Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
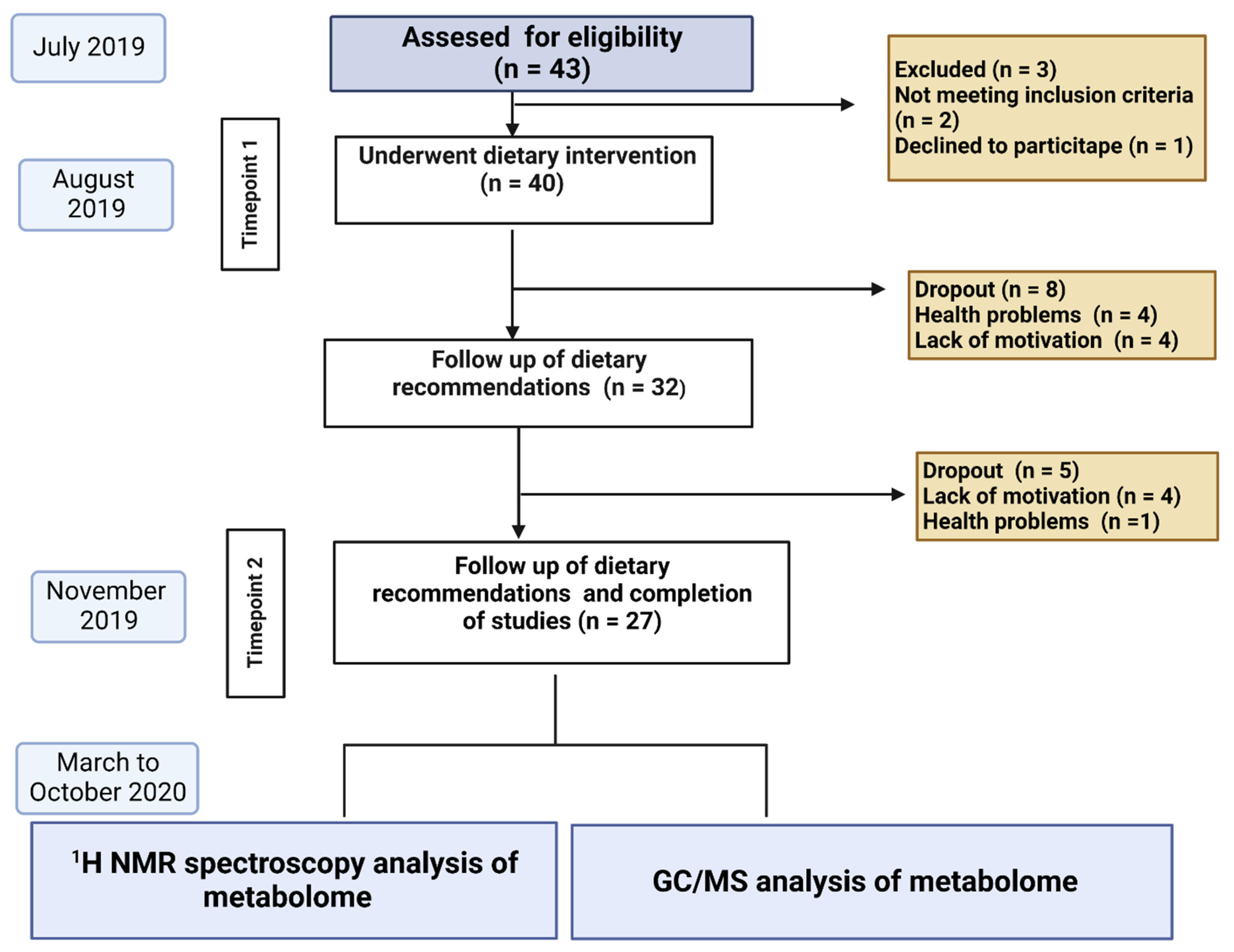
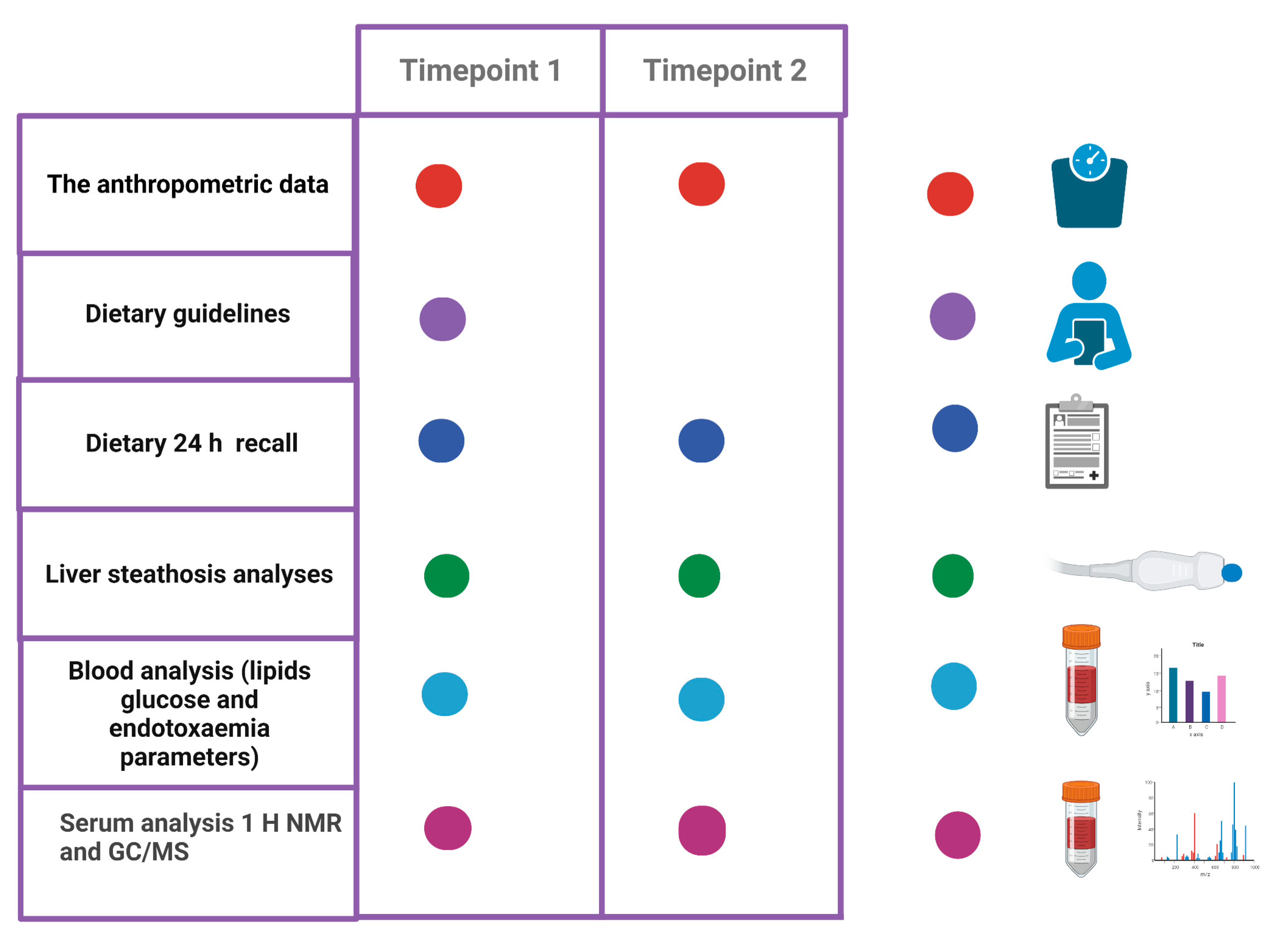
2.1. Study Design, Study Population Recruitment and Dietary Information
2.2. Intervention
2.3. The Anthropometric Data
2.4. Liver Stiffness and Steatosis Measurements
2.5. Dietary Guidelines and Control Visits
2.6. Blood Sample Collection
2.7. Outcome Measures
2.7.1. Serum Short-Chain Fatty Acids (SCFAs) and Branched Chain Fatty Acids (BCFAs) in Plasma Analysis by Means of GC/MS Chromatography
H NMR Spectroscopy Analysis of the Bacterial Metabolites
2.8. Data Processing and Multivariate Statistical Data Analysis
3. Results
3.1. The Dietary Intervention Contributed to a Reduction in Lipid Deposits in the Liver and a Reduction in Body Weight
3.2. The Caloric and Fiber Content of the Diet Explain the Change in Serum Metabolites
3.3. The Use of Precise Nutrition Had an Effect on the Concentration of SCFA and BCFA in the Serum
3.4. Metabolites Identification 1H NMR Spectrum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeVatte, M.; Keshteli, A.H.; Zarei, P.; Wishart, D.S. Applications of Metabolomics to Precision Nutrition. Lifestyle Genom. 2022, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Datz, C.; Reiberger, T.; Trauner, M. Diet and Exercise in NAFLD/NASH: Beyond the Obvious. Liver Int. 2021, 41, 2249–2268. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A Global View of the Interplay between Non-Alcoholic Fatty Liver Disease and Diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana per lo Studio del Fegato (AISF); Società Italiana di Diabetologia (SID); Società Italiana dell’Obesità (SIO). Non-Alcoholic Fatty Liver Disease in Adults 2021: A Clinical Practice Guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Eat. Weight Disord. 2021, 27, 1603–1619. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Younossi, Z.; Natarajan, Y.; Bugianesi, E.; Rinella, M.E.; Harrison, S.A.; Mantzoros, C.; Pfotenhauer, K.; Klein, S.; et al. Preparing for the NASH Epidemic: A Call to Action. Gastroenterology 2021, 161, 1030–1042.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Park, J.W.; Kim, H.S.; Jang, M.-K.; Suk, K.T.; Kim, D.J. The Role of Gut Dysbiosis in Acute-on-Chronic Liver Failure. Int. J. Mol. Sci. 2021, 22, 11680. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Khoshbin, K.; Camilleri, M. Effects of Dietary Components on Intestinal Permeability in Health and Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef]
