Caffeine Intake throughout Pregnancy, and Factors Associated with Non-Compliance with Recommendations: A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Caffeine Intake Calculations
2.3. Statistical Analyses
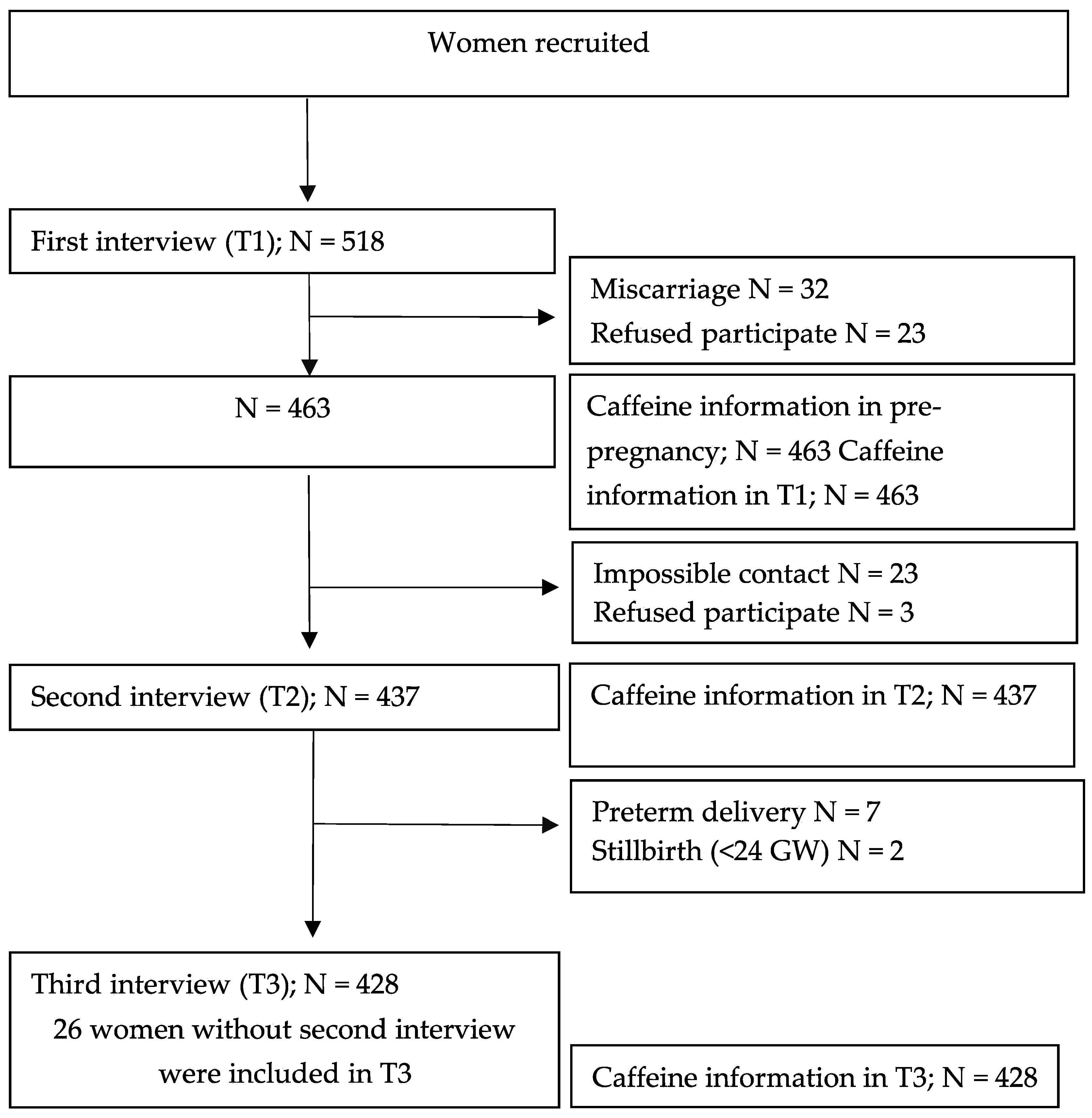
3. Results
3.1. Population Characteristics
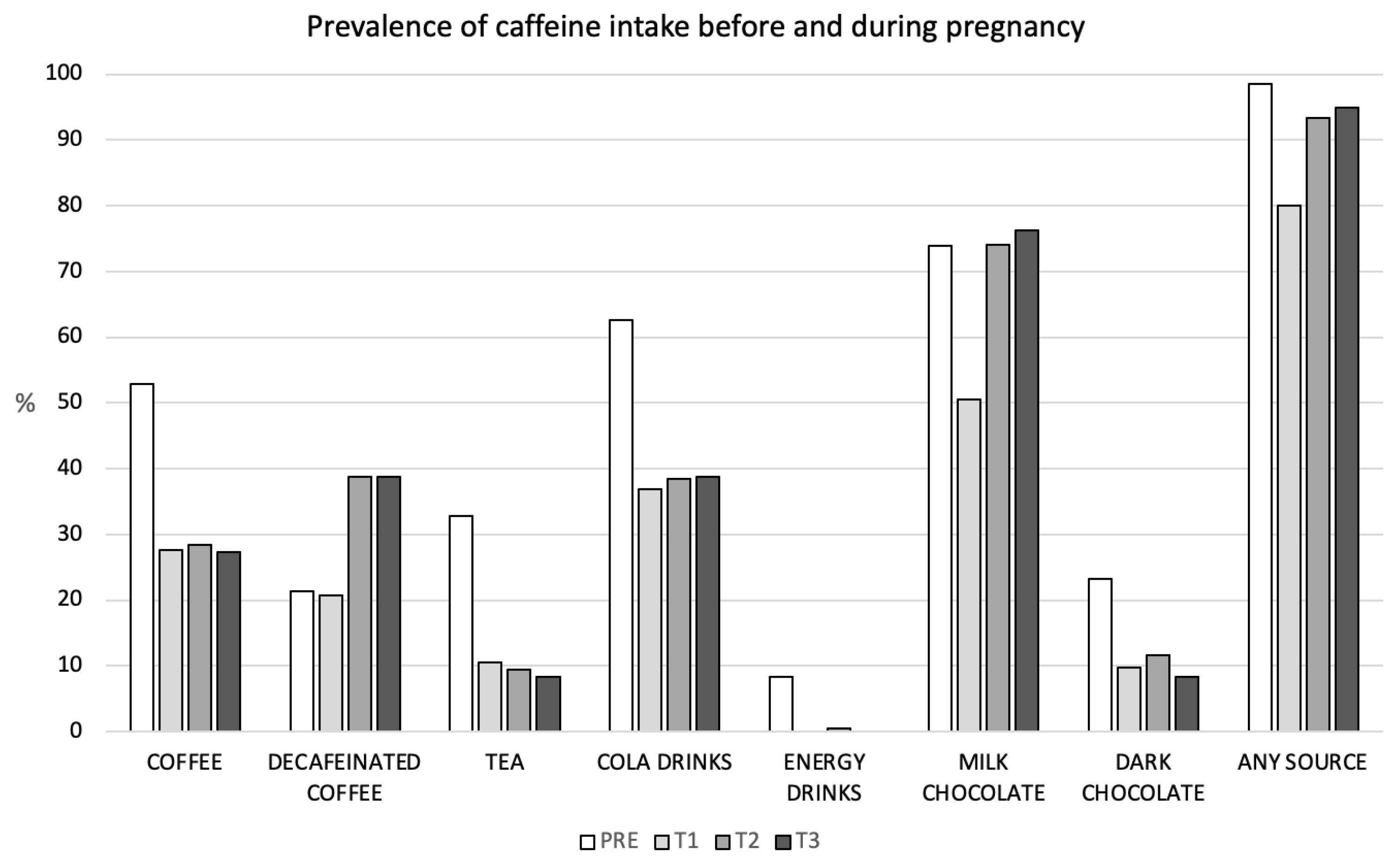
3.2. Prevalence and Changes in Caffeine Intake throughout Pregnancy
3.3. Non-Compliance with Recommendations and Factors Associated
4. Discussion
4.1. Study Limitations
4.2. Study Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahanfar, S.; Jaafar, S.H. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database Syst. Rev. 2015, 6, Cd006965. [Google Scholar] [CrossRef] [PubMed]
- Grosso, L.M.; Bracken, M.B. Caffeine metabolism, genetics, and perinatal outcomes: A review of exposure assessment considerations during pregnancy. Ann. Epidemiol. 2005, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Group, C.S. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, a2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslova, E.; Bhattacharya, S.; Lin, S.W.; Michels, K.B. Caffeine consumption during pregnancy and risk of preterm birth: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- ACOG. Committee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet. Gynecol. 2010, 116, 467–468. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 120. [Google Scholar]
- Frayer, N.C.; Kim, Y. Caffeine Intake during Pregnancy and Risk of Childhood Obesity: A Systematic Review. Int. J. MCH. AIDS 2020, 9, 364–380. [Google Scholar] [CrossRef]
- James, J.E. Maternal caffeine consumption and pregnancy outcomes: A narrative review with implications for advice to mothers and mothers-to-be. BMJ Evid. Based. Med. 2021, 26, 114–115. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Song, J.M.; Zhang, J.; Tang, Y.L.; Xin, C.M. A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int. J. Gynaecol. Obstet. 2015, 130, 116–122. [Google Scholar] [CrossRef]
- Jafari, A.; Naghshi, S.; Shahinfar, H.; Salehi, S.O.; Kiany, F.; Askari, M.; Surkan, P.J.; Azadbakht, L. Relationship between maternal caffeine and coffee intake and pregnancy loss: A grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of observational studies. Front. Nutr. 2022, 9, 886224. [Google Scholar] [CrossRef]
