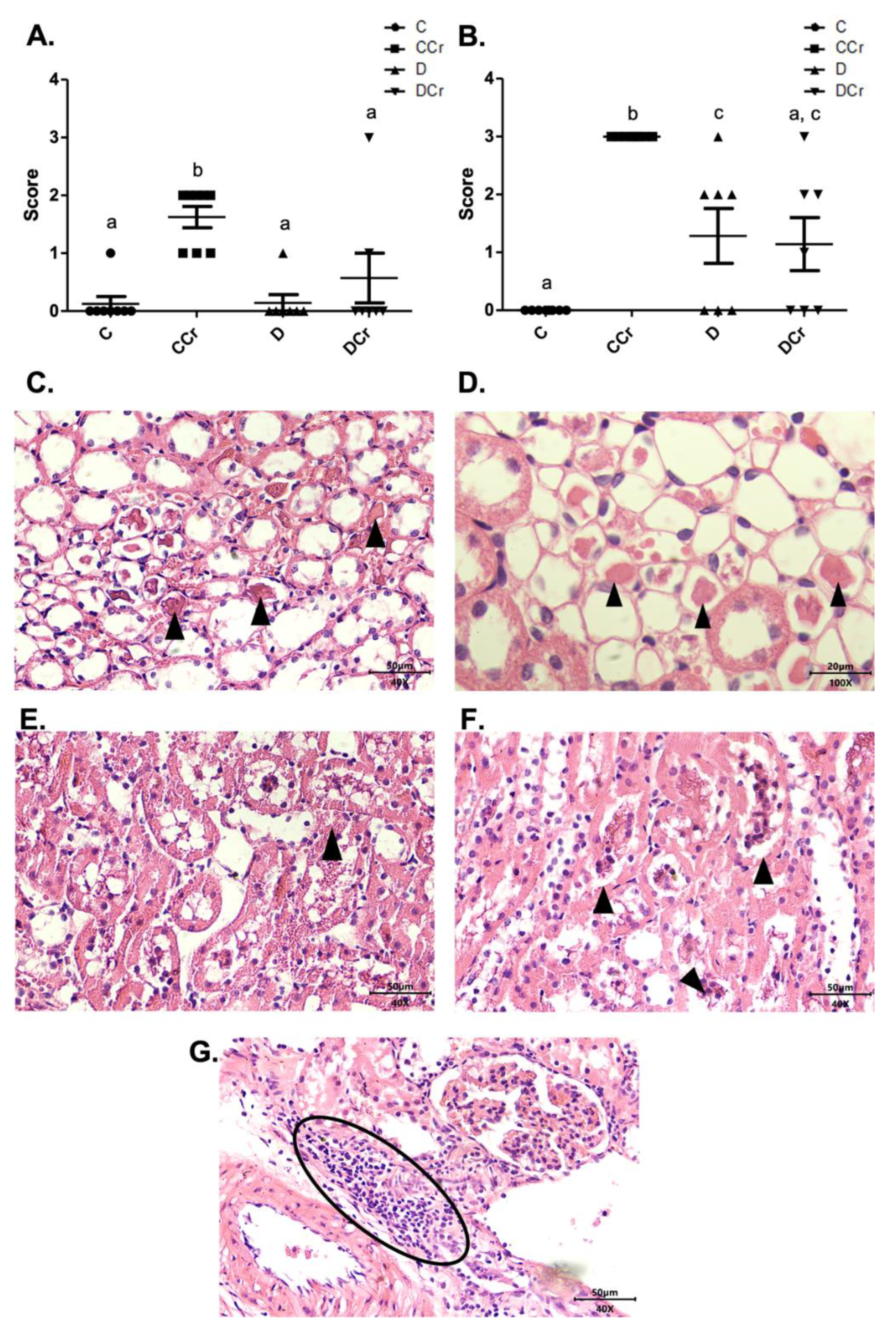
3.2. Morphological Analyses of the Pancreatic Tissue
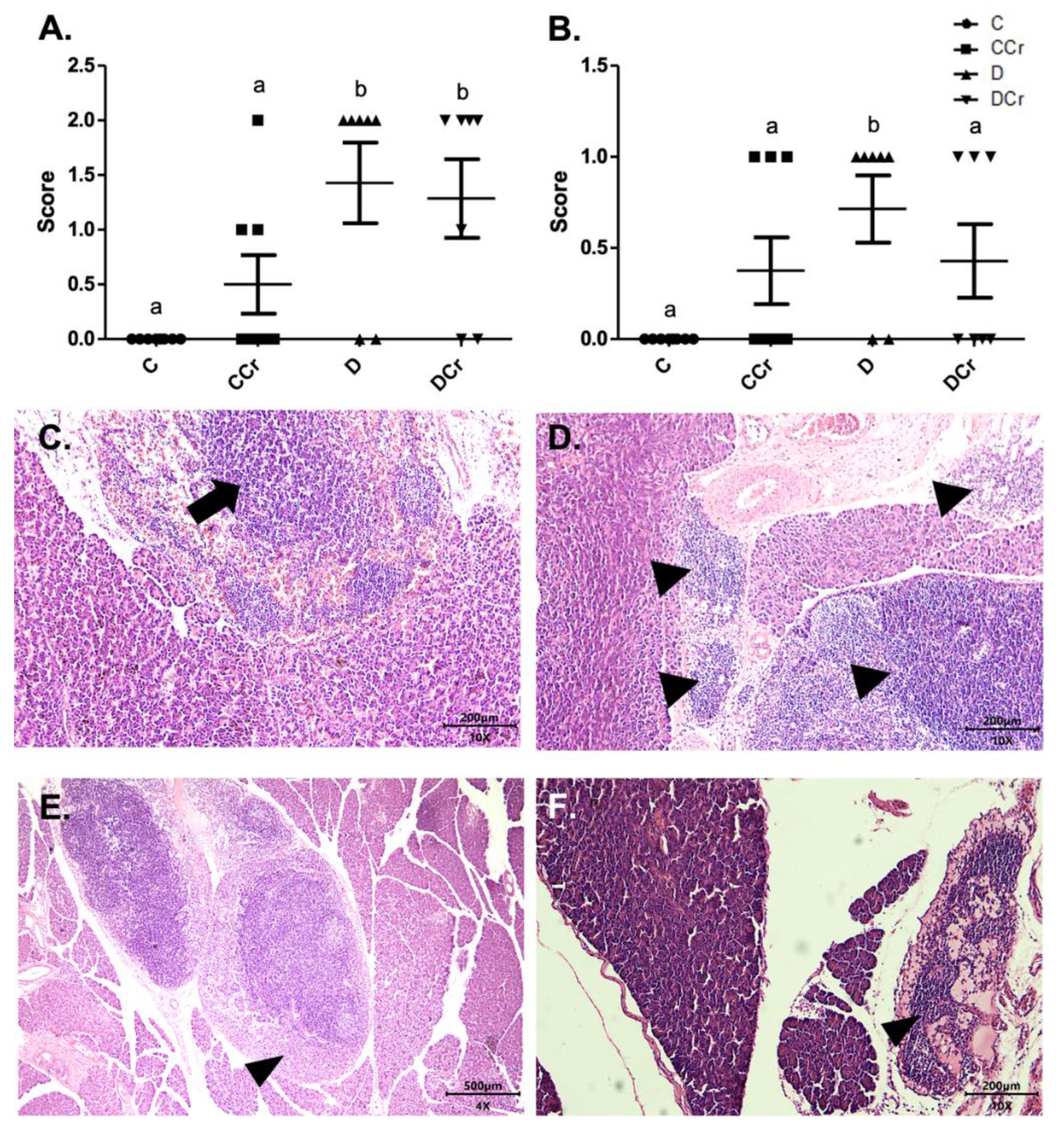
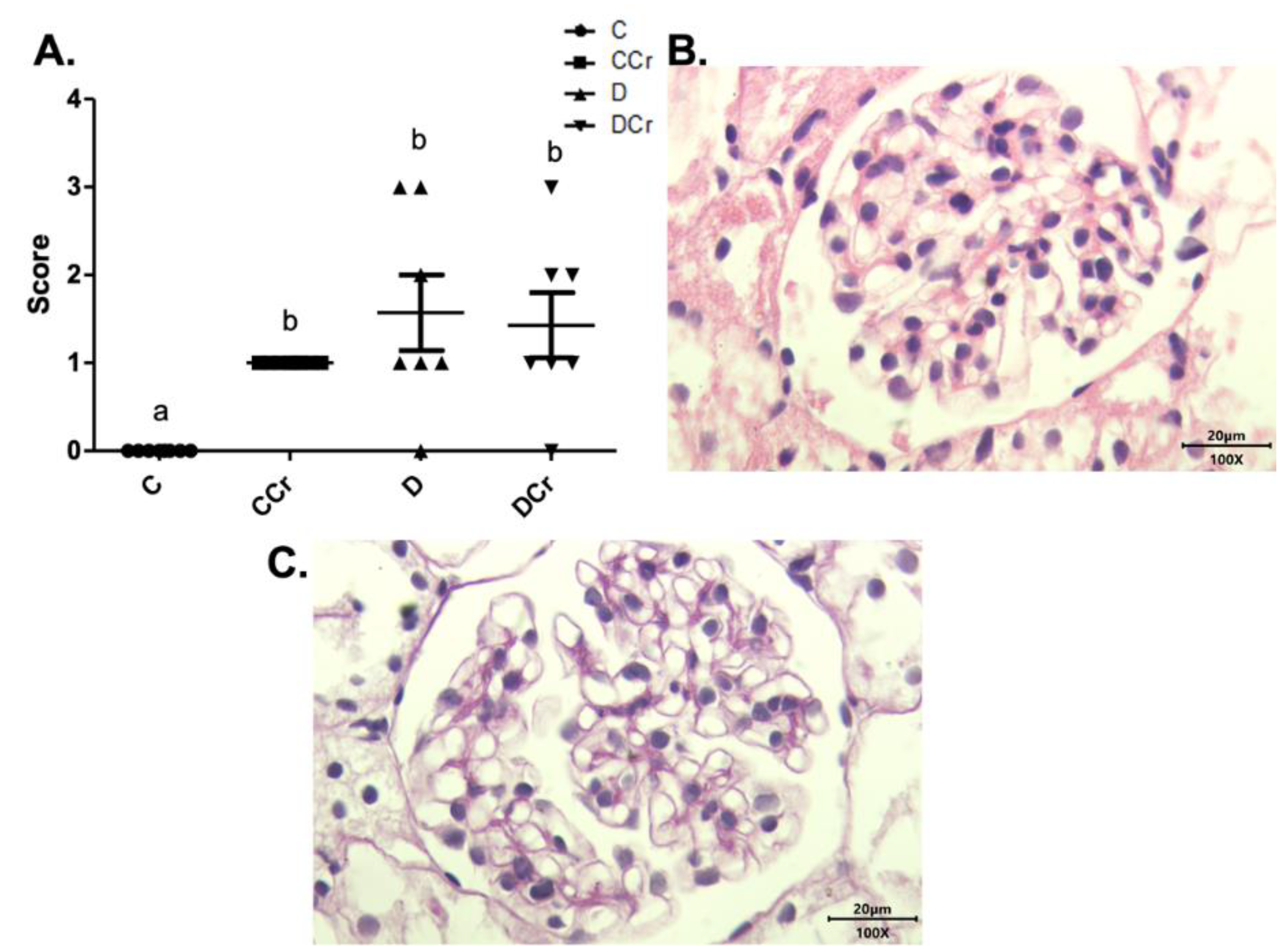
3.3. Morphological Analyses of Renal Tissues
3.4. Antioxidant Enzyme Activities and Redox State Parameters from Renal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Care, D. Classification and diagnosis of diabetes: Standards of medical care in diabetes 2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2019; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 9782930229812. [Google Scholar]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef]
- Schmidt, A.M. Highlighting Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Sociedade Brasileira de Diabetes. Diretrizes SBD 2017–2018; Clannad Editora Científica: São Paulo, Brazil, 2021; Volume 8, ISBN 9788593746024. [Google Scholar]
- Pearson, E.R. Type 2 diabetes: A multifaceted disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- De Beaufort, C.; Besançon, S.; Balde, N. Management of type 1 diabetes [Prise en charge du diabète de type 1]. Med. Sante Trop. 2019, 28, 359–362. [Google Scholar]
- Lucier, J.; Weinstock, R.S. Diabetes Mellitus Type 1; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhang, M.; Feng, L.; Gu, J.; Ma, L.; Qin, D.; Wu, C.; Jia, X. The attenuation of Moutan cortex on oxidative stress for renal injury in AGEs-induced mesangial cell dysfunction and streptozotocin-induced diabetic nephropathy rats. Oxid. Med. Cell. Longev. 2014, 2014, 463815. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Castro, E. O papel dos produtos finais de glicosilação avançada na nefropatia diabética. Arq. Med. 2011, 25, 27–37. [Google Scholar]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Bassey, I.E.; Ikpi, D.E.; Isong, I.K.P.; Akpan, U.O.; Onyeukwu, C.C.; Nwankwo, N.P.; Udofia, I.G. Effect of combined calcium, magnesium, vitamin C and E supplementation on seminal parameters and serum oxidative stress markers in fructose-induced diabetic Wistar rats. Arch. Physiol. Biochem. 2020, 126, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef]