- Yang, W.-T.; Yang, R.; Zhao, Q.; Li, X.-D.; Wang, Y.-T. A Systematic Review and Meta-Analysis of the Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide with the Incidence of Atrial Fibrillation. Ann. Palliat. Med. 2021, 10, 11512–11523. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Portincasa, P.; Jamioł-Milc, D.; Maciejewska-Markiewicz, D.; Skonieczna-Żydecka, K. The Relationship between Prebiotic Supplementation and Anthropometric and Biochemical Parameters in Patients with NAFLD-A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef]
- Yang, M.; Khoukaz, L.; Qi, X.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Diet and Gut Microbiota Interaction-Derived Metabolites and Intrahepatic Immune Response in NAFLD Development and Treatment. Biomedicines 2021, 9, 1893. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Garnov, N.; Böhm, S.; Tenckhoff, H.; Wittekind, C.; Wiese, M.; Schiefke, I.; Linder, N.; Schaudinn, A.; et al. Non-Invasive Assessment of Hepatic Steatosis in Patients with NAFLD Using Controlled Attenuation Parameter and 1H-MR Spectroscopy. PLoS ONE 2014, 9, e91987. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric Acid, a Gut Bacteria Metabolite, Lowers Arterial Blood Pressure via Colon-Vagus Nerve Signaling and GPR41/43 Receptors. Pflug. Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Augustyn, M.; Grys, I.; Kukla, M. Small Intestinal Bacterial Overgrowth and Nonalcoholic Fatty Liver Disease. Clin. Exp. Hepatol. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; Konstabel, K.; Lätt, E.; Mäestu, J.; Purge, P.; Jürimäe, J. Diet Misreporting Can Be Corrected: Confirmation of the Association between Energy Intake and Fat-Free Mass in Adolescents. Br. J. Nutr. 2016, 116, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related With Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.G.; Casafont, F.; Crespo, J.; Cayón, A.; Mayorga, M.; Estebanez, A.; Fernadez-Escalante, J.C.; Pons-Romero, F. Lipopolysaccharide-Binding Protein Plasma Levels and Liver TNF-Alpha Gene Expression in Obese Patients: Evidence for the Potential Role of Endotoxin in the Pathogenesis of Non-Alcoholic Steatohepatitis. Obes. Surg. 2007, 17, 1374–1380. [Google Scholar] [CrossRef]
