Effects of the Malnutrition—Eat Additional Meal (MEAM) Diet on the Serum Levels of Albumin and C-Reactive Protein in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Work Plan, Study Population and Ethical Approval
- -
- clinically stable patients (men and women), aged 35–70 years, who had undergone hemodialysis for ≥3 months before the study,
- -
- patients undergoing a standard procedure of hemodialysis 3 times/week,
- -
- duration of the hemodialysis session: 180–300 min, depending on the delivered dose of dialysis,
- -
- the surface area of the dialysis membrane: 1.6–2.2 m2,
- -
- a dialyzer of the low-flux type.
- -
- a BMI of >30 kg/m2,
- -
- diabetes type 1 or 2, chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis or inflammatory bowel disease, malignancy history in the past 5 years and/or severe cardiac insufficiency (NYHA 4),
- -
- alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≥ 3× the upper limit of normal (ULN), and total bilirubin ≥1.5 × ULN at randomization.
2.2. Calculation of Individual Dietary Recommendations for Patients Treated with Hemodialysis
2.3. Calculation of the Nutritional Value of the Meal Administered just before Dialysis in HG2
2.4. Nutritional Assessment and Body Composition
2.5. Blood Sampling
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen, K.L.; Chertow, G.M.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Li, S.; Li, S.; Liu, J.; et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2022, 79, A8–A12. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular Disease in Dialysis Patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. S3), iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L. Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Axelsson, J.; Machowska, A.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Lindström, K.; Stenvinkel, P.; Qureshi, A.R. Biomarkers of Cardiovascular Disease and Mortality Risk in Patients with Advanced CKD. CJASN 2016, 11, 1163–1172. [Google Scholar] [CrossRef]