- Choi, H.; Koo, S.; Park, H.Y. Maternal coffee intake and the risk of bleeding in early pregnancy: A cross-sectional analysis. BMC Pregnancy Childbirth 2020, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk-Bębenek, E.; Piórecka, B.; Kopytko, M.; Chadzińska, Z.; Jagielski, P.; Schlegel-Zawadzka, M. Evaluation of Caffeine Consumption among Pregnant Women from Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, A.; Hutchinson, D.; Wilson, J.; McCormack, C.; Bruno, R.; Olsson, C.A.; Allsop, S.; Elliott, S.; Burns, L.; Mattick, R.P. Adherence to the Caffeine Intake Guideline during Pregnancy and Birth Outcomes: A Prospective Cohort Study. Nutrients 2018, 10, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichelberger, K.Y.; Baker, A.M.; Woodham, P.C.; Haeri, S.; Strauss, R.A.; Stuebe, A.M. Second-Trimester Maternal Serum Paraxanthine, CYP1A2 Activity, and the Risk of Severe Preeclampsia. Obstet. Gynecol. 2015, 126, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Peraita-Costa, I.; Llopis-González, A.; Perales-Marín, A.; Sanz, F.; Llopis-Morales, A.; Morales-Suárez-Varela, M. A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn. Int. J. Environ. Res. Public Health 2018, 15, 1530. [Google Scholar] [CrossRef] [Green Version]
- Gleason, J.L.; Tekola-Ayele, F.; Sundaram, R.; Hinkle, S.N.; Vafai, Y.; Buck Louis, G.M.; Gerlanc, N.; Amyx, M.; Bever, A.M.; Smarr, M.M.; et al. Association Between Maternal Caffeine Consumption and Metabolism and Neonatal Anthropometry: A Secondary Analysis of the NICHD Fetal Growth Studies–Singletons. JAMA Netw. Open 2021, 4, e213238. [Google Scholar] [CrossRef]
- Román-Gálvez, R.M.; Amezcua-Prieto, C.; Olmedo-Requena, R.; Lewis-Mikhael Saad, A.M.; Martínez-Galiano, J.M.; Bueno-Cavanillas, A. Partner smoking influences whether mothers quit smoking during pregnancy: A prospective cohort study. BJOG 2018, 125, 820–827. [Google Scholar] [CrossRef]
- Román-Gálvez, R.M.; Amezcua-Prieto, C.; Salcedo-Bellido, I.; Martínez-Galiano, J.M.; Khan, K.S.; Bueno-Cavanillas, A. Factors associated with insomnia in pregnancy: A prospective Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 70–75. [Google Scholar] [CrossRef]
- Román-Gálvez, M.R.; Amezcua-Prieto, C.; Salcedo-Bellido, I.; Olmedo-Requena, R.; Martínez-Galiano, J.M.; Khan, K.S.; Bueno-Cavanillas, A. Physical activity before and during pregnancy: A cohort study. Int. J. Gynaecol. Obstet. 2021, 152, 374–381. [Google Scholar] [CrossRef]