- Karalis, D.T. The Beneficiary Role of Selenium in Type II Diabetes: A Longitudinal Study. Cureus 2019, 11, e6443. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Chen, P.Y.; Wang, C.M.; Chen, W.Y.; Chen, C.W.; Owaga, E.; Chang, J.S. Dose-related effects of ferric citrate supplementation on endoplasmic reticular stress responses and insulin signalling pathways in streptozotocin-nicotinamide-induced diabetes. Food Funct. 2016, 7, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.J.; Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Assaf, A.; Parmar, M.Y.; Ahmed, M.M. Lutein Dietary Supplementation Attenuates Streptozotocin-induced testicular damage and oxidative stress in diabetic rats. BMC Complement. Altern. Med. 2015, 15, 204. [Google Scholar] [CrossRef]
- Matter, R.M.; Elbarbary, N.S.; Ismail, E.A.R.; Darwish, Y.W.; Nada, A.S.; Banoub, V.P. Zinc supplementation improves glucose homeostasis in patients with β-thalassemia major complicated with diabetes mellitus: A randomized controlled trial. Nutrition 2020, 73, 110702. [Google Scholar] [CrossRef]
- Rašković, A.; Ćućuz, V.; Torović, L.; Tomas, A.; Gojković-Bukarica, L.; Ćebović, T.; Milijašević, B.; Stilinović, N.; Cvejić Hogervorst, J. Resveratrol supplementation improves metabolic control in rats with induced hyperlipidemia and type 2 diabetes. Saudi Pharm. J. 2019, 27, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.Y.; Artioli, G.G.; Gualano, B. Potential of creatine in glucose management and diabetes. Nutrients 2021, 13, 570. [Google Scholar] [CrossRef]
- Gualano, B.; Novaes, R.B.; Artioli, G.G.; Freire, T.O.; Coelho, D.F.; Scagliusi, F.B.; Rogeri, P.S.; Roschel, H.; Ugrinowitsch, C.; Lancha, A.H. Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 2008, 34, 245–250. [Google Scholar] [CrossRef]
- Snow, R.J.; Murphy, R.M. Creatine and the creatine transporter: A review. Mol. Cell. Biochem. 2001, 224, 169–181. [Google Scholar] [CrossRef]
- Balsom, P.D.; Söderlund, K.; Ekblom, B. Creatine in Humans with Special Reference to Creatine Supplementation. Sport. Med. 1994, 18, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.B. Creatine: Biosynthesis, regulation and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar] [PubMed]
- De Souza e Silva, A.; Pertille, A.; Reis Barbosa, C.G.; Aparecida de Oliveira Silva, J.; de Jesus, D.V.; Ribeiro, A.G.S.V.; Baganha, R.J.; de Oliveira, J.J. Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019, 29, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; Portari, G.V.; Vannucchi, H.; Jordao, A.A. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br. J. Nutr. 2009, 102, 110–116. [Google Scholar] [CrossRef]