- Herselman, M.; Esau, N.; Kruger, J.-M.; Labadarios, D.; Moosa, M.R. Relationship between Serum Protein and Mortality in Adults on Long-Term Hemodialysis: Exhaustive Review and Meta-Analysis. Nutrition 2010, 26, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Poor Nutritional Status and Inflammation: Serum Albumin: Relationship to Inflammation and Nutrition: Serum Albumin. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Inflammation in End-Stage Renal Disease-What Have We Learned in 10 Years?: Inflammation in End-Stage Renal Disease. Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef]
- Machowska, A.; Sun, J.; Qureshi, A.R.; Isoyama, N.; Leurs, P.; Anderstam, B.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Lindholm, B. Plasma Pentosidine and Its Association with Mortality in Patients with Chronic Kidney Disease. PLoS ONE 2016, 11, e0163826. [Google Scholar] [CrossRef]
- Cobo, G.; Qureshi, A.R.; Lindholm, B.; Stenvinkel, P. C-Reactive Protein: Repeated Measurements Will Improve Dialysis Patient Care. Semin. Dial. 2016, 29, 7–14. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Chertow, G.M.; Adhikarla, R.; Young, B.; Ronco, C.; Levin, N.W. Inflammation and Dietary Protein Intake Exert Competing Effects on Serum Albumin and Creatinine in Hemodialysis Patients. Kidney Int. 2001, 60, 333–340. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong Association between Malnutrition, Inflammation, and Atherosclerosis in Chronic Renal Failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Graterol Torres, F.; Molina, M.; Soler-Majoral, J.; Romero-González, G.; Rodríguez Chitiva, N.; Troya-Saborido, M.; Socias Rullan, G.; Burgos, E.; Paúl Martínez, J.; Urrutia Jou, M.; et al. Evolving Concepts on Inflammatory Biomarkers and Malnutrition in Chronic Kidney Disease. Nutrients 2022, 14, 4297. [Google Scholar] [CrossRef] [PubMed]