- Grupo de trabajo de la Sociedad Española de Epidemiología y de la Sociedad Española de Medicina de Familia y Comunitaria. Una propuesta de medida de la clase social (Proposal to measure social class). Atención Primaria 2000, 25, 350–363. [Google Scholar] [CrossRef] [Green Version]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gesteiro, E.; Bastida, S.; Rodríguez Bernal, B.; Sánchez-Muniz, F.J. Adherence to Mediterranean diet during pregnancy and serum lipid, lipoprotein and homocysteine concentrations at birth. Eur. J. Nutr. 2015, 54, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [Green Version]
- Martin-Moreno, J.M.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Chen, L.; Bell, E.M.; Browne, M.L.; Druschel, C.M.; Romitti, P.A. Exploring maternal patterns of dietary caffeine consumption before conception and during pregnancy. Matern. Child Health J. 2014, 18, 2446–2455. [Google Scholar] [CrossRef] [Green Version]
- Cnattingius, S.; Signorello, L.B.; Annerén, G.; Clausson, B.; Ekbom, A.; Ljunger, E.; Blot, W.J.; McLaughlin, J.K.; Petersson, G.; Rane, A.; et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N. Engl. J. Med. 2000, 343, 1839–1845. [Google Scholar] [CrossRef]
- Boylan, S.M.; Cade, J.E.; Kirk, S.F.L.; Greenwood, D.C.; White, K.L.M.; Shires, S.; Simpson, N.A.B.; Wild, C.P.; Hay, A.W.M. Assessing caffeine exposure in pregnant women. Br. J. Nutr. 2008, 100, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Wierzejska, R.; Jarosz, M.; Wojda, B. Caffeine Intake during Pregnancy and Neonatal Anthropometric Parameters. Nutrients 2019, 11, 806. [Google Scholar] [CrossRef] [Green Version]
- Sengpiel, V.; Elind, E.; Bacelis, J.; Nilsson, S.; Grove, J.; Myhre, R.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Jacobsson, B.; et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: Results from a large prospective observational cohort study. BMC Med. 2013, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Hinkle, S.N.; Laughon, S.K.; Catov, J.M.; Olsen, J.; Bech, B.H. First trimester coffee and tea intake and risk of gestational diabetes mellitus: A study within a national birth cohort. BJOG 2015, 122, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, E.; Martín-Grau, C.; Hernández-Martinez, C.; Voltas, N.; Canals, J.; Arija, V. Changes in fatty acid levels (saturated, monounsaturated and polyunsaturated) during pregnancy. BMC Pregnancy Childbirth 2021, 21, 778. [Google Scholar] [CrossRef] [PubMed]
