Clearing Steatosis Prior to Liver Surgery for Colorectal Metastasis: A Narrative Review and Case Illustration
Abstract
:1. Introduction
2. Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease
3. Molecular Pathways of NAFLD
3.1. Gene Variants
3.2. Gut Microbiota and NAFLD
3.3. Hepatic Lipid Accumulation and Adipose Tissue Dysfunction
3.4. Influence of Metabolic Disease and NAFLD on Colorectal Cancer
3.5. Chemotherapy-Associated Steatosis (CAS)
3.6. Impact of Hepatic Steatosis in Liver Surgery
3.7. Clearing Steatosis Prior to Liver Surgery
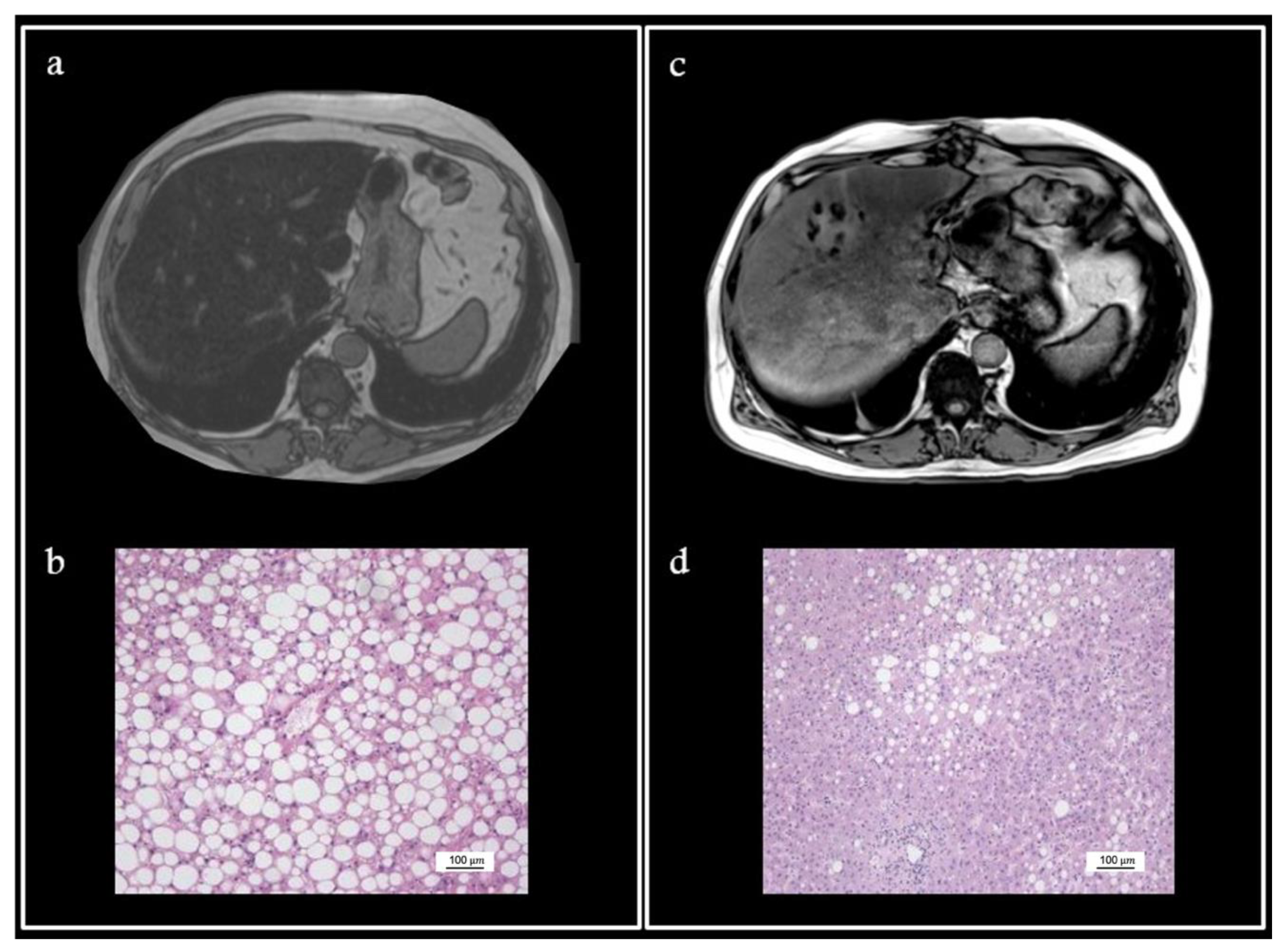
4. Illustrative Case
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ACOX1 | acid acyl-CoA oxidase 1 |
| CAS | chemotherapy-associated steatosis |
| ChREBP | carbohydrate response element binding protein |
| CRC | colorectal cancer |
| CRLM | colorectal liver metastasis |
| FA | fatty acids |
| FFAs | free fatty acids |
| FIT2 | fat storage-inducing transmembrane protein 2 |
| GCKR | glucokinase regulatory protein |
| HDL | high-density lipoprotein |
| HS | hepatic steatosis |
| MBOAT7 | membrane-bound O-acyltransferase domain-containing 7 |
| MetS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NLRC4 | NOD-like receptor 4 |
| PNPAL3 | patatin-like phospholipase domain-containing protein 3 |
| PUFA | polyunsatured fatty acids |
| SREBP1c | sterol regulatory element binding protein 1c |
| T2DM | type 2 diabetes mellitus |
| TM6SF2 | transmembrane 6 superfamily member 2 |
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Metabolic syndrome and non-alcoholic fatty liver disease. Ann. Hepatol. 2009, 8 (Suppl. S1), S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Lipka, S.; Kumar, A.; Mustacchia, P. Association between nonalcoholic fatty liver disease and colorectal adenoma: A systemic review and meta-analysis. J. Gastrointest. Oncol. 2014, 5, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Yakov, G.; Alao, H.; Haydek, J.P.; Fryzek, N.; Cho, M.H.; Hemmati, M.; Samala, V.; Shovlin, M.; Dunleavy, K.; Wilson, W.; et al. Development of Hepatic Steatosis After Chemotherapy for Non-Hodgkin Lymphoma. Hepatol. Commun. 2018, 3, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Koh, Y.X.; Tan, H.J.; Liew, Y.X.; Syn, N.; Teo, J.Y.; Lee, S.Y.; Goh, B.K.P.; Goh, G.B.B.; Chan, C.Y. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. J. Am. Coll. Surg. 2019, 229, 467–478.e1. [Google Scholar] [CrossRef]
- Yamanaka-Okumura, H.; Urano, E.; Kawaura, A.; Imura, S.; Utsunomiya, T.; Shimada, M.; Takeda, E. Treatment of rapid weight loss in a donor with hepatic steatosis in living donor liver transplantation: A case report. Hepatogastroenterology 2012, 59, 869–871. [Google Scholar] [CrossRef]
- Turati, F.; Talamini, R.; Pelucchi, C.; Polesel, J.; Franceschi, S.; Crispo, A.; Izzo, F.; La Vecchia, C.; Boffetta, P.; Montella, M. Metabolic syndrome and hepatocellular carcinoma risk. Br. J. Cancer 2013, 108, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Dhamija, E.; Paul, S.B.; Kedia, S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J. Med. Res. 2019, 149, 9–17. [Google Scholar] [CrossRef]
- Masaki, S.; Hashimoto, Y.; Kunisho, S.; Kimoto, A.; Kitadai, Y. Fatty change of the liver microenvironment influences the metastatic potential of colorectal cancer. Int. J. Exp. Pathol. 2020, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Romeijn, M.M.; Kolen, A.M.; Holthuijsen, D.D.B.; Janssen, L.; Schep, G.; Leclercq, W.K.G.; van Dielen, F.M.H. Effectiveness of a Low-Calorie Diet for Liver Volume Reduction Prior to Bariatric Surgery: A Systematic Review. Obes. Surg. 2021, 31, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Oshita, A.; Tashiro, H.; Amano, H.; Kobayashi, T.; Onoe, T.; Ide, K.; Takaki, S.; Takahashi, S.; Arihiro, K.; Chayama, K.; et al. Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation. Transplantation 2012, 27, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Nam, G.E.; Kwon, H.S. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci. Rep. 2020, 10, 2313. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, E.; O’Reilly, D.A.; Adam, R.; Kaiser, G.M.; Laurent, C.; Elias, D.; Capussotti, L.; Renehan, A.G.; LiverMetSurvey Centres. Equivalent survival in patients with and without steatosis undergoing resection for colorectal liver metastases following pre-operative chemotherapy. Eur. J. Surg. Oncol. 2014, 40, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Malik, H.Z.; Bonney, G.K.; Wong, V.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br. J. Surg. 2007, 94, 1395–1402. [Google Scholar] [CrossRef]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Kleiner, D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016, 65, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedossa, P. Histological Assessment of NAFLD. Dig. Dis. Sci. 2016, 61, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.; Abrams, G.A. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2007, 13, 3540–3553. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Nick, A.; Hölttä-Vuori, M.; Thiele, C.; Isokuortti, E.; Lallukka-Brück, S.; Zhou, Y.; Hakkarainen, A.; Lundbom, N.; Peltonen, M.; et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. J. Clin. Investig. 2019, 4, e127902. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, G.; Liu, P.; Li, X.; Zhou, X.; He, S. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease (NAFLD) susceptibility and severity: A meta-analysis. Medicine 2019, 98, e14324. [Google Scholar] [CrossRef] [PubMed]
- Mahdessian, H.; Taxiarchis, A.; Popov, S.; Silveira, A.; Franco-Cereceda, A.; Hamsten, A.; Eriksson, P.; van’t Hooft, F. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc. Natl. Acad. Sci. USA 2014, 111, 8913–8918. [Google Scholar] [CrossRef] [Green Version]
- Smagris, E.; Gilyard, S.; BasuRay, S.; Cohen, J.C.; Hobbs, H.H. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2016, 291, 10659–10676. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Reeves, H.L.; Burt, A.D.; Tiniakos, D.; McPherson, S.; Leathart, J.B.; Allison, M.E.; Alexander, G.J.; Piguet, A.C.; Anty, R.; et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basaranoglu, M. Lean and Nonobese NAFLD/NASH From a Hepatologist’s Point of View. J. Clin. Gastroenterol. 2021, 55, 93–94. [Google Scholar] [CrossRef]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef]
- Pirola, C.J.; Sookoian, S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: A meta-analysis. Hepatology 2015, 62, 1742–1756. [Google Scholar] [CrossRef]
- Valenti, L.; Alisi, A.; Nobili, V. Unraveling the genetics of fatty liver in obese children: Additive effect of P446L GCKR and I148M PNPLA3 polymorphisms. Hepatology 2012, 55, 661–663, Erratum in Hepatology 2012, 55, 1311. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Bantel, H.; Rau, M.; Schattenberg, J.M.; Grünhage, F.; Pathil, A.; Demir, M.; Kluwe, J.; Boettler, T.; Weber, S.N.; et al. Could inherited predisposition drive non-obese fatty liver disease? Results from German tertiary referral centers. J. Hum. Genet. 2018, 63, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Prakash, S.; Tomaro-Duchesneau, C.; Saha, S.; Cantor, A. The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells. J. Biomed. Biotechnol. 2011, 2011, 981214. [Google Scholar] [CrossRef] [Green Version]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [Green Version]