- de Mutsert, R.; Grootendorst, D.C.; Indemans, F.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. Association Between Serum Albumin and Mortality in Dialysis Patients Is Partly Explained by Inflammation, and Not by Malnutrition. J. Ren. Nutr. 2009, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-Analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.; Yap, K.B.; Pan, F.; Ng, T.P. Malnutrition Risk and Kidney Function and Decline in Community-Dwelling Older Adults. J. Ren. Nutr. 2022, 32, 560–568. [Google Scholar] [CrossRef]
- Locatelli, F.; Fouque, D.; Heimburger, O.; Drüeke, T.B.; Cannata-Andía, J.B.; Hörl, W.H.; Ritz, E. Nutritional Status in Dialysis Patients: A European Consensus. Nephrol. Dial. Transpl. 2002, 17, 563–572. [Google Scholar] [CrossRef]
- Kyle, U.G.; Pirlich, M.; Schuetz, T.; Luebke, H.J.; Lochs, H.; Pichard, C. Prevalence of Malnutrition in 1760 Patients at Hospital Admission: A Controlled Population Study of Body Composition. Clin. Nutr. 2003, 22, 473–481. [Google Scholar] [CrossRef]
- Beddhu, S.; Cheung, A.K.; Larive, B.; Greene, T.; Kaysen, G.A.; Levey, A.S.; Rocco, M.; Sarnak, M.; Toto, R.; Eknoyan, G. Inflammation and Inverse Associations of Body Mass Index and Serum Creatinine With Mortality in Hemodialysis Patients. J. Ren. Nutr. 2007, 17, 372–380. [Google Scholar] [CrossRef][Green Version]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of Protein-Energy Wasting in Non-Dialysis-Dependent Chronic Kidney Disease: Reconciling Low Protein Intake with Nutritional Therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef]
- Lopes, A.A.; Bragg-Gresham, J.L.; Elder, S.J.; Ginsberg, N.; Goodkin, D.A.; Pifer, T.; Lameire, N.; Marshall, M.R.; Asano, Y.; Akizawa, T.; et al. Independent and Joint Associations of Nutritional Status Indicators With Mortality Risk Among Chronic Hemodialysis Patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). J. Ren. Nutr. 2010, 20, 224–234. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A. Let Them Eat During Dialysis: An Overlooked Opportunity to Improve Outcomes in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2013, 23, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Riella, M.C. Malnutrition in Dialysis: Malnourishment or Uremic Inflammatory Response? Kidney Int. 2000, 57, 1211–1232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bossola, M.; Muscaritoli, M.; Tazza, L.; Panocchia, N.; Liberatori, M.; Giungi, S.; Tortorelli, A.; Fanelli, F.R.; Luciani, G. Variables Associated with Reduced Dietary Intake in Hemodialysis Patients. J. Ren. Nutr. 2005, 15, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A Proposed Nomenclature and Diagnostic Criteria for Protein–Energy Wasting in Acute and Chronic Kidney Disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.M.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Laville, M.; Fouque, D. Nutritional Aspects in Hemodialysis. Kidney Int. 2000, 58, S133–S139. [Google Scholar] [CrossRef]
- Veeneman, J.M.; Kingma, H.A.; Boer, T.S.; Stellaard, F.; De Jong, P.E.; Reijngoud, D.-J.; Huisman, R.M. Protein Intake during Hemodialysis Maintains a Positive Whole Body Protein Balance in Chronic Hemodialysis Patients. Am. J. Physiol.-Endocrinol. Metab. 2003, 284, E954–E965. [Google Scholar] [CrossRef][Green Version]
- Pupim, L.B.; Majchrzak, K.M.; Flakoll, P.J.; Ikizler, T.A. Intradialytic Oral Nutrition Improves Protein Homeostasis in Chronic Hemodialysis Patients with Deranged Nutritional Status. JASN 2006, 17, 3149–3157. [Google Scholar] [CrossRef]
- Cherry, N.; Shalansky, K. Efficacy of Intradialytic Parenteral Nutrition in Malnourished Hemodialysis Patients. Am. J. Health-Syst. Pharm. 2002, 59, 1736–1741. [Google Scholar] [CrossRef]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-Term Oral Nutrition Supplementation Improves Outcomes in Malnourished Patients With Chronic Kidney Disease on Hemodialysis. J. Parenter. Enteral. Nutr. 2014, 38, 960–965. [Google Scholar] [CrossRef]