- Tryggvadottir, E.A.; Gunnarsdottir, I.; Birgisdottir, B.E.; Hrolfsdottir, L.; Landberg, R.; Hreidarsdottir, I.T.; Hardardottir, H.; Halldorsson, T.I. Early pregnancy plasma fatty acid profiles of women later diagnosed with gestational diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002326. [Google Scholar] [CrossRef]
- Dong, J.Y.; Kimura, T.; Ikehara, S.; Cui, M.; Kawanishi, Y.; Yamagishi, K.; Ueda, K.; Iso, H.; Japan Environment and Children’s Study Group. Chocolate consumption and risk of gestational diabetes mellitus: The Japan Environment and Children’s Study. Br. J. Nutr. 2019, 122, 936–941. [Google Scholar] [CrossRef]
- Zielinsky, P.; Piccoli, A.L.; Vian, I.; Zílio, A.M.; Naujorks, A.A.; Nicoloso, L.H.; Barbisan, C.W.; Busato, S.; Lopes, M.; Klein, C. Maternal restriction of polyphenols and fetal ductal dynamics in normal pregnancy: An open clinical trial. Arq. Bras. Cardiol. 2013, 101, 217–225. [Google Scholar] [CrossRef]
- Zielinsky, P.; Martignoni, F.V.; Markoski, M.; Zucatti, K.P.; Dos Santos Marinho, G.; Pozzobon, G.; Magno, P.R.; de Bittencourt Antunes, V.; Sulis, N.M.; Cardoso, A.; et al. Maternal ingestion of cocoa causes constriction of fetal ductus arteriosus in rats. Sci. Rep. 2021, 11, 9929. [Google Scholar] [CrossRef]
- Modzelewska, D.; Bellocco, R.; Elfvin, A.; Brantsæter, A.L.; Meltzer, H.M.; Jacobsson, B.; Sengpiel, V. Caffeine exposure during pregnancy, small for gestational age birth and neonatal outcome–results from the Norwegian Mother and Child Cohort Study. BMC Pregnancy Childbirth 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.; Uusitalo, L.; Auriola, S.; Backman, K.; Heinonen, S.; Keski-Nisula, L.; Pasanen, M.; Pekkanen, J.; Tuomainen, T.P.; Voutilainen, R.; et al. Caffeine content in newborn hair correlates with maternal dietary intake. Eur. J. Nutr. 2021, 60, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, N.; Jiménez-Moleón, J.J.; Olmedo-Requena, R.; Martínez-Ruiz, V.; Bueno-Cavanillas, A.; Mozas, J. Perinatal outcomes of prematurity and birth weight according to maternal caffeine consumption. Nutr. Hosp. 2015, 32, 2658–2664. [Google Scholar]