- Schwimmer, J.B.; Johnson, J.S.; Angeles, J.E.; Behling, C.; Belt, P.H.; Borecki, I.; Bross, C.; Durelle, J.; Goyal, N.P.; Hamilton, G.; et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157, 1109–1122. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Ajmera, V.; Loomba, R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin. Gastroenterol. Hepatol. 2019, 17, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080, Erratum in Nat. Med. 2018, 24, 1628. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D. Role of the Gut Microbiota in Regulating Non-alcoholic Fatty Liver Disease in Children and Adolescents. Front. Nutr. 2021, 8, 700058. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2019, 30, 607, Erratum in Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef] [Green Version]
- Aragonès, G.; González-García, S.; Aguilar, C.; Richart, C.; Auguet, T. Gut Microbiota-Derived Mediators as Potential Markers in Nonalcoholic Fatty Liver Disease. Biomed Res. Int. 2019, 2019, 8507583. [Google Scholar] [CrossRef] [Green Version]
- Reid, D.T.; McDonald, B.; Khalid, T.; Vo, T.; Schenck, L.P.; Surette, M.G.; Beck, P.L.; Reimer, R.A.; Probert, C.S.; Rioux, K.P.; et al. Unique microbial-derived volatile organic compounds in portal venous circulation in murine non-alcoholic fatty liver disease. Biochim. Biophys. Acta 2016, 1862, 1337–1344. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Dai, X.; Hou, H.; Zhang, W.; Liu, T.; Li, Y.; Wang, S.; Wang, B.; Cao, H. Microbial Metabolites: Critical Regulators in NAFLD. Front. Microbiol. 2020, 11, 567654. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef]
- Tsay, C.J.; Lim, J.K. NASH and the Gut Microbiome: Implications for New Therapies. Clin. Liver Dis. 2022, 19, 97–100. [Google Scholar] [CrossRef]
- Seebacher, F.; Zeigerer, A.; Kory, N.; Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020, 108, 72–81. [Google Scholar] [CrossRef]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef]
- Man, W.C.; Miyazaki, M.; Chu, K.; Ntambi, J. Colocalization of SCD1 and DGAT2: Implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J. Lipid Res. 2006, 47, 1928–1939. [Google Scholar] [CrossRef]
- Hayes, M.; Choudhary, V.; Ojha, N.; Shin, J.J.; Han, G.S.; Carman, G.M.; Loewen, C.J.; Prinz, W.A.; Levine, T. Fat storage-inducing transmembrane (FIT or FITM) proteins are related to lipid phosphatase/phosphotransferase enzymes. Microb. Cell 2017, 5, 88–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Arlt, H.; Brock, K.P.; Lai, Z.W.; DiMaio, F.; Marks, D.S.; Liao, M.; Farese, R.V., Jr.; Walther, T.C. Cryo-electron microscopy structure of the lipid droplet-formation protein seipin. J. Cell Biol. 2018, 217, 4080–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Wang, Y.; Tang, Y.; Liu, Y.; Zhao, L.; Deng, J.; Xu, G.; Peng, X.; Ju, S.; Liu, G.; et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 2011, 20, 3022–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Hazlehurst, J.M.; Hull, D.; Guo, K.; Borrows, S.; Yu, J.; Gough, S.C.; Newsome, P.N.; Tomlinson, J.W. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes. Metab. 2014, 16, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe HR 3rd Heidal, K.; Choi, M.D.; Kraus, R.M.; Boyle, K.; Hickner, R.C. Increased adipose tissue lipolysis after a 2-week high-fat diet in sedentary overweight/obese men. Metabolism 2011, 60, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Wang, Y.; Jones Voy, B.; Urs, S.; Kim, S.; Soltani-Bejnood, M.; Quigley, N.; Heo, Y.R.; Standridge, M.; Andersen, B.; Dhar, M.; et al. The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr. 2004, 134, 1032–1038. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; He, J.; Jiang, H.; Zu, L.; Zhai, W.; Pu, S.; Xu, G. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 2009, 23, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef]