- Tomayko, E.J.; Kistler, B.M.; Fitschen, P.J.; Wilund, K.R. Intradialytic Protein Supplementation Reduces Inflammation and Improves Physical Function in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Calegari, A.; Barros, E.G.; Veronese, F.V.; Thomé, F.S. Malnourished Patients on Hemodialysis Improve after Receiving a Nutritional Intervention. J. Bras. Nefrol. 2011, 33, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sundell, M.B.; Pupim, L.B.; Wu, P.; Shintani, A.; Ikizler, T.A. The Effect of Resistance Exercise to Augment Long-Term Benefits of Intradialytic Oral Nutritional Supplementation in Chronic Hemodialysis Patients. J. Ren. Nutr. 2011, 21, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Caglar, K.; Fedje, L.; Dimmitt, R.; Hakim, R.M.; Shyr, Y.; Ikizler, T.A. Therapeutic Effects of Oral Nutritional Supplementation during Hemodialysis. Kidney Int. 2002, 62, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, L. Chronic Renal Disease. In Dietotherapy; Włodarek, D., Lange, E., Kozłowska, L., Głąbska, D., Eds.; PZWL: Warszawa, Poland, 2014. [Google Scholar]
- Fouque, D.; Vennegoor, M.; Ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A.; et al. EBPG Guideline on Nutrition. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S2), ii45–ii87. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stos, K.; Charzewska, J. Nutrition Standards for the Polish Population; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020. [Google Scholar]
- Jo, I.Y.; Kim, W.J.; Park, H.C.; Choi, H.Y.; Lee, J.E.; Lee, S.M. Effect of Personalized Nutritional Counseling on the Nutritional Status of Hemodialysis Patients. Clin. Nutr. Res. 2017, 6, 285–295. [Google Scholar] [CrossRef][Green Version]
- Chertow, G.M.; Ling, J.; Lew, N.L.; Lazarus, J.M.; Lowrie, E.G. The Association of Intradialytic Parenteral Nutrition Administration with Survival in Hemodialysis Patients. Am. J. Kidney Dis. 1994, 24, 912–920. [Google Scholar] [CrossRef]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is Controlling Phosphorus by Decreasing Dietary Protein Intake Beneficial or Harmful in Persons with Chronic Kidney Disease? Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and Enteral Supplements for Improving Outcomes in Chronic Kidney Disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef]