- Hoyt, A.T.; Browne, M.; Richardson, S.; Romitti, P.; Druschel, C. National Birth Defects Prevention Study. Maternal caffeine consumption and small for gestational age births: Results from a population-based case-control study. Matern. Child Health J. 2014, 18, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Lamy, S.; Houivet, E.; Benichou, J.; Marret, S.; Thibaut, F. Perinatal network of Upper-Normandy. Caffeine use during pregnancy: Prevalence of use and newborn consequences in a cohort of French pregnant women. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, I.; North, M.; Kothe, E.; Chai, T.L.; Pirotta, S.; Lim, S.; Hill, B. The Relationship between Pregnancy Intentions and Diet or Physical Activity Behaviors in the Preconception and Antenatal Periods: A Systematic Review and Meta-Analysis. J. Midwifery Women’s Health 2020, 65, 660–680. [Google Scholar] [CrossRef] [PubMed]
| N = 463 (Pre-Pregnancy) | N = 463 (T1) | N = 437 (T2) | N = 428 (T3) | |||||
|---|---|---|---|---|---|---|---|---|
| mg/day Caffeine Median (IQR) | 100.01 (181.05) | 9.42 (66.24) | 12.1 (65.57) | 14.0 (61.1) | ||||
| Age | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| 18–25 | 53 | 100 (128.18) | 53 | 13.28 (49.85) | 49 | 18.71 (40.55) | 49 | 20.14 (36.88) |
| 26–30 | 137 | 75.85 (154.85) | 137 | 6.85 (37.41) | 132 | 12 (28.57) | 125 | 12.72 (40.42) |
| 31–35 | 185 | 100.22 (194.26) | 185 | 8.49 (59.97) | 171 | 10.22 (66) | 169 | 12 (62.28) |
| >35 | 88 | 105.59 (182.73) | 88 | 19.42 (99.56) | 85 | 29.42 (96.95) | 85 | 19.28 (96.48) |
| p = 0.83 | p = 0.12 | p = 0.06 | p = 0.78 | |||||
| Social Class | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| I–II | 157 | 102 (184.34) | 157 | 6.28 (99.93) | 152 | 13.29 (96.28) | 149 | 14.5 (84.14) |
| III | 90 | 90 (187.86) | 90 | 11.37 (41.43) | 86 | 10.81 (74.57) | 83 | 12 (96.71) |
| IV–V | 216 | 91.85 (170.48) | 216 | 10.45 (55.91) | 199 | 12.28 (47.42) | 196 | 14.17 (33.73) |
| p = 0.90 | p = 0.77 | p = 0.65 | p = 0.63 | |||||
| Paid work | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| Yes | 327 | 102.57 (184.57) | 327 | 9.42 (99.78) | 306 | 12.28 (96.57) | 302 | 13.42 (45.85) |
| No | 136 | 69.43 (115.07) | 136 | 9.42 (45.75) | 131 | 12.14 (41.57) | 126 | 14 (30.57) |
| p = 0.23 | p = 0.89 | p = 0.48 | p = 0.17 | |||||
| Parity | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| 0 | 239 | 102.57 (172.97) | 239 | 6.28 (59.86) | 227 | 12 (55.14) | 221 | 12.85 (45.14) |
| 1 | 197 | 68.72 (187.57) | 197 | 11.14 (48) | 185 | 13.14 (56.77) | 182 | 14.64 (69.85) |
| >1 | 27 | 115.67 (132.71) | 27 | 57 (97.96) | 25 | 48 (96.2) | 25 | 7.42 (46) |
| p = 0.45 | p = 0.07 | p = 0.20 | p = 0.46 | |||||