- Männistö, V.; Färkkilä, M.; Pussinen, P.; Jula, A.; Männistö, S.; Lundqvist, A.; Valsta, L.; Salomaa, V.; Perola, M.; Åberg, F. Serum Lipopolysaccharides Predict Advanced Liver Disease in the General Population. JHEP Rep. 2019, 1, 345–352. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Kedenko, L.; Paulweber, B.; Stadlmayr, A.; Huber-Schönauer, U.; Niederseer, D.; Stickel, F.; Auer, S.; et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-Alcoholic Fatty Liver. Am. J. Gastroenterol. 2017, 112, 102–110. [Google Scholar] [CrossRef]
- Piras, C.; Noto, A.; Ibba, L.; Deidda, M.; Fanos, V.; Muntoni, S.; Leoni, V.P.; Atzori, L. Contribution of Metabolomics to the Understanding of NAFLD and NASH Syndromes: A Systematic Review. Metabolites 2021, 11, 694. [Google Scholar] [CrossRef]
- Hasegawa, T.; Iino, C.; Endo, T.; Mikami, K.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Changed Amino Acids in NAFLD and Liver Fibrosis: A Large Cross-Sectional Study without Influence of Insulin Resistance. Nutrients 2020, 12, 1450. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched Chain Amino Acid Metabolism Profiles in Progressive Human Nonalcoholic Fatty Liver Disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline Metabolism Provides Novel Insights into Non-Alcoholic Fatty Liver Disease and Its Progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Noga, A.A.; Vance, D.E. A Gender-Specific Role for Phosphatidylethanolamine N-Methyltransferase-Derived Phosphatidylcholine in the Regulation of Plasma High Density and Very Low Density Lipoproteins in Mice. J. Biol. Chem. 2003, 278, 21851–21859. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine Homeostasis and Liver Failure. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.A.; Garner, S.C.; Chang, J.; Zeisel, S.H. Effects of Prolonged (1 Year) Choline Deficiency and Subsequent Re-Feeding of Choline on 1,2-Sn-Diradylglycerol, Fatty Acids and Protein Kinase C in Rat Liver. Carcinogenesis 1995, 16, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.K.; Rushmore, T.H.; Farber, E. Initiation of Carcinogenesis by a Dietary Deficiency of Choline in the Absence of Added Carcinogens. Cancer Lett. 1987, 36, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Fitriakusumah, Y.; Lesmana, C.R.A.; Bastian, W.P.; Jasirwan, C.O.M.; Hasan, I.; Simadibrata, M.; Kurniawan, J.; Sulaiman, A.S.; Gani, R.A. The Role of Small Intestinal Bacterial Overgrowth (SIBO) in Non-Alcoholic Fatty Liver Disease (NAFLD) Patients Evaluated Using Controlled Attenuation Parameter (CAP) Transient Elastography (TE): A Tertiary Referral Center Experience. BMC Gastroenterol. 