- Clarke, H.; Kim, D.H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The evolving applications of creatine supplementation: Could creatine improve vascular health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; Van Dusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Taner, B.; Aysim, O.; Abdulkadir, U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2011, 4, 23–24. [Google Scholar] [CrossRef]
- Thorsteinsdottir, B.; Grande, J.P.; Garovic, V.D. Acute Renal Failure in a Young Weight Lifter Taking Multiple Food Supplements, Including Creatine Monohydrate. J. Ren. Nutr. 2006, 16, 341–345. [Google Scholar] [CrossRef]
- Robinson, T.M.; Sewell, D.A.; Casey, A.; Steenge, G.; Greenhaff, P.L. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br. J. Sports Med. 2000, 34, 284–288. [Google Scholar] [CrossRef]
- Carvalho, A.P.P.F.; Molina, G.E.; Fontana, K.E. Creatine supplementation associated with resistance training does not alter renal and hepatic functions. Rev. Bras. Med. Esporte 2011, 17, 237–241. [Google Scholar] [CrossRef][Green Version]
- Domingues, W.J.R.; Ritti-Dias, R.M.; Cucato, G.G.; Wolosker, N.; Zerati, A.E.; Puech-Leão, P.; Nunhes, P.M.; Moliterno, A.A.; Avelar, A. Does Creatine Supplementation Affect Renal Function in Patients with Peripheral Artery Disease? A Randomized, Double Blind, Placebo-controlled, Clinical Trial. Ann. Vasc. Surg. 2020, 63, 45–52. [Google Scholar] [CrossRef]
- Gualano, B.; De Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; Da Silva, M.E.R.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Ugrinowitsch, C.; Novaes, R.B.; Artioli, G.G.; Shimizu, M.H.; Seguro, A.C.; Harris, R.C.; Lancha, A.H. Effects of creatine supplementation on renal function: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Appl. Physiol. 2008, 103, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sujithra, K.; Srinivasan, S.; Indumathi, D.; Vinothkumar, V. Allyl methyl sulfide, a garlic active component mitigates hyperglycemia by restoration of circulatory antioxidant status and attenuating glycoprotein components in streptozotocin-induced experimental rats. Toxicol. Mech. Methods 2019, 29, 165–176. [Google Scholar] [CrossRef]