| Active smokers | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| Yes | 167 | 121.42 (215.42) | 398 | 6.38 (49.50) | 54 | 42.53 (104.93) | 51 | 33.2 (103.42) |
| No | 245 | 66.4 (126.16) | 65 | 39.79 (98.52) | 383 | 11.83 (47.42) | 377 | 12.06 (45.42) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |||||
| AMD | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| Yes | 218 | 100.01 (215.42) | 275 | 6.28 (49.53) | 263 | 10.22 (58.6) | 258 | 12 (40.28) |
| No | 245 | 100 (176.71) | 188 | 14.44 (98) | 173 | 16.14 (61.68) | 166 | 24.28 (70.42) |
| p = 0.32 | p < 0.001 | p = 0.03 | p < 0.001 | |||||
| BMI | n | median (IQR) | n | median (IQR) | n | median (IQR) | n | median (IQR) |
| Normal | 287 | 100 (182.58) | 287 | 7.31 (56.76) | 273 | 12.71 (59.95) | 268 | 12.79 (56.85) |
| Overweight | 128 | 80.79 (183.08) | 128 | 14.24 (98.26) | 118 | 13.65 (59.95) | 116 | 15.75 (71.55) |
| Obesity | 45 | 119.28 (166.79) | 45 | 10.62 (58.90) | 44 | 6.65 (23.92) | 41 | 16.71 (51.14) |
| p = 0.39 | p = 0.18 | p = 0.22 | p = 0.91 | |||||
| Compared to Pre-Pregnancy Values | T1 | T2 | T3 |
|---|---|---|---|
| n = 463 | n = 437 | n = 428 | |
| Coffee | |||
| No consumers | n = 218 | n = 207 | n = 201 |
| Start consuming | 3.7% | 3.4% | 5.5% |
| Consumers | n = 245 | n = 230 | n = 227 |
| Increase intake | 1.2% | 0.8% | 1.8% |
| Decrease intake | 75.5% | 80.0% | 83.7% |
| Decaffeinated coffee | |||
| No consumers | n = 364 | n = 342 | n = 335 |
| Start consuming | 17.0% | 30.4% | 31.0% |
| Consumers | n = 99 | n = 95 | n = 93 |
| Increase intake | 0.0% | 24.2% | 24.7% |
| Decrease intake | 69.7% | 53.7% | 50.5% |
| Tea | |||
| No consumers | n = 311 | n = 291 | n = 286 |
| Start consuming | 0.3% | 3.8% | 2.1% |
| Consumers | n = 152 | n = 146 | n = 142 |
| Increase intake | 1.3% | 5.5% | 5.6% |
| Decrease intake | 76.3% | 88.4% | 90.9% |
| Cola drinks | |||
| No consumers | n = 173 | n = 162 | n = 163 |
| Start consuming | 9.3% | 18.5% | 22.1% |
| Consumers | n = 290 | n = 275 | n = 265 |
| Increase intake | 1.7% | 13.8% | 14.3% |
| Decrease intake | 71.0% | 77.1% | 77.7% |
| Energy drinks | |||
| No consumers | n = 424 | n = 400 | n = 390 |
| Start consuming | 0.0% | 0.0% | 0.00% |
| Consumers | n = 39 | n = 37 | n = 38 |
| Increase intake | 0.0% | 2.7% | 0.0% |
| Decrease intake | 100% | 97.3% | 100% |
| Milk Chocolate | |||
| No consumers | n = 121 | n = 114 | n = 112 |
| Start consuming | 4.1% | 53.5% | 58.9% |
| Consumers | n = 342 | n = 323 | n = 316 |