- Soltani, G.; Poursheikhani, A.; Yassi, M.; Hayatbakhsh, A.; Kerachian, M.; Kerachian, M.A. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr. Disord. 2019, 19, 113. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Stimac, D.; Hrstic, I.; Jakopcic, I.; Milic, S. Non-alcoholic fatty liver disease and colorectal cancer. Postgrad. Med. J. 2017, 93, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, Y.M.; Yun, J.S.; Ahn, Y.B.; Lee, K.M.; Kim, D.B.; Lee, J.M.; Han, K.; Ko, S.H. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: National population-based cohort study. PLoS ONE 2020, 15, e0226351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Zhai, M.Z.; Weltzien, E.K.; Feliciano, E.M.C.; Meyerhardt, J.A.; Giovannucci, E.; Caan, B.J. Non-alcoholic fatty liver disease and colorectal cancer survival. Cancer Causes Control 2019, 30, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Author Correction: Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci. Rep. 2020, 10, 7606, Erratum in Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Behrns, K.E.; Tsiotos, G.G.; DeSouza, N.F.; Krishna, M.K.; Ludwig, J.; Nagorney, D.M. Hepatic steatosis as a potential risk factor for major hepatic resection. J. Gastrointest. Surg. 1998, 2, 292–298. [Google Scholar] [CrossRef]
- Pathak, S.; Tang, J.M.; Terlizzo, M.; Poston, G.J.; Malik, H.Z. Hepatic steatosis, body mass index and long term outcome in patients undergoing hepatectomy for colorectal liver metastases. Eur. J. Surg. Oncol. 2010, 36, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Kooby, D.A.; Fong, Y.; Suriawinata, A.; Gonen, M.; Allen, P.J.; Klimstra, D.S.; DeMatteo, R.P.; D’Angelica, M.; Blumgart, L.H.; Jarnagin, W.R. Impact of steatosis on perioperative outcome following hepatic resection. J. Gastrointest. Surg. 2003, 7, 1034–1044. [Google Scholar] [CrossRef]
- Fagenson, A.M.; Pitt, H.A.; Moten, A.S.; Karhadkar, S.S.; Di Carlo, A.; Lau, K.N. Fatty liver: The metabolic syndrome increases major hepatectomy mortality. Surgery 2021, 169, 1054–1060. [Google Scholar] [CrossRef]
- Nishio, T.; Hatano, E.; Sakurai, T.; Taura, K.; Okuno, M.; Kasai, Y.; Seo, S.; Yasuchika, K.; Mori, A.; Kaido, T.; et al. Impact of Hepatic Steatosis on Disease-Free Survival in Patients with Non-B Non-C Hepatocellular Carcinoma Undergoing Hepatic Resection. Ann. Surg. Oncol. 2015, 22, 2226–2234. [Google Scholar] [CrossRef]
- Reeves, J.G.; Suriawinata, A.A.; Ng, D.P.; Holubar, S.D.; Mills, J.B.; Barth, R.J. Short-term preoperative diet modification reduces steatosis and blood loss in patients undergoing liver resection. Surgery 2013, 154, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.J., Jr.; Mills, J.B.; Suriawinata, A.A.; Putra, J.; Tosteson, T.D.; Axelrod, D.; Freeman, R.; Whalen, G.F.; LaFemina, J.; Tarczewski, S.M.; et al. Short-term Preoperative Diet Decreases Bleeding After Partial Hepatectomy: Results From a Multi-institutional Randomized Controlled Trial. Ann. Surg. 2019, 269, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Cauchy, F.; Zalinski, S.; Dokmak, S.; Fuks, D.; Farges, O.; Castera, L.; Paradis, V.; Belghiti, J. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br. J. Surg. 2013, 100, 113–121. [Google Scholar] [CrossRef]
- Ohashi, K.; Wang, Z.; Yang, Y.M.; Billet, S.; Tu, W.; Pimienta, M.; Cassel, S.L.; Pandol, S.J.; Lu, S.C.; Sutterwala, F.S.; et al. NOD-like receptor C4 Inflammasome Regulates the Growth of Colon Cancer Liver Metastasis in NAFLD. Hepatology 2019, 70, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, Y.; Shi, X.; Zhang, N.; Zu, G.; Li, Z.; Zhou, J.; Gao, D.; Lv, L.; Tian, X.; et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016, 6, 28734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubbia-Brandt, L.; Audard, V.; Sartoretti, P.; Roth, A.D.; Brezault, C.; Le Charpentier, M.; Dousset, B.; Morel, P.; Soubrane, O.; Chaussade, S.; et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann. Oncol. 2004, 15, 460–466. [Google Scholar] [CrossRef]