2019, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Lou, S.; Watthanasuntorn, K.; Kroner, P.T.; Cheungpasitporn, W.; Lukens, F.J.; Pungpapong, S.; Keaveny, A.P.; Ungprasert, P. Small Intestinal Bacterial Overgrowth and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 601–608. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Gut Biofactory—Neurocompetent Metabolites within the Gastrointestinal Tract. A Scoping Review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.; Risby, T.; Diehl, A.M. Increased Gastrointestinal Ethanol Production in Obese Mice: Implications for Fatty Liver Disease Pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
| Parameter (n = 27) | T1 Median (Range) | T2 Median (Range) | FDR Corrected p-Value |
|---|---|---|---|
| Anthropometry | |||
| Age | 51.1 (29–68) | - | |
| BMI (kg/m2) | 29.5 (23.2–35.7) | 28.9 (22.8–35.2) | 0.057 |
| Body weight (kg) | 87.3 (60.3–115.6) | 85.2 (59–113.9) | 0.35 |
| Visceral obesity (kg) | 10 (5–18) | 10 (4–18) | 0.79 |
| PBF (%) | 29.8 (15.2–43.8) | 27.4 (11.7–43.6) | 0.18 |
| Lean mass (kg) | 55.9 (39.5–76.7) | 56.8 (38.4–76) | 1 |
| TBW (%) | 50.9 (41.9–60.2) | 51.8 (42–63.4) | 0.24 |
| Dietary parameters | |||
| Energy (kcal/day) | 1464 (775.5–2323.8) | 1364 (847.5–2051) | 0.18 |
| Total fiber in diet with rolls (g/day) | 18.7 (10–33.6) | 27.4 (17.3–44.8) | 5.87 × 10−5 |
| Liver function and metabolites | |||
| Fibroscan CAP (dB/m) | 309.3 (242–400)/95% CI: 290.40–328.14 | 277 (224–371)/95% CI: 274.94–310.68 | 0.04 |
| Fibroscan (VCTE) | 6 (3.9–9.4)/95% CI: 5.43–6.67 | 5.3 (3.6–9.7)/95% CI: 5.02–6.13 | 0.20 |
| GGTP (IU/L) Norm (40–60 IU/L) | 29 (12–100) | 30 (11–102) | 0.18 |
| ALT (IU/L) Norm 35–40 IU/L | 34 (11–136) | 35 (11–86) | 1 |
| AST (IU/L) Norm (5 do 40 IU/L) | 26 (11–52) | 24 (13–40) | 1 |
| Endotoxemia parameters | |||
| LPS (pg/mL) | 96 (13–8856) | 153 (0–481) | 1 |
| LBP (pg/mL) | 25 (19–31) | 23 (20–28) | 0.004 |
| Lipids and glucose metabolism | |||
| Glucose (mg/dL) Norm (70–99 mg/dL) | 93.3 (80.4–276.5) | 96.1 (76.3–272.6) | 0.63 |
| Insulin (mIU/L) Norm (<25 mIU/L) | 21.1 (6.7–152) | 18.5 (4.3–129) | 0.52 |