- Grant, G.M. Leksykon Memetyczny. In Teksty z Ulicy. Zeszyt Memetyczny; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005; Volume 9, pp. 12–18. ISSN 2081-397X. [Google Scholar]
- Stefánsson, B.V.; Brunelli, S.M.; Cabrera, C.; Rosenbaum, D.; Anum, E.; Ramakrishnan, K.; Jensen, D.E.; Stålhammar, N.-O. Intradialytic Hypotension and Risk of Cardiovascular Disease. CJASN 2014, 9, 2124–2132. [Google Scholar] [CrossRef]
- Sars, B.; van der Sande, F.M.; Kooman, J.P. Intradialytic Hypotension: Mechanisms and Outcome. Blood Purif. 2020, 49, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sornmo, L.; Sandberg, F.; Gil, E.; Solem, K. Noninvasive Techniques for Prevention of Intradialytic Hypotension. IEEE Rev. Biomed. Eng. 2012, 5, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.B.; Mc Causland, F.R. Mechanisms, Clinical Implications, and Treatment of Intradialytic Hypotension. CJASN 2018, 13, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Ertuglu, L.A.; Afsar, B.; Ozdogan, E.; Siriopol, D.; Covic, A.; Basile, C.; Ortiz, A. An Update Review of Intradialytic Hypotension: Concept, Risk Factors, Clinical Implications and Management. Clin. Kidney J. 2020, 13, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Kistler, B.; Benner, D.; Burgess, M.; Stasios, M.; Kalantar-Zadeh, K.; Wilund, K.R. To Eat or Not to Eat—International Experiences With Eating During Hemodialysis Treatment. J. Ren. Nutr. 2014, 24, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wizemann, V. Regular Dialysis Treatment in Germany: The Role of Non-Profit Organisations. J. Nephrol. 2000, 13 (Suppl. S3), S16–S19. [Google Scholar]
- Kopple, J.D.; Swendseid, M.E.; Shinaberger, J.H.; Umezawa, C.Y. The Free and Bound Amino Acids Removed by Hemodialysis. ASAIO J. 1973, 19, 309–313. [Google Scholar] [CrossRef]