- Narayan, R.R.; Harris, J.W.; Chou, J.F.; Gönen, M.; Bao, F.; Shia, J.; Allen, P.J.; Balachandran, V.P.; Drebin, J.A.; Jarnagin, W.R.; et al. Prediction of Recurrence Patterns from Hepatic Parenchymal Disease After Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2020, 27, 188–195. [Google Scholar] [CrossRef]
- Yang, S.; Peng, R.; Zhou, L. The impact of hepatic steatosis on outcomes of colorectal cancer patients with liver metastases: A systematic review and meta-analysis. Front. Med. 2022, 9, 938718. [Google Scholar] [CrossRef]
- Kim, H.P.; Navarro, V.; Zacks, S.; Odin, J.; Kleiner, D.E.; Hayashi, P.H.; Drug-Induced Liver Injury Network Investigators. The Clinical Spectrum and Diagnosis of Oxaliplatin Liver Injury in the Era of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2199–2201. [Google Scholar] [CrossRef]
- Khan, A.Z.; Morris-Stiff, G.; Makuuchi, M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 137–144. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Kneuertz, P.J.; Maithel, S.K.; Staley, C.A.; Kooby, D.A. Chemotherapy-associated liver injury: Impact on surgical management of colorectal cancer liver metastases. Ann. Surg. Oncol. 2011, 18, 181–190. [Google Scholar] [CrossRef]
- Wolf, P.S.; Park, J.O.; Bao, F.; Allen, P.J.; DeMatteo, R.P.; Fong, Y.; Jarnagin, W.R.; Kingham, T.P.; Gönen, M.; Kemeny, N.; et al. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: A single institution experience. J. Am. Coll. Surg. 2013, 216, 41–49. [Google Scholar] [CrossRef]
- Sommer, J.; Mahli, A.; Freese, K.; Schiergens, T.S.; Kuecuekoktay, F.S.; Teufel, A.; Thasler, W.E.; Müller, M.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget 2017, 8, 13059–13072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloia, T.; Sebagh, M.; Plasse, M.; Karam, V.; Lévi, F.; Giacchetti, S.; Azoulay, D.; Bismuth, H.; Castaing, D.; Adam, R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J. Clin. Oncol. 2006, 24, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.A.; Gentner, B.; Wu, T.T.; Curley, S.A.; Ellis, L.M.; Vauthey, J.N. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J. Gastrointest. Surg. 2003, 7, 1082–1088. [Google Scholar] [CrossRef]
- Meunier, L.; Larrey, D. Chemotherapy-associated steatohepatitis. Ann. Hepatol. 2020, 19, 597–601. [Google Scholar] [CrossRef]
- de Meijer, V.E.; Kalish, B.T.; Puder, M.; Ijzermans, J.N. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br. J. Surg. 2010, 97, 1331–1339. [Google Scholar] [CrossRef]
- Sultana, A.; Brooke-Smith, M.; Ullah, S.; Figueras, J.; Rees, M.; Vauthey, J.N.; Conrad, C.; Hugh, T.J.; Garden, O.J.; Fan, S.T.; et al. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: An international multicentre study. HPB 2018, 20, 462–469. [Google Scholar] [CrossRef]
- Morine, Y.; Shimada, M.; Utsunomiya, T. Evaluation and management of hepatic injury induced by oxaliplatin-based chemotherapy in patients with hepatic resection for colorectal liver metastasis. Hepatol. Res. 2014, 44, 59–69. [Google Scholar] [CrossRef]