| HDL mg/dL Norm (>50 mg/dL) | 45.2 (25–71.3) | 44.9 (25.8–77.5) | 0.36 |
| LDL (mg/dL) Norm (<115 mg/dL) | 132.3 (43.5–282.2) | 114.4 (47.9–258.3) | 0.06 |
| Total cholesterol (mg/dL) Norm (<190 mg/dL) | 195.2 (110–394.4) | 178.2 (98–340.2) | 0.04 |
| Triglycerides (mg/dL) (Norm < 150 mg/dL) | 191.5 (76.1–700.5) | 150.5 (50.9–452) | 0.14 |
| Parameter (n = 27) | Timepoint 1 | Timepoint 2 | FDR Corrected p-Value |
|---|---|---|---|
| 2_MeB | 0.13 (0–0.67) | 0 (0–0.29) | 0.0013 |
| VA | 0.06 (0–0.29) | 0 (0–0.05) | 0.0056 |
| CA | 0.55 (0.33–1.29) | 0.58 (0.34–0.88) | 0.52 |
| ICA | 0 (0–0.26) | 0 (0–0.15) | 0.026 |
| IVA | 0.38 (0–1.53) | 0.23 (0–0.9) | 0.056 |
| BA | 0.44 (0.14–1.65) | 0.31 (0.07–1.21) | 0.033 |
| PA | 1.15 (0.41–2.59) | 0.81 (0.5–2.38) | 0.036 |
| IBA | 0.08 (0–0.49) | 0.1 (0.05–0.26) | 0.52 |
| AA | 26.13 (8.66–47.25) | 21.95 (6.43–52.14) | 0.56 |
| MeVA | 0 (0–0.13) | 0 (0–0) | NA |
| Metabolite |
Mean Relative Concentration T1 |
Mean Relative Concentration T2 | RSD T1 [%] | RSD T2 [%] | p-Value |
FDR Corrected p-Value |
|---|---|---|---|---|---|---|
| choline | 0.58 | 0.48 | 27.13 | 19.90 | 5.41 × 10−3 | 7.14 × 10−2 |
| proline | 0.29 | 0.26 | 18.44 | 24.11 | 2.17 × 10−2 | 1.79 × 10−1 |
| Unk_2 | 0.69 | 0.80 | 24.04 | 11.56 | 6.49 × 10−3 | 7.14 × 10−2 |
| acetate | 0.36 | 0.34 | 31.15 | 31.08 | 6.00 × 10−1 | 7.34 × 10−1 |
| acetone | 0.06 | 0.05 | 54.55 | 46.04 | 3.85 × 10−1 | 7.34 × 10−1 |
| alanine | 1.96 | 2.02 | 18.74 | 18.20 | 5.55 × 10−1 | 7.34 × 10−1 |
| creatinine | 0.28 | 0.30 | 24.95 | 22.47 | 2.39 × 10−1 | 6.06 × 10−1 |
| ethanol | 0.39 | 0.43 | 36.82 | 51.20 | 6.23 × 10−1 | 7.34 × 10−1 |
| formate | 0.06 | 0.05 | 29.16 | 30.71 | 5.93 × 10−2 | 3.69 × 10−1 |
| glucose | 3.21 | 3.16 | 25.13 | 21.16 | 8.44 × 10−1 | 8.44 × 10−1 |
| glutamine | 0.56 | 0.60 | 22.31 | 20.69 | 2.13 × 10−1 | 6.06 × 10−1 |
| glycerol | 0.10 | 0.12 | 37.99 | 31.79 | 1.01 × 10−1 | 3.69 × 10−1 |
| glycine | 0.89 | 1.01 | 28.71 | 25.34 | 9.12 × 10−2 | 3.69 × 10−1 |
| homoserine | 0.28 | 0.27 | 18.84 | 23.27 | 3.25 × 10−1 | 7.15 × 10−1 |
| imidazole | 0.27 | 0.26 | 15.80 | 15.59 | 4.92 × 10−1 | 7.34 × 10−1 |
| isobutyrate | 1.37 | 1.34 | 13.29 | 18.12 | 5.44 × 10−1 | 7.34 × 10−1 |
| isoleucine | 0.19 | 0.18 | 20.84 | 29.35 | 1.74 × 10−1 | 5.73 × 10−1 |
| lactate | 10.43 | 11.43 | 63.66 | 57.07 | 4.03 × 10−1 | 7.34 × 10−1 |
| leucine | 1.25 | 1.17 | 18.15 | 26.46 | 6.87 × 10−2 | 3.69 × 10−1 |