- Hultman, E.; Söderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Vandenberghe, K.; Goris, M.; Van Hecke, P.; Van Leemputte, M.; Vangerven, L.; Hespel, P. Long-term creatine intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. 1997, 83, 2055–2063. [Google Scholar] [CrossRef]
- Araújo, M.B.; Moura, L.P.; Junior, R.C.; Junior, M.C.; Dalia, R.A.; Sponton, A.C.; Ribeiro, C.; Mello, M.A. Creatine supplementation and oxidative stress in rat liver. J. Int. Soc. Sports Nutr. 2013, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.S.; Smith, J.L.; Oppelt, P.J.; Fisher, J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E347–E352. [Google Scholar] [CrossRef][Green Version]
- Marzuca-Nassr, G.N.; Fortes, M.A.S.; Guimarães-Ferreira, L.; Murata, G.M.; Vitzel, K.F.; Vasconcelos, D.A.A.; Bassit, R.A.; Curi, R. Short-term creatine supplementation changes protein metabolism signaling in hindlimb suspension. Braz. J. Med. Biol. Res. 2019, 52, e8391. [Google Scholar] [CrossRef] [PubMed]
- Eijnde, B.O.; Richter, E.A.; Henquin, J.C.; Kiens, B.; Hespel, P. Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol. Scand. 2001, 171, 169–176. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Olivia, F.; Denaro, V.; Maffulli, N. Oxygen species and overuse tendinopathy in athletes. Disabil. Rehabil. 2008, 30, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.I.; Monteiro, I.C.; Valença, S.; Leal-Cardoso, J.H.; Fortunato, R.S.; Carvalho, D.P.; Teodoro, B.G.; Ceccatto, V.M. Effect of exercise training on liver antioxidant enzymes in STZ-diabetic rats. Life Sci. 2015, 128, 64–71. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Exogenous application of triadimefon affects the antioxidant defense system of Withania somnifera Dunal. Pestic. Biochem. Physiol. 2008, 91, 170–174. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Riddles, P.W.; Blakeley, R.L.; Zerner, B. Ellman’s Reagent: 5,5’-Dithiobis(2-nitrobenzoic Acid)—A Reexamination. Anal. Biochem. 1979, 94, 75–81. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.M.; Heck, T.G.; Wronski, E.C.; Ulbrich, A.Z.; Boff, E. Effects of creatine supplementation on biomarkers of hepatic and renal function in young trained rats. Toxicol. Mech. Methods 2013, 23, 697–701. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Deminice, R.; Vilhena, R.; Portari, G.V.; Jordão, A.A. Suplementação de creatina, homocisteína e estresse oxidativo. Medicina 2007, 40, 368–377. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct Antioxidant Properties of Creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Bravi, G.; Piccoli, G.; Curci, R.; Battistelli, M.; Falcieri, E.; Agostini, D.; Gioacchini, A.M.; Stocchi, V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006, 40, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Stefani, G.P.; Nunes, R.B.; Dornelles, A.Z.; Alves, J.P.; Piva, M.O.; Di Domenico, M.; Rhoden, C.R.; Lago, P.D. Effects of creatine supplementation associated with resistance training on oxidative stress in different tissues of rats. J. Int. Soc. Sports Nutr. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shibata, W.; Maeda, S. Adhesion molecules and pancreatitis. J. Gastroenterol. 2019, 54, 99–107. [Google Scholar] [CrossRef]
- Mohapatra, S.; Majumder, S.; Smyrk, T.C.; Matveyenko, A.; Kudva, Y.C.; Chari, S.T. Diabetes Mellitus Causes an Exocrine Pancreatopathy: Conclusions Based on a Systematic Review. Am. J. Gastroenterol. 2015, 110, S2–S3. [Google Scholar] [CrossRef]
- Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965, 14, 619–633. [Google Scholar] [CrossRef]
- Davison, L.J. Diabetes mellitus and pancreatitis—Cause or effect? J. Small Anim. Pract. 2015, 56, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Willcox, A.; Gillespie, K.M. Histology of type 1 diabetes pancreas. Methods Mol. Biol. 2016, 1433, 105–117. [Google Scholar] [CrossRef]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Ferber, S.; Johnson, J.H.; Newgard, C.B. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 1994, 43, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Paul, J.; Shihaz, A.V.H. Pancreatic steatosis: A new diagnosis and therapeutic challenge in gastroenterology. Arq. Gastroenterol. 2020, 57, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Skog, O.; Korsgren, O. On the dynamics of the human endocrine pancreas and potential consequences for the development of type 1 diabetes. Acta Diabetol. 2020, 57, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol. Int. 2019, 69, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Hemmer, W. Creatine kinase in non-muscle tissues and cells. Mol. Cell. Biochem. 1994, 133–134, 193–220. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef] [PubMed]