- Cole, D.J.; Ferguson, C.M. Complications of hepatic resection for colorectal carcinoma metastasis. Am. Surg. 1992, 58, 88–91. [Google Scholar]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edholm, D.; Kullberg, J.; Haenni, A.; Karlsson, F.A.; Ahlström, A.; Hedberg, J.; Ahlström, H.; Sundbom, M. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes. Surg. 2011, 21, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Dhampalwar, S.; Saigal, S.; Choudhary, N.; Saraf, N.; Chaudhary, A.; Soin, A. Successful Outcome of Bariatric Surgery in Living Donor Liver Transplant Recipients With Multidisciplinary Approach: A Preliminary Experience. J. Clin. Exp. Hepatol. 2021, 11, 144–148. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Saraf, N.; Saigal, S.; Gautam, D.; Lipi, L.; Rastogi, A.; Goja, S.; Menon, P.B.; Bhangui, P.; Ramchandra, S.K.; et al. Rapid Reversal of Liver Steatosis With Life Style Modification in Highly Motivated Liver Donors. J. Clin. Exp. Hepatol. 2015, 5, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyanarayana, P.; Jogi, M.; Muthupillai, R.; Krishnamurthy, R.; Samson, S.L.; Bajaj, M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity 2011, 19, 2310–2315. [Google Scholar] [CrossRef]
- Cussons, A.J.; Watts, G.F.; Mori, T.A.; Stuckey, B.G. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: A randomized controlled trial employing proton magnetic resonance spectroscopy. J. Clin. Endocrinol. Metab. 2009, 94, 3842–3848. [Google Scholar] [CrossRef]
- Garcia-Caraballo, S.C.; Comhair, T.M.; Verheyen, F.; Gaemers, I.; Schaap, F.G.; Houten, S.M.; Hakvoort, T.B.; Dejong, C.H.; Lamers, W.H.; Koehler, S.E. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim. Biophys. Acta 2013, 1832, 685–695. [Google Scholar] [CrossRef]
- Garcia Caraballo, S.C.; Comhair, T.M.; Houten, S.M.; Dejong, C.H.; Lamers, W.H.; Koehler, S.E. High-protein diets prevent steatosis and induce hepatic accumulation of monomethyl branched-chain fatty acids. J. Nutr. Biochem. 2014, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Modulation of hepatic steatosis by dietary fatty acids. World J. Gastroenterol. 2014, 20, 1746–1755. [Google Scholar] [CrossRef]
- Battezzati, A.; Riso, P. Amino acids: Fuel, building blocks for proteins, and signals. Nutrition 2002, 18, 773–774. [Google Scholar] [CrossRef]
- Sikalidis, A.K. Amino acids and immune response: A role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bella, D.L.; Hirschberger, L.L.; Hosokawa, Y.; Stipanuk, M.H. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am. J. Physiol. 1999, 276, E326–E335. [Google Scholar] [CrossRef] [PubMed]
- Cresenzi, C.L.; Lee, J.I.; Stipanuk, M.H. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J. Nutr. 2003, 133, 2697–2702. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W.; Eck, H.P.; Gmünder, H.; Mihm, S. Modulation of lymphocyte functions and immune responses by cysteine and cysteine derivatives. Am. J. Med. 1991, 91, 140S–144S. [Google Scholar] [CrossRef]
- Mihm, S.; Galter, D.; Dröge, W. Modulation of transcription factor NF kappa B activity by intracellular glutathione levels and by variations of the extracellular cysteine supply. FASEB J. 1995, 9, 246–252. [Google Scholar] [CrossRef]
- Grimble, R.F. The effects of sulfur amino acid intake on immune function in humans. J. Nutr. 2006, 136 (Suppl. S6), 1660S–1665S. [Google Scholar] [CrossRef] [Green Version]
- Kontny, E.; Szczepańska, K.; Kowalczewski, J.; Kurowska, M.; Janicka, I.; Marcinkiewicz, J.; Maśliński, W. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum. 2000, 43, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131 (Suppl. S9), 2515S–2522S, Erratum in 2001, 131 (Suppl. S9), 2523S–2524S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammami, I.; Chen, J.; Bronte, V.; DeCrescenzo, G.; Jolicoeur, M. L-glutamine is a key parameter in the immunosuppression phenomenon. Biochem. Biophys. Res. Commun. 2012, 425, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Popovic, P.J.; Zeh, H.J., 3rd; Ochoa, J.B. Arginine and immunity. J. Nutr. 2007, 137 (Suppl. S2), 1681S–1686S. [Google Scholar] [CrossRef] [Green Version]