| lysine | 0.83 | 0.80 | 15.61 | 19.63 | 4.96 × 10−1 | 7.34 × 10−1 |
| methionine | 0.29 | 0.30 | 19.64 | 16.73 | 3.19 × 10−1 | 7.15 × 10−1 |
| phenylalanine | 0.31 | 0.30 | 10.45 | 10.69 | 2.37 × 10−1 | 6.06 × 10−1 |
| pyroglutamate | 0.22 | 0.24 | 22.65 | 19.63 | 9.92 × 10−2 | 3.69 × 10−1 |
| pyruvate | 0.26 | 0.24 | 49.97 | 51.61 | 5.60 × 10−1 | 7.34 × 10−1 |
| succinate | 0.23 | 0.21 | 55.43 | 62.05 | 4.81 × 10−1 | 7.34 × 10−1 |
| tryptophan | 0.20 | 0.20 | 32.25 | 27.07 | 6.02 × 10−1 | 7.34 × 10−1 |
| tyrosine | 0.49 | 0.50 | 14.21 | 15.71 | 7.07 × 10−1 | 7.78 × 10−1 |
| Unk_1 | 0.18 | 0.17 | 44.74 | 48.79 | 7.31 × 10−1 | 7.78 × 10−1 |
| Unk_3 | 0.10 | 0.10 | 33.85 | 29.53 | 7.71 × 10−1 | 7.95 × 10−1 |
| uracil | 0.17 | 0.17 | 16.81 | 23.13 | 6.52 × 10−1 | 7.42 × 10−1 |
| valine | 1.36 | 1.33 | 13.85 | 18.61 | 6.16 × 10−1 | 7.34 × 10−1 |
| π-methylhistidine | 0.12 | 0.11 | 36.11 | 48.86 | 5.23 × 10−1 | 7.34 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowska, E.; Maciejewska-Markiewicz, D.; Palma, J.; Mielko, K.A.; Qasem, B.; Kozłowska-Petriczko, K.; Ufnal, M.; Sokolowska, K.E.; Hawryłkowicz, V.; Załęska, P.; et al. Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study. Nutrients 2022, 14, 5355. https://doi.org/10.3390/nu14245355
Stachowska E, Maciejewska-Markiewicz D, Palma J, Mielko KA, Qasem B, Kozłowska-Petriczko K, Ufnal M, Sokolowska KE, Hawryłkowicz V, Załęska P, et al. Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study. Nutrients. 2022; 14(24):5355. https://doi.org/10.3390/nu14245355
Chicago/Turabian StyleStachowska, Ewa, Dominika Maciejewska-Markiewicz, Joanna Palma, Karolina Anna Mielko, Badr Qasem, Katarzyna Kozłowska-Petriczko, Marcin Ufnal, Katarzyna Ewa Sokolowska, Victoria Hawryłkowicz, Patrycja Załęska, and et al. 2022. "Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study" Nutrients 14, no. 24: 5355. https://doi.org/10.3390/nu14245355
APA StyleStachowska, E., Maciejewska-Markiewicz, D., Palma, J., Mielko, K. A., Qasem, B., Kozłowska-Petriczko, K., Ufnal, M., Sokolowska, K. E., Hawryłkowicz, V., Załęska, P., Jakubczyk, K., Wunsch, E., Ryterska, K., Skonieczna-Żydecka, K., & Młynarz, P. (2022). Precision Nutrition in NAFLD: Effects of a High-Fiber Intervention on the Serum Metabolome of NAFD Patients—A Pilot Study. Nutrients, 14(24), 5355. https://doi.org/10.3390/nu14245355