- Obineche, E.N.; Mensah-Brown, E.; Chandranath, S.I.; Ahmed, I.; Naseer, O.; Adem, A. Morphological changes in the rat kidney following long-term diabetes. Arch. Physiol. Biochem. 2001, 109, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Najafian, B.; Mauer, M. Morphologic Features of Declining Renal Function in Type 1 Diabetes. Semin. Nephrol. 2012, 32, 415–422. [Google Scholar] [CrossRef]
- Caramori, M.L.; Kim, Y.; Huang, C.; Fish, A.J.; Rich, S.S.; Miller, M.E.; Russell, G.; Mauer, M. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 2002, 51, 506–513. [Google Scholar] [CrossRef]
- Nephropathy, P.O.D. Fisiopatologia da nefropatia diabética. Rev. Méd. Minas Gerais 2004, 14, 180–185. [Google Scholar]
- Craven, P.A.; DeRubertis, F.R.; Kagan, V.E.; Melhem, M.; Studer, R.K. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. J. Am. Soc. Nephrol. 1997, 8, 1405–1414. [Google Scholar] [CrossRef]
- Osterby, R.; Gundersen, H.J. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 1975, 11, 225–229. [Google Scholar] [CrossRef]
- McPhee, S.J.; Ganong, W.F. Fisiopatologia da Doença; McGraw-Hill: New York, NY, USA, 2011; pp. 367–388. ISBN 978-85-7726-010-2. [Google Scholar]
- Fioretto, P.; Mauer, M. Glomerular changes in normo- and microalbuminuric patients with long-standing insulin-dependent diabetes mellitus. Adv. Nephrol. Necker Hosp. 1997, 26, 247–263. [Google Scholar] [PubMed]
- Steinke, J.M.; Sinaiko, A.R.; Kramer, M.S.; Suissa, S.; Chavers, B.M.; Mauer, M. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 2005, 54, 2164–2171. [Google Scholar] [CrossRef]
- Gualano, B.; DE Salles Painneli, V.; Roschel, H.; Artioli, G.G.; Neves, M.J.; De Sá Pinto, A.L.; Da Silva, M.E.R.; Cunha, M.R.; Otaduy, M.C.G.; Leite, C.D.C.; et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef]
- Op’t Eijnde, B.; Ursø, B.; Richter, E.A.; Greenhaff, P.L.; Hespel, P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes 2001, 50, 18–23. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Adewole, O.S. Hibiscus sabdariffa renews pancreatic β-cells in experimental type 1 diabetic model rats. Morphologie 2019, 103, 80–93. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Kaikini, A.A.; Dhodi, D.; Muke, S.; Peshattiwar, V.; Bagle, S.; Korde, A.; Sarnaik, J.; Kadwad, V.; Sachdev, S.; Sathaye, S. Standardization of type 1 and type 2 diabetic nephropathy models in rats: Assessment and characterization of metabolic features and renal injury. J. Pharm. Bioallied Sci. 2020, 12, 295–307. [Google Scholar] [CrossRef]