- Hendriks, F.K.; Smeets, J.S.J.; Broers, N.J.H.; van Kranenburg, J.M.X.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J.C. End-Stage Renal Disease Patients Lose a Substantial Amount of Amino Acids during Hemodialysis. J. Nutr. 2020, 150, 1160–1166. [Google Scholar] [CrossRef]
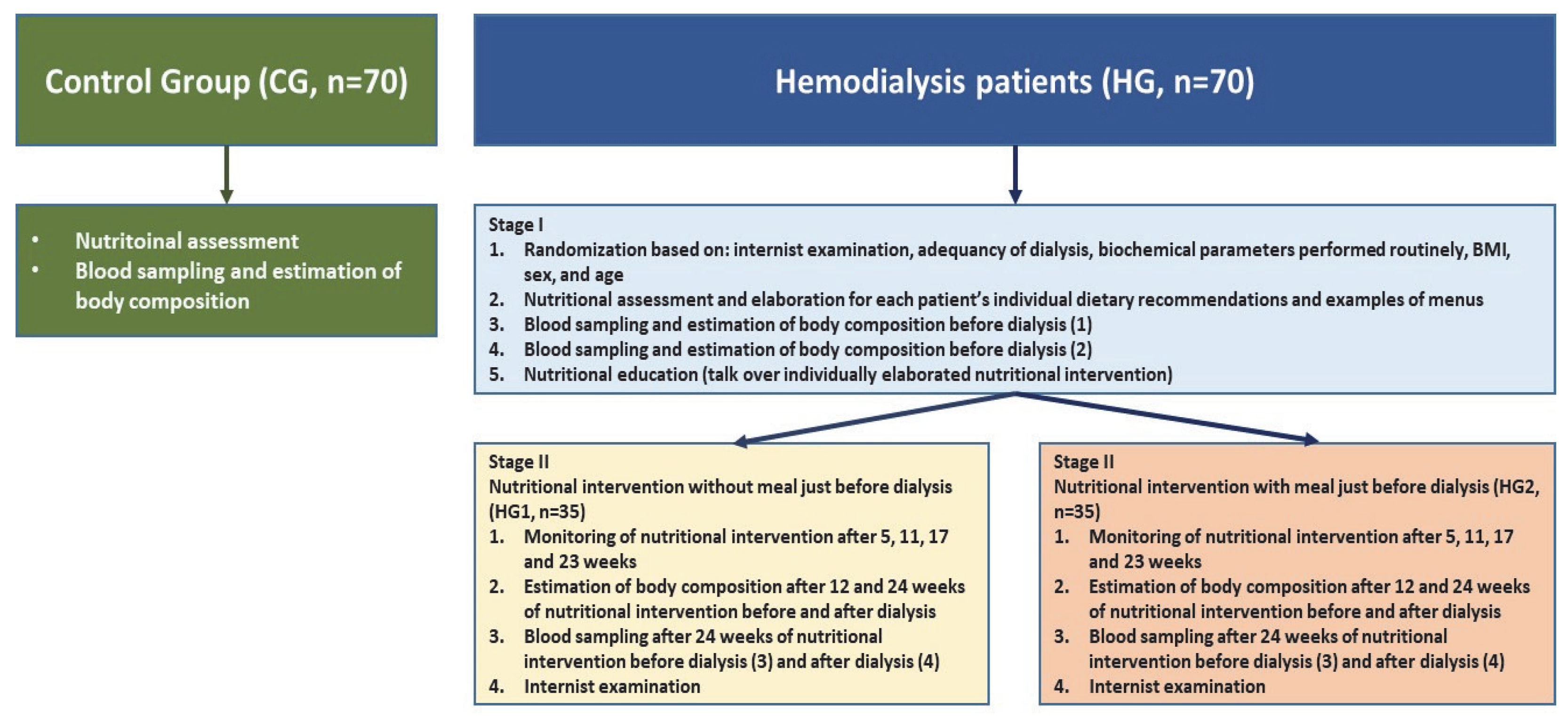
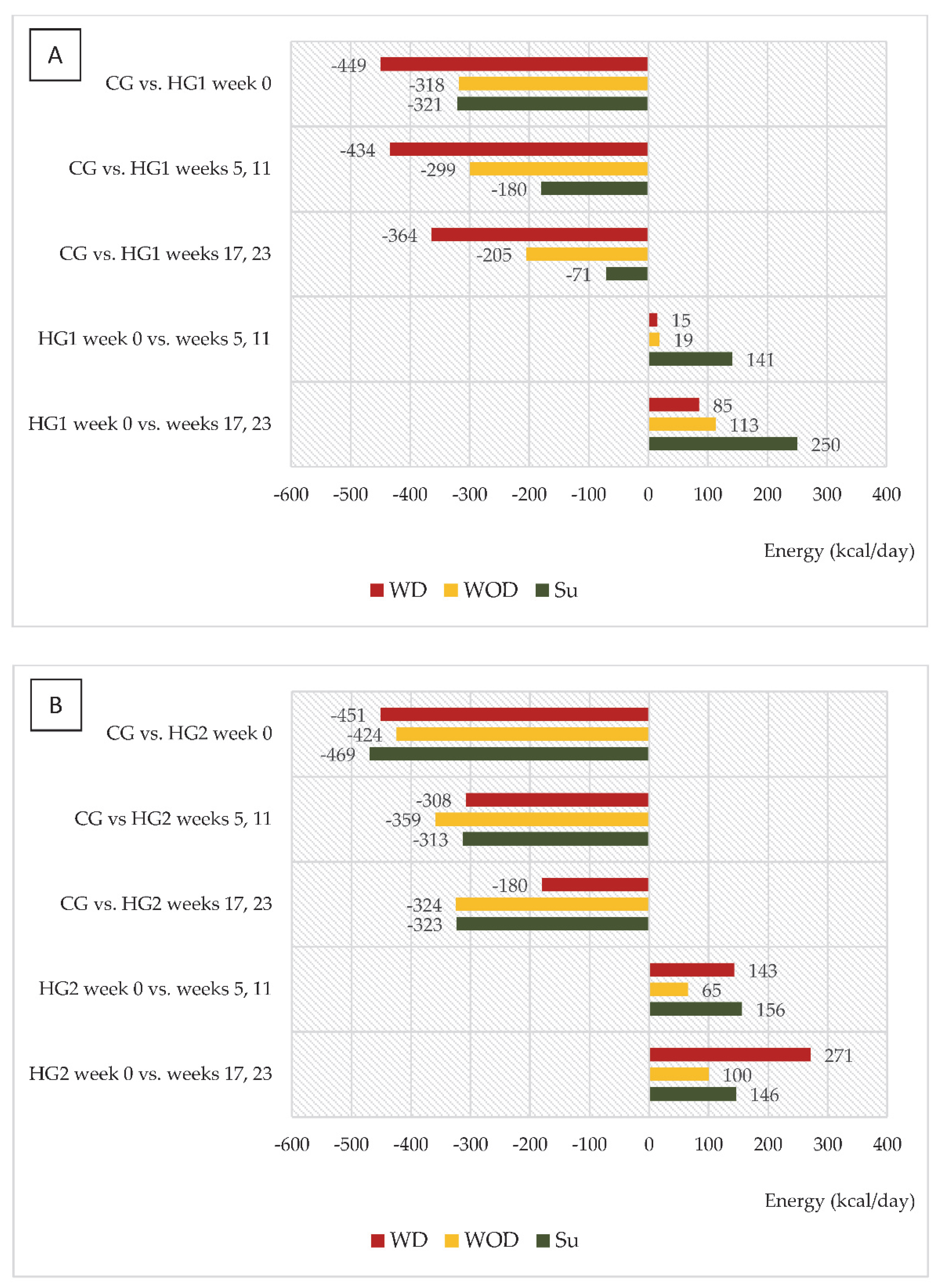
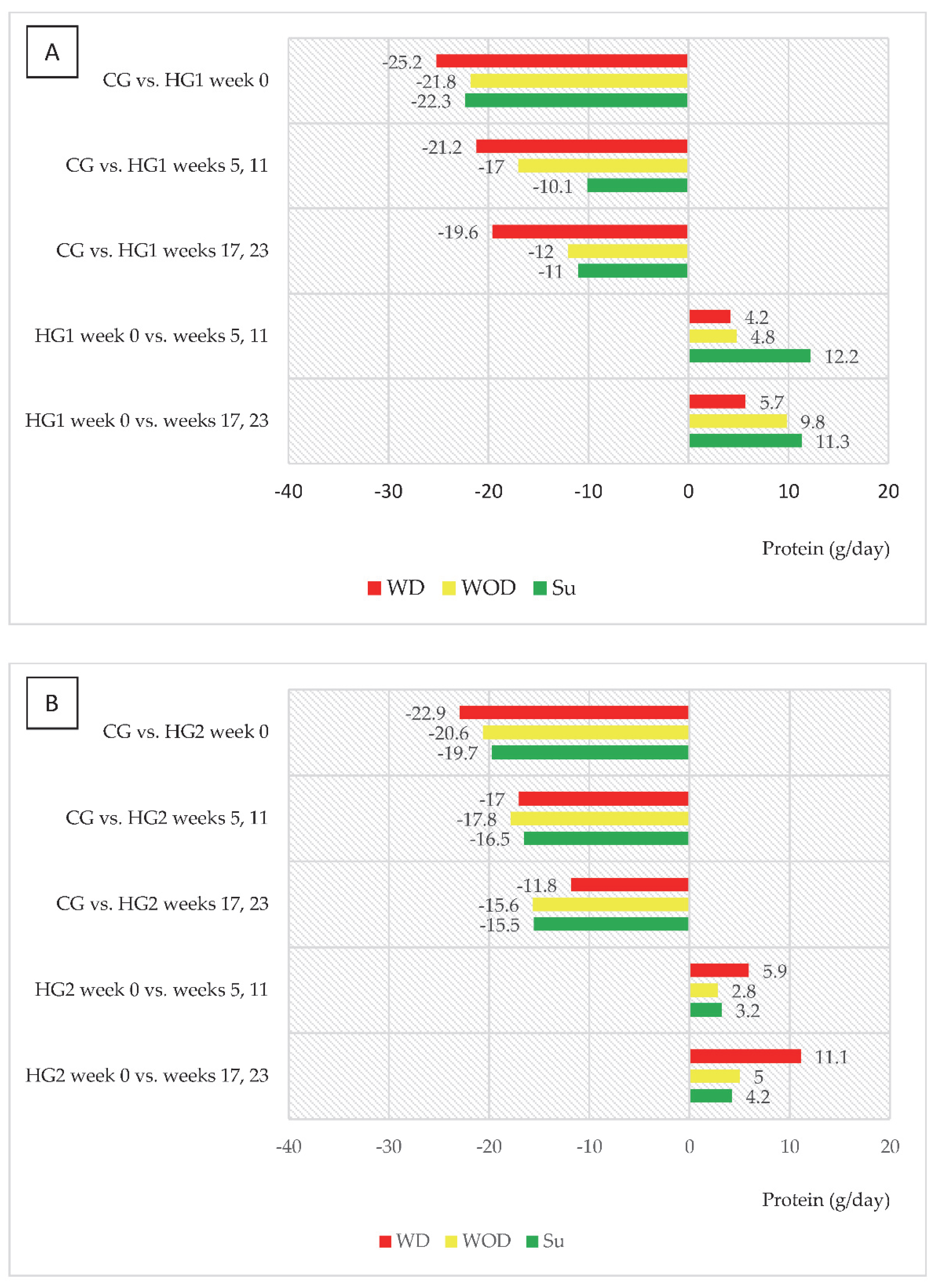
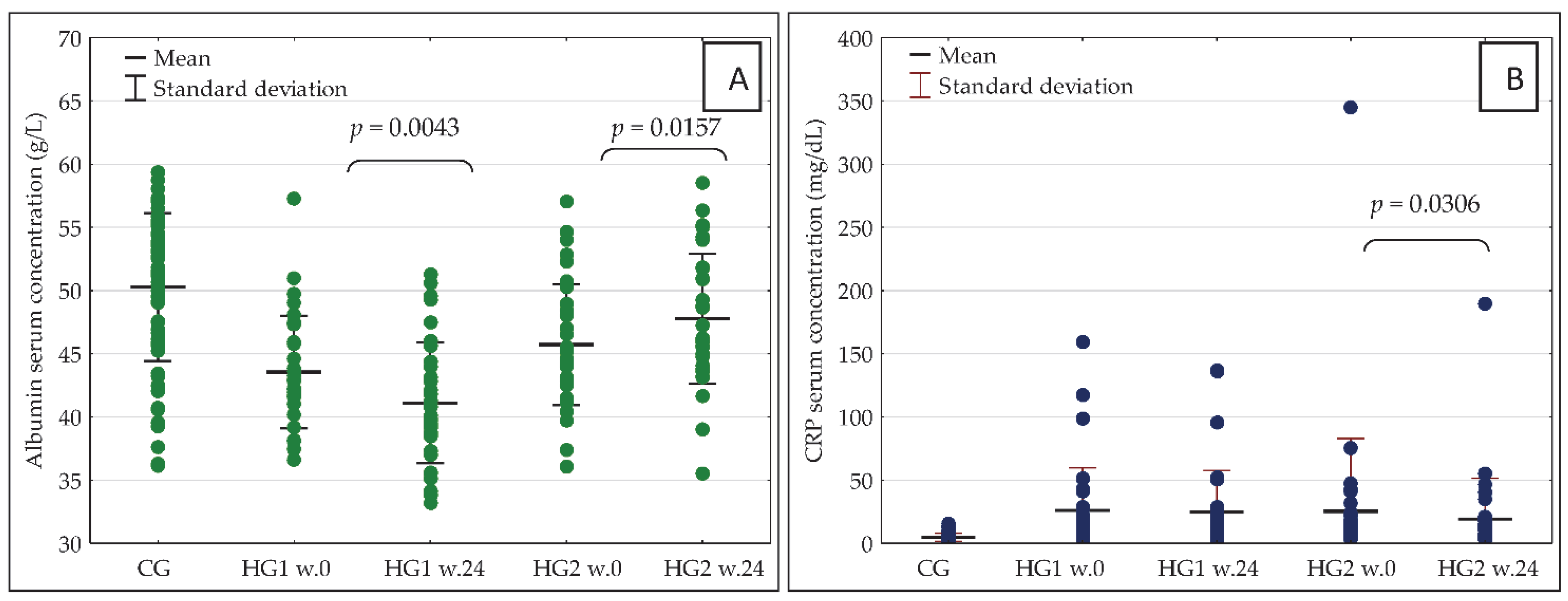
| Variables | CG | HG1 | HG2 |
|---|---|---|---|
| Sex: men/women (n) | 38/32 | 21/14 | 18/17 |
| Sex: men/women (%) | 54.3/45.7 | 60.0/40.0 | 51.4/48.6 |
| Age (years) | 54.4 ± 7.0 | 59.7 ± 11.5 | 58.1 ± 15.7 |
| Height (cm) | 170.4 ± 8.9 | 168.1 ± 9.5 | 169.8 ± 10.5 |
| BMI (kg/m2) | 26.7 ± 4.4 | 26.6 ± 4.9 | 25.2 ± 4.9 |
| Variables | Stage/Week | CG | HG1 | HG2 | ||
|---|---|---|---|---|---|---|
| BD | AD | BD | AD | |||
| Body mass (kg) | I/0 | 77.9 ± 15.9 | 74.9 ± 19.4 | 74.5 ± 19.0 | 71.9 ± 13.2 | 71.3 ± 14.2 |
| II/12 | 75.2 ± 19.7 | 75.3 ± 18.9 | 71.7 ± 13.0 | 71.5 ± 14.3 | ||
| II/24 | 75.7 ± 19.5 | 75.4 ± 18.9 | 71.6 ± 13.0 | 71.2 ± 14.0 | ||
| p# | 0.4325 | 0.0161 | 0.5541 | 0.6689 | ||
| p## | 0.0205 | 0.0042 | 0.3232 | 0.5662 | ||
| Fat free mass (kg) | I/0 | 57.2 ± 11.4 | 59.2 ± 13.1 | 55.9 ± 13.0 | 55.9 ± 9.1 | 52.4 ± 8.6 |
| II/12 | 59.7 ± 13.3 | 56.1 ± 13.0 | 56.3 ± 10.0 | 53.2 ± 9.6 | ||
| II/24 | 59.3 ± 13.4 | 55.9 ± 13.0 | 56.5 ± 9.7 | 53.0 ± 9.1 | ||
| p# | 0.4709 | 0.5982 | 0.4193 | 0.0715 | ||
| p## | 0.8279 | 0.8967 | 0.1714 | 0.3361 | ||
| Total body water (kg) | I | 40.4 ± 7.9 | 42.2 ± 9.6 | 39.2 ± 9.1 | 39.7 ± 6.8 | 36.9 ± 6.2 |
| II/12 | 42.7 ± 10.0 | 39.4 ± 9.2 | 40.1 ± 7.6 | 37.7 ± 7.3 | ||
| II/24 | 42.4 ± 10.0 | 39.2 ± 9.1 | 40.1 ± 7.2 | 37.2 ± 6.5 | ||
| p# | 0.3170 | 0.5807 | 0.3665 | 0.0467 | ||
| p## | 0.6796 | 0.9092 | 0.3030 | 0.5298 | ||
| Stage/Weeks | Group | CG 1 | WD | WOD | Su |
|---|---|---|---|---|---|
| Daily energy intake (kcal/kg/day) | |||||
| Stage I, week 0 | CG | 29.8 ± 7.7 | |||
| HG1 | 23.4 ± 5.6 ** | 25.2 ± 6.1 * | 25.3 ± 6.1 * | ||
| HG2 | 23.4 ± 8.3 ** | 23.6 ± 10.1 ** | 23.0 ± 8.0 ** | ||