| Increase intake | 5.60% | 40.6% | 42.1% |
| Decrease intake | 44.2% | 48.0% | 45.6% |
| Dark Chocolate | |||
| No consumers | n = 355 | n = 335 | n = 330 |
| Start consuming | 0.5% | 5.7% | 5.5% |
| Consumers | n = 108 | n = 102 | n = 98 |
| Increase intake | 10.2% | 16.7% | 12.2% |
| Decrease intake | 65.7% | 77.5% | 86.7% |
| All sources | |||
| No consumers | n = 7 | n = 6 | n = 7 |
| Start consuming | 28.6% | 83.3% | 100% |
| Consumers | n = 456 | n = 431 | n = 421 |
| Increase intake | 5.3% | 16.5% | 18.1% |
| Decrease intake | 84% | 82.8% | 80.8% |
| T1 (>200 mg/day) | T2 (>200 mg/day) | T3 (>200 mg/day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||||
| cOR a | CI 95% | aOR b | CI 95% | cOR | CI 95% | aOR | CI 95% | cOR | CI 95% | aOR | CI 95% | |
| Age | ||||||||||||
| 18–25 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| 26–30 | 0.49 | 0.19–1.27 | 0.62 | 0.23–1.67 | 0.39 | 0.08–1.79 | 0.59 | 0.11–2.99 | 1.09 | 0.27–4.43 | 1.07 | 0.22–5.02 |
| 31–35 | 0.39 | 0.16–0.99 | 0.58 | 0.22–1.54 | 0.76 | 0.15–3.66 | 1.65 | 0.29–9.50 | 2.13 | 0.49–9.28 | 1.81 | 0.35–9.30 |
| >35 | 0.73 | 0.26–2.07 | 0.98 | 0.33–2.94 | 0.68 | 0.12–3.64 | 1.75 | 0.25–11.91 | 0.72 | 0.17–2.94 | 0.55 | 0.11–2.68 |
| Social Class | ||||||||||||
| I–II | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| III | 1.07 | 0.58–2.00 | 0.95 | 0.50–1.82 | 0.76 | 0.29–1.97 | 0.95 | 0.26–3.48 | 1.99 | 0.40–9.83 | 1.74 | 0.33–9.12 |
| IV–V | 1.59 | 0.95–2.67 | 1.03 | 0.55–1.92 | 1.47 | 0.60–3.56 | 1.4 | 0.46–4.30 | 0.69 | 0.27–1.78 | 0.93 | 0.28–3.09 |
| Parity | ||||||||||||
| 0 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| 1 | 1.52 | 0.94–2.45 | 1.41 | 0.63–3.17 | 1.49 | 0.82–2.70 | 1.21 | 0.50–2.96 | 1.03 | 0.39–2.66 | 1 | 0.35–2.86 |
| ≥ 2 | 2.44 | 0.71–8.43 | 0.93 | 0.20–4.28 | 0.75 | 0.16–3.37 | 0.47 | 0.09–2.48 | 0.24 | 0.07–0.86 | 0.33 | 0.07–1.53 |
| Paid Work | ||||||||||||
| Yes | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| No | 1.76 | 1.02–3.06 | 2.09 | 1.10–3.98 | 1.13 | 0.48–2.62 | 1.16 | 0.46–2.90 | 0.32 | 0.13–0.77 | 0.34 | 0.12–0.93 |
| AMD | ||||||||||||
| Low (<8) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| High (≥8) | 0.47 | 0.28–0.78 | 0.5 | 0.28–0.88 | 0.37 | 0.15–0.94 | 0.39 | 0.15–1.02 | 1.43 | 0.59–3.46 | 1.36 | 0.52–3.55 |
| Active smokers | ||||||||||||