- Miaffo, D.; Ntchapda, F.; Mahamad, T.A.; Maidadi, B.; Kamanyi, A. Hypoglycemic, antidyslipidemic and antioxydant effects of Vitellaria paradoxa barks extract on high-fat diet and streptozotocin-induced type 2 diabetes rats. Metab. Open 2021, 9, 100071. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, Z.; Hadjzadeh, M.-A.-R.; Moradi, R.; Ghorbani, A.; Saghebi, A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in streptozotocin-induced diabetic rats. Adv. Biomed. Res. 2015, 4, 33. [Google Scholar] [CrossRef]
- Dalbo, V.; Roberts, M.; Stout, J.; Kerksick, C. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br. J. Sports Med. 2008, 42, 567–573. [Google Scholar] [CrossRef]
- Powers, M.E.; Arnold, B.L.; Weltman, A.L.; Perrin, D.H.; Mistry, D.; Kahler, D.M.; Kraemer, W.; Volek, J. Creatine supplementation increases total body water without altering fluid distribution. J. Athletic Train. 2003, 38, 44–50. [Google Scholar]
- Carlos Sales Zanelli, J.; Alves Cordeiro, B.; Teles Soares Beserra, B.; de Moraes, B.S.; Trindade, E. Creatine and Resistance Training: Effect on Hydration and Lean Body Mass. Rev. Bras. Med. Esporte 2015, 21, 27–31. [Google Scholar]
- Lopez, R.M.; Casa, D.J.; McDermott, B.P.; Ganio, M.S.; Armstrong, L.E.; Maresh, C.M. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J. Athl. Train. 2009, 44, 215–223. [Google Scholar] [CrossRef]
- Dabla, P.K. Renal function in diabetic nephropathy. World J. Diabetes 2010, 1, 48–56. [Google Scholar] [CrossRef]
- Ji, E.; Kim, Y.S. Prevalence of chronic kidney disease defined by using CKD-EPI equation and albumin-to-creatinine ratio in the Korean adult population. Korean J. Intern. Med. 2016, 31, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Persky, A.M.; Rawson, E.S. Safety of creatine supplementation. Subcell. Biochem. 2007, 46, 275–289. [Google Scholar] [CrossRef]
- Lala, V.; Goyal, A.; Bansal, P.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K.H. Abnormal liver function tests associated with severe rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef]
- Alsahli, M.; Gerich, J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res. Clin. Pract. 2017, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef]