- Taheri, F.; Ochoa, J.B.; Faghiri, Z.; Culotta, K.; Park, H.J.; Lan, M.S.; Zea, A.H.; Ochoa, A.C. L-Arginine regulates the expression of the T-cell receptor zeta chain (CD3zeta) in Jurkat cells. Clin. Cancer Res. 2001, 7, 958s–965s. [Google Scholar]
- Martí ILíndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. J. Reprod. Immunol. 2001, 52, 5–13. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. Modulation of Macrophage Immunometabolism: A New Approach to Fight Infections. Front. Immunol. 2022, 13, 780839. [Google Scholar] [CrossRef]
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer 2020, 122, 30–44. [Google Scholar] [CrossRef]
- Yan, T.; Yan, N.; Wang, P.; Xia, Y.; Hao, H.; Wang, G.; Gonzalez, F.J. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm. Sin. B 2020, 10, 3–18. [Google Scholar] [CrossRef]
- Wah Kheong, C.; Nik Mustapha, N.R.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949.e8. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Nellemann, B.; Bibby, B.M.; Stødkilde-Jørgensen, H.; Pedersen, S.B.; Grønbaek, H.; Nielsen, S. No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 20, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Tang, X.; Li, Y.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [Green Version]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The Role of Resveratrol in Liver Disease: A Comprehensive Review from In Vitro to Clinical Trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Bakker, N.; van den Helder, R.S.; Geenen, R.W.F.; Hunfeld, M.A.; Cense, H.A.; Demirkiran, A.; Houdijk, A.P.J. Four Weeks of Preoperative Omega-3 Fatty Acids Reduce Liver Volume: A Randomised Controlled Trial. Obes. Surg. 2019, 29, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Gugenheim, J.; Petrucciani, N. Clinical impact of omega-3 fatty acids (Ω3 FA) supplementation on liver surgery. Hepatobiliary Surg. Nutr. 2020, 9, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [Green Version]
- El-Badry, A.M.; Moritz, W.; Contaldo, C.; Tian, Y.; Graf, R.; Clavien, P.A. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology 2007, 45, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Marsman, H.A.; de Graaf, W.; Heger, M.; van Golen, R.F.; Ten Kate, F.J.; Bennink, R.; van Gulik, T.M. Hepatic regeneration and functional recovery following partial liver resection in an experimental model of hepatic steatosis treated with omega-3 fatty acids. Br. J. Surg. 2013, 100, 674–683. [Google Scholar] [CrossRef]
- Linecker, M.; Limani, P.; Botea, F.; Popescu, I.; Alikhanov, R.; Efanov, M.; Kim, P.; Khatkov, I.; Raptis, D.A.; Tschuor, C.; et al. A randomized, double-blind study of the effects of omega-3 fatty acids (Omegaven) on outcome after major liver resection. BMC Gastroenterol. 2015, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Han, W.; Yue, Q.; Ke, J.; Jia, B.; Fu, X. Perioperative omega-3 fatty acids for liver surgery: A meta-analysis of randomized controlled trials. Medicine 2021, 100, e25743. [Google Scholar] [CrossRef]
- Nakamuta, M.; Morizono, S.; Soejima, Y.; Yoshizumi, T.; Aishima, S.; Takasugi, S.; Yoshimitsu, K.; Enjoji, M.; Kotoh, K.; Taketomi, A.; et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation 2005, 80, 608–612. [Google Scholar] [CrossRef]
- Perkins, J.D. Saying “Yes” to obese living liver donors: Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Liver Transplant. 2006, 12, 1012–1013. [Google Scholar] [CrossRef]
- Griffin, S.B.; Palmer, M.A.; Strodl, E.; Lai, R.; Burstow, M.J.; Ross, L.J. Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review. Nutrients 2021, 13, 3775. [Google Scholar] [CrossRef]