| Yes | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| No | 0.16 | 0.05–0.54 | 0.17 | 0.05–0.59 | 0.5 | 0.11–2.19 | 0.22 | 0.09–0.53 | 0.33 | 0.04–2.57 | 0.32 | 0.04–2.58 |
| BMI | ||||||||||||
| Normal | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| Overweight | 1.13 | 0.67–1.90 | 1.11 | 0.59–2.12 | 0.91 | 0.38–2.17 | 0.56 | 0.22–1.46 | 0.66 | 0.25–1.76 | 0.6 | 0.21–1.69 |
| Obesity | 2.2 | 0.83–5.82 | 1.47 | 0.60–3.55 | 0.66 | 0.21–2.07 | 0.43 | 0.13–1.47 | 0.54 | 0.14–2.03 | 0.64 | 0.15–2.64 |
| IPAQ | ||||||||||||
| Light | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| Moderate | 0.63 | 0.39–1.02 | 0.63 | 0.39–1.02 | 0.57 | 0.26–1.25 | 0.67 | 0.30–1.49 | 0.95 | 0.40–2.24 | 0.76 | 0.29–1.95 |
| Vigorous | 0.9 | 0.13–2.70 | 0.8 | 0.24–2.64 | - | - | - | - | - | - | - | - |
| Insomnia | ||||||||||||
| No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| Yes | 1.01 | 0.961.07 | 1 | 0.93–1.06 | 0.93 | 0.87–1.01 | 1.02 | 0.95–1.12 | 0.92 | 0.85–1.00 | 0.93 | 0.85–1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Gálvez, M.R.; Martín-Peláez, S.; Hernández-Martínez, L.; Cano-Ibáñez, N.; Olmedo-Requena, R.; Martínez-Galiano, J.M.; Bueno-Cavanillas, A.; Amezcua-Prieto, C. Caffeine Intake throughout Pregnancy, and Factors Associated with Non-Compliance with Recommendations: A Cohort Study. Nutrients 2022, 14, 5384. https://doi.org/10.3390/nu14245384
Román-Gálvez MR, Martín-Peláez S, Hernández-Martínez L, Cano-Ibáñez N, Olmedo-Requena R, Martínez-Galiano JM, Bueno-Cavanillas A, Amezcua-Prieto C. Caffeine Intake throughout Pregnancy, and Factors Associated with Non-Compliance with Recommendations: A Cohort Study. Nutrients. 2022; 14(24):5384. https://doi.org/10.3390/nu14245384
Chicago/Turabian StyleRomán-Gálvez, María Rosario, Sandra Martín-Peláez, Loreto Hernández-Martínez, Naomi Cano-Ibáñez, Rocío Olmedo-Requena, Juan Miguel Martínez-Galiano, Aurora Bueno-Cavanillas, and Carmen Amezcua-Prieto. 2022. "Caffeine Intake throughout Pregnancy, and Factors Associated with Non-Compliance with Recommendations: A Cohort Study" Nutrients 14, no. 24: 5384. https://doi.org/10.3390/nu14245384
APA StyleRomán-Gálvez, M. R., Martín-Peláez, S., Hernández-Martínez, L., Cano-Ibáñez, N., Olmedo-Requena, R., Martínez-Galiano, J. M., Bueno-Cavanillas, A., & Amezcua-Prieto, C. (2022). Caffeine Intake throughout Pregnancy, and Factors Associated with Non-Compliance with Recommendations: A Cohort Study. Nutrients, 14(24), 5384. https://doi.org/10.3390/nu14245384