- Singh, R.M.; Howarth, F.C.; Adeghate, E.; Bidasee, K.; Singh, J.; Waqar, T. Type 1 diabetes mellitus induces structural changes and molecular remodelling in the rat kidney. Mol. Cell. Biochem. 2018, 449, 9–25. [Google Scholar] [CrossRef]
- Portero-Otín, M.; Pamplona, R.; Ruiz, M.C.; Cabiscol, E.; Prat, J.; Bellmunt, M.J. Diabetes induces an impairment in the proteolytic activity against oxidized proteins and a heterogeneous effect in nonenzymatic protein modifications in the cytosol of rat liver and kidney. Diabetes 1999, 48, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, K.; Rodgers, K.J. Oxidised protein metabolism: Recent insights. Biol. Chem. 2017, 398, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.V.C.; Santos, A.S.R., Jr.; Corso, C.R.; da Silva, F.S.; Capote, A.; Ribeiro, C.D.; Abreu, B.J.D.G.A.; Acco, A.; Fogaça, R.H.; Lavezzo-Dias, F.A. Thirty-day experimental diabetes impairs contractility and increases fatigue resistance in rat diaphragm muscle associated with increased anti-oxidative activity. Can. J. Physiol. Pharmacol. 2020, 98, 490–497. [Google Scholar] [CrossRef]
- Castro, V.M.D.; Medeiros, K.C.P.; Lemos, L.I.C.; Pedrosa, L.F.C.; Ladd, F.V.L.; Carvalho, T.G.; Araújo Júnior, R.F.; Abreu, B.J.; Farias, N.B.D.S. S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue Cell 2021, 69, 101483. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.G.; Medeiros, M.A.; de Lemos, L.I.C.; de Fátima Campos Pedrosa, L.; de Andrade Santos, P.P.; Abreu, B.J.; Lima, J.P.M.S. Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 431. https://doi.org/10.3390/nu14030431
Gonçalves MG, Medeiros MA, de Lemos LIC, de Fátima Campos Pedrosa L, de Andrade Santos PP, Abreu BJ, Lima JPMS. Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats. Nutrients. 2022; 14(3):431. https://doi.org/10.3390/nu14030431
Chicago/Turabian StyleGonçalves, Meline Gomes, Matheus Anselmo Medeiros, Licyanne Ingrid Carvalho de Lemos, Lucia de Fátima Campos Pedrosa, Pedro Paulo de Andrade Santos, Bento João Abreu, and João Paulo Matos Santos Lima. 2022. "Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats" Nutrients 14, no. 3: 431. https://doi.org/10.3390/nu14030431
APA StyleGonçalves, M. G., Medeiros, M. A., de Lemos, L. I. C., de Fátima Campos Pedrosa, L., de Andrade Santos, P. P., Abreu, B. J., & Lima, J. P. M. S. (2022). Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats. Nutrients, 14(3), 431. https://doi.org/10.3390/nu14030431