| Author | Year | Type of Study | Population Enrolled | Main Findings | Identifier | Ref. |
|---|---|---|---|---|---|---|
| Berhns et al. | 1998 | Retrospective | 135 patients who had undergone major hepatic resection (4 or more liver segments) | HS has been associated to longer surgeries, higher rate of blood transfusion, post-operative bilirubine ans AST levels | PMID: 9841987 | [86] |
| Pathak et al. | 2010 | Retrospective | 102 patients undergoing hepatectomy for CRLM | HS does not influence post operative long-term survival | PMID: 19879103 | [87] |
| Kooby et al. | 2003 | Retrospective matched case control | 325 patients who had undergone hepatectomy for HCC, biliary cancer or CRLM | HS has been associated to higher rate of wound, hepatobiliary and gastro-intestinal complications. HS does not influence 5 yr survival rate. | PMID: 14675713 | [88] |
| Fagenson et al. | 2021 | Retrospective propensity-score matched analysis | 2927 patients undergoing major hepatectomy (3 or more liver segments) | HS has been associated with significant higher rate of biliary and pulmonary complications. HS has been conferred risk of postoperative mortality | PMID: 33358472 | [89] |
| Nishio et al. | 2015 | Retrospective | 518 HCC patients who underwent hepatic resection | Absence of HS has a significant impact on disease-free survival in non-b, non-c HCC patients | PMID: 25395147 | [90] |
| Gomez et al. | 2007 | Retrospective | 386 patients undergoing hepatic resection for CRLM | HS was associated with increased morbidity following hepatic resection | PMID: 17607707 | [23] |
| Parkin et al. | 2013 | Retrospective | 1793 patients who underwent first-time liver resection with background HS | HS was associated with improved 5 yr survival compared to normal background liver | UK charity chamber N°1054556 | [22] |
| Reeves et al. | 2013 | Prospective | 111 consecutive patients who had major elective hepatic resections | Short-term caloric restriction before liver resection significantly reduces both hepatic steatosis and steatohepatitis. Dietary modification also was associated with decreased intraoperative blood loss. | Darmouth Commitee for Protection of Human Subject n°22273 | [91] |
| Barth et al | 2019 | Randomized | 60 patients undergone liver liver surgey | Short-term, low-fat, and low-calorie diet significantly decreased blood loss with liver easier to manipulate | NCT01645852 | [92] |
| Cauchy et al. | 2013 | Retrospective | 560 patients undergoing liver resection for HCC | In presence of HS liver resection is still appropriate bau carries a high risk | PMID: 23147992 | [93] |
| Koh et al. | 2019 | Retrospective analysis | 996 patients who underwent liver resection for HCC | NAFLD-related HCC is associated with greater surgical morbidity and post-hepatectomy liver failure. Despite this, long-term survival outcomes are favorable compared with non-NAFLD etiologies | PMID: 31398386 | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peloso, A.; Tihy, M.; Moeckli, B.; Rubbia-Brandt, L.; Toso, C. Clearing Steatosis Prior to Liver Surgery for Colorectal Metastasis: A Narrative Review and Case Illustration. Nutrients 2022, 14, 5340. https://doi.org/10.3390/nu14245340
Peloso A, Tihy M, Moeckli B, Rubbia-Brandt L, Toso C. Clearing Steatosis Prior to Liver Surgery for Colorectal Metastasis: A Narrative Review and Case Illustration. Nutrients. 2022; 14(24):5340. https://doi.org/10.3390/nu14245340
Chicago/Turabian StylePeloso, Andrea, Matthieu Tihy, Beat Moeckli, Laura Rubbia-Brandt, and Christian Toso. 2022. "Clearing Steatosis Prior to Liver Surgery for Colorectal Metastasis: A Narrative Review and Case Illustration" Nutrients 14, no. 24: 5340. https://doi.org/10.3390/nu14245340