| Stage II, mean values in weeks 5 and 11 | HG1 | 23.6 ± 6.0 ** | 25.6 ± 7.9 * | 27.4 ± 7.3 | |
| HG2 | 25.4 ± 5.8 * | 24.7 ± 6.6 * | 25.4 ± 6.5 * | ||
| Stage II, mean values in weeks 17 and 23 | HG1 | 24.7 ± 5.0 ** | 27.3 ± 6.0 | 28.8 ± 8.6 | |
| HG2 | 27.1 ± 6.4 | 25.1 ± 6.3 * | 24.7 ± 5.7 ** | ||
| Daily protein intake (g/kg/day) | |||||
| Stage I, week 0 | CG | 1.36 ± 0.34 | |||
| HG1 | 1.01 ± 0.26 ** | 1.06 ± 0.33 ** | 1.05 ± 0.30 ** | ||
| HG2 | 1.04 ± 0.45 ** | 1.07 ± 0.51 ** | 1.08 ± 0.35 ** | ||
| Stage II, mean values in weeks 5 and 11 | HG1 | 1.07 ± 0.26 ** | 1.13 ± 0.34 * | 1.24 ± 0.34 | |
| HG2 | 1.12 ± 0.30 ** | 1.11 ± 0.30 ** | 1.14 ± 0.31 * | ||
| Stage II, mean values in weeks 17 and 23 | HG1 | 1.11 ± 0.32 * | 1.22 ± 0.35 | 1.22 ± 0.35 | |
| HG2 | 1.19 ± 0.30 * | 1.14 ± 0.33 * | 1.13 ± 0.39 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlowska, L.; Gromadzinska, J.; Zwiech, R.; Zbrog, Z.; Wasowicz, W. Effects of the Malnutrition—Eat Additional Meal (MEAM) Diet on the Serum Levels of Albumin and C-Reactive Protein in Hemodialysis Patients. Nutrients 2022, 14, 5352. https://doi.org/10.3390/nu14245352
Kozlowska L, Gromadzinska J, Zwiech R, Zbrog Z, Wasowicz W. Effects of the Malnutrition—Eat Additional Meal (MEAM) Diet on the Serum Levels of Albumin and C-Reactive Protein in Hemodialysis Patients. Nutrients. 2022; 14(24):5352. https://doi.org/10.3390/nu14245352
Chicago/Turabian StyleKozlowska, Lucyna, Jolanta Gromadzinska, Rafal Zwiech, Zbigniew Zbrog, and Wojciech Wasowicz. 2022. "Effects of the Malnutrition—Eat Additional Meal (MEAM) Diet on the Serum Levels of Albumin and C-Reactive Protein in Hemodialysis Patients" Nutrients 14, no. 24: 5352. https://doi.org/10.3390/nu14245352
APA StyleKozlowska, L., Gromadzinska, J., Zwiech, R., Zbrog, Z., & Wasowicz, W. (2022). Effects of the Malnutrition—Eat Additional Meal (MEAM) Diet on the Serum Levels of Albumin and C-Reactive Protein in Hemodialysis Patients. Nutrients, 14(24), 5352. https://doi.org/10.3390/nu14245352







